 Found 440 hits with Last Name = 'staels' and Initial = 'b'
Found 440 hits with Last Name = 'staels' and Initial = 'b' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM28681

(5-[(4-{2-[methyl(pyridin-2-yl)amino]ethoxy}phenyl)...)Show InChI InChI=1S/C18H19N3O3S/c1-21(16-4-2-3-9-19-16)10-11-24-14-7-5-13(6-8-14)12-15-17(22)20-18(23)25-15/h2-9,22H,10-12H2,1H3,(H,20,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SPOT-EA3857
Curated by ChEMBL
| Assay Description
Displacement of [3H]Rosiglitazone from human PPARgamma |
Bioorg Med Chem Lett 18: 1617-22 (2008)
Article DOI: 10.1016/j.bmcl.2008.01.067
BindingDB Entry DOI: 10.7270/Q2222TGW |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50234375

((S)-2-ethoxy-3-(4-(2-(6-((methoxyimino)(phenyl)met...)Show SMILES CCO[C@@H](Cc1ccc(OCCN2CCC(C)(C)c3cc(ccc23)C(=N/OC)\c2ccccc2)cc1)C(O)=O Show InChI InChI=1S/C32H38N2O5/c1-5-38-29(31(35)36)21-23-11-14-26(15-12-23)39-20-19-34-18-17-32(2,3)27-22-25(13-16-28(27)34)30(33-37-4)24-9-7-6-8-10-24/h6-16,22,29H,5,17-21H2,1-4H3,(H,35,36)/b33-30-/t29-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 18 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SPOT-EA3857
Curated by ChEMBL
| Assay Description
Displacement of [3H]Rosiglitazone from human PPARgamma |
Bioorg Med Chem Lett 18: 1617-22 (2008)
Article DOI: 10.1016/j.bmcl.2008.01.067
BindingDB Entry DOI: 10.7270/Q2222TGW |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50294690
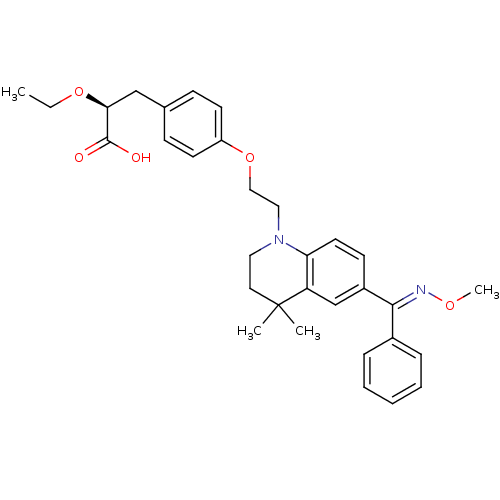
((S)-2-ethoxy-3-(4-(2-(6-((methoxyimino)(phenyl)met...)Show SMILES CCO[C@@H](Cc1ccc(OCCN2CCC(C)(C)c3cc(ccc23)C(=N\OC)\c2ccccc2)cc1)C(O)=O |r| Show InChI InChI=1S/C32H38N2O5/c1-5-38-29(31(35)36)21-23-11-14-26(15-12-23)39-20-19-34-18-17-32(2,3)27-22-25(13-16-28(27)34)30(33-37-4)24-9-7-6-8-10-24/h6-16,22,29H,5,17-21H2,1-4H3,(H,35,36)/b33-30+/t29-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 18 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
UMR GICC
Curated by ChEMBL
| Assay Description
Displacement of [3H]rosiglitazone from human PPARgamma receptor |
Bioorg Med Chem Lett 19: 2683-7 (2009)
Article DOI: 10.1016/j.bmcl.2009.03.143
BindingDB Entry DOI: 10.7270/Q2KP826B |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM28798

((2S)-2-ethoxy-3-(4-{2-[4-(methanesulfonyloxy)pheny...)Show SMILES CCO[C@@H](Cc1ccc(OCCc2ccc(OS(C)(=O)=O)cc2)cc1)C(O)=O |r| Show InChI InChI=1S/C20H24O7S/c1-3-25-19(20(21)22)14-16-6-8-17(9-7-16)26-13-12-15-4-10-18(11-5-15)27-28(2,23)24/h4-11,19H,3,12-14H2,1-2H3,(H,21,22)/t19-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
| MMDB
PDB
Article
PubMed
| 18 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SPOT-EA3857
Curated by ChEMBL
| Assay Description
Displacement of [3H]Rosiglitazone from human PPARgamma |
Bioorg Med Chem Lett 18: 1617-22 (2008)
Article DOI: 10.1016/j.bmcl.2008.01.067
BindingDB Entry DOI: 10.7270/Q2222TGW |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50294688
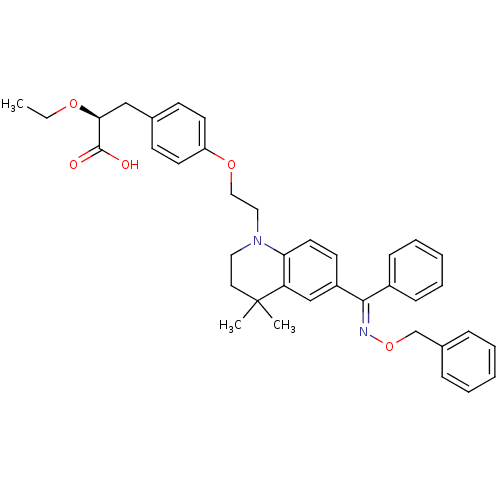
((S)-3-(4-(2-(6-((benzyloxyimino)(phenyl)methyl)-4,...)Show SMILES CCO[C@@H](Cc1ccc(OCCN2CCC(C)(C)c3cc(ccc23)C(=N\OCc2ccccc2)\c2ccccc2)cc1)C(O)=O |r| Show InChI InChI=1S/C38H42N2O5/c1-4-43-35(37(41)42)25-28-15-18-32(19-16-28)44-24-23-40-22-21-38(2,3)33-26-31(17-20-34(33)40)36(30-13-9-6-10-14-30)39-45-27-29-11-7-5-8-12-29/h5-20,26,35H,4,21-25,27H2,1-3H3,(H,41,42)/b39-36+/t35-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 18.4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
UMR GICC
Curated by ChEMBL
| Assay Description
Displacement of [3H]rosiglitazone from human PPARgamma receptor |
Bioorg Med Chem Lett 19: 2683-7 (2009)
Article DOI: 10.1016/j.bmcl.2009.03.143
BindingDB Entry DOI: 10.7270/Q2KP826B |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50294692

((S)-3-(4-(2-(6-((methoxyimino)(phenyl)methyl)-4,4-...)Show SMILES CO\N=C(/c1ccccc1)c1ccc2N(CCOc3ccc(C[C@H](OCC(F)(F)F)C(O)=O)cc3)CCC(C)(C)c2c1 |r| Show InChI InChI=1S/C32H35F3N2O5/c1-31(2)15-16-37(27-14-11-24(20-26(27)31)29(36-40-3)23-7-5-4-6-8-23)17-18-41-25-12-9-22(10-13-25)19-28(30(38)39)42-21-32(33,34)35/h4-14,20,28H,15-19,21H2,1-3H3,(H,38,39)/b36-29+/t28-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 33 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
UMR GICC
Curated by ChEMBL
| Assay Description
Displacement of [3H]rosiglitazone from human PPARgamma receptor |
Bioorg Med Chem Lett 19: 2683-7 (2009)
Article DOI: 10.1016/j.bmcl.2009.03.143
BindingDB Entry DOI: 10.7270/Q2KP826B |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50234377

(2-ethoxy-3-(4-(2-(6-((methoxyimino)(phenyl)methyl)...)Show SMILES CCOC(Cc1ccc(OCCN2CCC(C)(C)c3cc(ccc23)C(=N/OC)\c2ccccc2)cc1)C(O)=O |w:3.3| Show InChI InChI=1S/C32H38N2O5/c1-5-38-29(31(35)36)21-23-11-14-26(15-12-23)39-20-19-34-18-17-32(2,3)27-22-25(13-16-28(27)34)30(33-37-4)24-9-7-6-8-10-24/h6-16,22,29H,5,17-21H2,1-4H3,(H,35,36)/b33-30- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 48 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SPOT-EA3857
Curated by ChEMBL
| Assay Description
Displacement of [3H]Rosiglitazone from human PPARgamma |
Bioorg Med Chem Lett 18: 1617-22 (2008)
Article DOI: 10.1016/j.bmcl.2008.01.067
BindingDB Entry DOI: 10.7270/Q2222TGW |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50294691
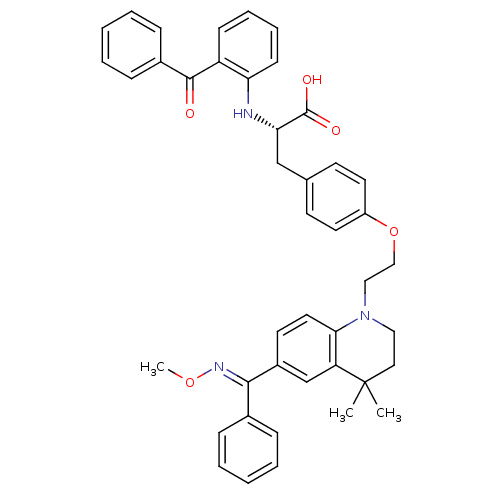
((S)-2-(2-benzoylphenylamino)-3-(4-(2-(6-((methoxyi...)Show SMILES CO\N=C(/c1ccccc1)c1ccc2N(CCOc3ccc(C[C@H](Nc4ccccc4C(=O)c4ccccc4)C(O)=O)cc3)CCC(C)(C)c2c1 |r| Show InChI InChI=1S/C43H43N3O5/c1-43(2)24-25-46(39-23-20-33(29-36(39)43)40(45-50-3)31-12-6-4-7-13-31)26-27-51-34-21-18-30(19-22-34)28-38(42(48)49)44-37-17-11-10-16-35(37)41(47)32-14-8-5-9-15-32/h4-23,29,38,44H,24-28H2,1-3H3,(H,48,49)/b45-40+/t38-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 54 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
UMR GICC
Curated by ChEMBL
| Assay Description
Displacement of [3H]rosiglitazone from human PPARgamma receptor |
Bioorg Med Chem Lett 19: 2683-7 (2009)
Article DOI: 10.1016/j.bmcl.2009.03.143
BindingDB Entry DOI: 10.7270/Q2KP826B |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50294689
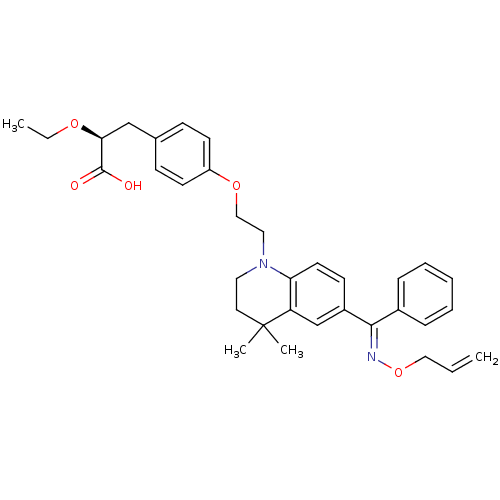
((S)-3-(4-(2-(6-((allyloxyimino)(phenyl)methyl)-4,4...)Show SMILES CCO[C@@H](Cc1ccc(OCCN2CCC(C)(C)c3cc(ccc23)C(=N\OCC=C)\c2ccccc2)cc1)C(O)=O |r| Show InChI InChI=1S/C34H40N2O5/c1-5-21-41-35-32(26-10-8-7-9-11-26)27-14-17-30-29(24-27)34(3,4)18-19-36(30)20-22-40-28-15-12-25(13-16-28)23-31(33(37)38)39-6-2/h5,7-17,24,31H,1,6,18-23H2,2-4H3,(H,37,38)/b35-32+/t31-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 54.2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
UMR GICC
Curated by ChEMBL
| Assay Description
Displacement of [3H]rosiglitazone from human PPARgamma receptor |
Bioorg Med Chem Lett 19: 2683-7 (2009)
Article DOI: 10.1016/j.bmcl.2009.03.143
BindingDB Entry DOI: 10.7270/Q2KP826B |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50234372
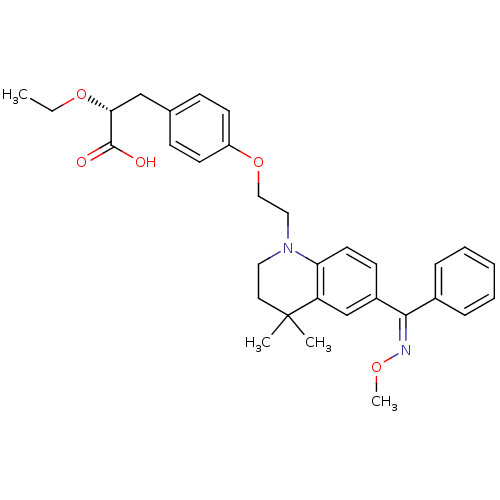
((R)-2-ethoxy-3-(4-(2-(6-((methoxyimino)(phenyl)met...)Show SMILES CCO[C@H](Cc1ccc(OCCN2CCC(C)(C)c3cc(ccc23)C(=N/OC)\c2ccccc2)cc1)C(O)=O Show InChI InChI=1S/C32H38N2O5/c1-5-38-29(31(35)36)21-23-11-14-26(15-12-23)39-20-19-34-18-17-32(2,3)27-22-25(13-16-28(27)34)30(33-37-4)24-9-7-6-8-10-24/h6-16,22,29H,5,17-21H2,1-4H3,(H,35,36)/b33-30-/t29-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 89 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SPOT-EA3857
Curated by ChEMBL
| Assay Description
Displacement of [3H]Rosiglitazone from human PPARgamma |
Bioorg Med Chem Lett 18: 1617-22 (2008)
Article DOI: 10.1016/j.bmcl.2008.01.067
BindingDB Entry DOI: 10.7270/Q2222TGW |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50294693

((S)-3-(4-(2-(6-((cyclopropylmethoxyimino)(phenyl)m...)Show SMILES CCO[C@@H](Cc1ccc(OCCN2CCC(C)(C)c3cc(ccc23)C(=N\OCC2CC2)\c2ccccc2)cc1)C(O)=O |r| Show InChI InChI=1S/C35H42N2O5/c1-4-40-32(34(38)39)22-25-12-15-29(16-13-25)41-21-20-37-19-18-35(2,3)30-23-28(14-17-31(30)37)33(27-8-6-5-7-9-27)36-42-24-26-10-11-26/h5-9,12-17,23,26,32H,4,10-11,18-22,24H2,1-3H3,(H,38,39)/b36-33+/t32-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 254 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
UMR GICC
Curated by ChEMBL
| Assay Description
Displacement of [3H]rosiglitazone from human PPARgamma receptor |
Bioorg Med Chem Lett 19: 2683-7 (2009)
Article DOI: 10.1016/j.bmcl.2009.03.143
BindingDB Entry DOI: 10.7270/Q2KP826B |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50234373

(CHEMBL253851 | ethyl 2-ethoxy-3-(4-(2-(6-((hydroxy...)Show SMILES CCOC(Cc1ccc(OCCN2CCC(C)(C)c3cc(ccc23)C(N=O)c2ccccc2)cc1)C(=O)OCC Show InChI InChI=1S/C33H40N2O5/c1-5-38-30(32(36)39-6-2)22-24-12-15-27(16-13-24)40-21-20-35-19-18-33(3,4)28-23-26(14-17-29(28)35)31(34-37)25-10-8-7-9-11-25/h7-17,23,30-31H,5-6,18-22H2,1-4H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 618 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SPOT-EA3857
Curated by ChEMBL
| Assay Description
Displacement of [3H]Rosiglitazone from human PPARgamma |
Bioorg Med Chem Lett 18: 1617-22 (2008)
Article DOI: 10.1016/j.bmcl.2008.01.067
BindingDB Entry DOI: 10.7270/Q2222TGW |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50234379
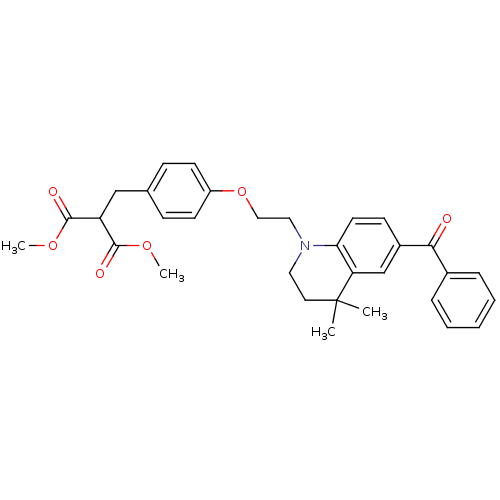
(CHEMBL252995 | dimethyl 2-(4-(2-(6-benzoyl-4,4-dim...)Show SMILES COC(=O)C(Cc1ccc(OCCN2CCC(C)(C)c3cc(ccc23)C(=O)c2ccccc2)cc1)C(=O)OC Show InChI InChI=1S/C32H35NO6/c1-32(2)16-17-33(28-15-12-24(21-27(28)32)29(34)23-8-6-5-7-9-23)18-19-39-25-13-10-22(11-14-25)20-26(30(35)37-3)31(36)38-4/h5-15,21,26H,16-20H2,1-4H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SPOT-EA3857
Curated by ChEMBL
| Assay Description
Displacement of [3H]Rosiglitazone from human PPARgamma |
Bioorg Med Chem Lett 18: 1617-22 (2008)
Article DOI: 10.1016/j.bmcl.2008.01.067
BindingDB Entry DOI: 10.7270/Q2222TGW |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50294694

((S)-2-(2-ethoxy-2-oxoethylamino)-3-(4-(2-(6-((hydr...)Show SMILES CCOC(=O)CN(Cc1ccc(OCCN2CCC(C)(C)c3cc(ccc23)C(N=O)c2ccccc2)cc1)C(O)=O Show InChI InChI=1S/C32H37N3O6/c1-4-40-29(36)22-35(31(37)38)21-23-10-13-26(14-11-23)41-19-18-34-17-16-32(2,3)27-20-25(12-15-28(27)34)30(33-39)24-8-6-5-7-9-24/h5-15,20,30H,4,16-19,21-22H2,1-3H3,(H,37,38) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
UMR GICC
Curated by ChEMBL
| Assay Description
Displacement of [3H]rosiglitazone from human PPARgamma receptor |
Bioorg Med Chem Lett 19: 2683-7 (2009)
Article DOI: 10.1016/j.bmcl.2009.03.143
BindingDB Entry DOI: 10.7270/Q2KP826B |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50234376

(CHEMBL252996 | dimethyl 2-(4-(2-(6-((hydroxyimino)...)Show SMILES COC(=O)C(Cc1ccc(OCCN2CCC(C)(C)c3cc(ccc23)C(N=O)c2ccccc2)cc1)C(=O)OC Show InChI InChI=1S/C32H36N2O6/c1-32(2)16-17-34(28-15-12-24(21-27(28)32)29(33-37)23-8-6-5-7-9-23)18-19-40-25-13-10-22(11-14-25)20-26(30(35)38-3)31(36)39-4/h5-15,21,26,29H,16-20H2,1-4H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SPOT-EA3857
Curated by ChEMBL
| Assay Description
Displacement of [3H]Rosiglitazone from human PPARgamma |
Bioorg Med Chem Lett 18: 1617-22 (2008)
Article DOI: 10.1016/j.bmcl.2008.01.067
BindingDB Entry DOI: 10.7270/Q2222TGW |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50294695

((S)-2-acetoxy-3-(4-(2-(6-((methoxyimino)(phenyl)me...)Show SMILES CO\N=C(/c1ccccc1)c1ccc2N(CCOc3ccc(C[C@H](OC(C)=O)C(O)=O)cc3)CCC(C)(C)c2c1 |r| Show InChI InChI=1S/C32H36N2O6/c1-22(35)40-29(31(36)37)20-23-10-13-26(14-11-23)39-19-18-34-17-16-32(2,3)27-21-25(12-15-28(27)34)30(33-38-4)24-8-6-5-7-9-24/h5-15,21,29H,16-20H2,1-4H3,(H,36,37)/b33-30+/t29-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
UMR GICC
Curated by ChEMBL
| Assay Description
Displacement of [3H]rosiglitazone from human PPARgamma receptor |
Bioorg Med Chem Lett 19: 2683-7 (2009)
Article DOI: 10.1016/j.bmcl.2009.03.143
BindingDB Entry DOI: 10.7270/Q2KP826B |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50234374

((S)-ethyl 2-ethoxy-3-(4-(2-(6-((methoxyimino)(phen...)Show SMILES CCO[C@@H](Cc1ccc(OCCN2CCC(C)(C)c3cc(ccc23)C(=N/OC)\c2ccccc2)cc1)C(=O)OCC Show InChI InChI=1S/C34H42N2O5/c1-6-39-31(33(37)40-7-2)23-25-13-16-28(17-14-25)41-22-21-36-20-19-34(3,4)29-24-27(15-18-30(29)36)32(35-38-5)26-11-9-8-10-12-26/h8-18,24,31H,6-7,19-23H2,1-5H3/b35-32-/t31-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SPOT-EA3857
Curated by ChEMBL
| Assay Description
Displacement of [3H]Rosiglitazone from human PPARgamma |
Bioorg Med Chem Lett 18: 1617-22 (2008)
Article DOI: 10.1016/j.bmcl.2008.01.067
BindingDB Entry DOI: 10.7270/Q2222TGW |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50234378
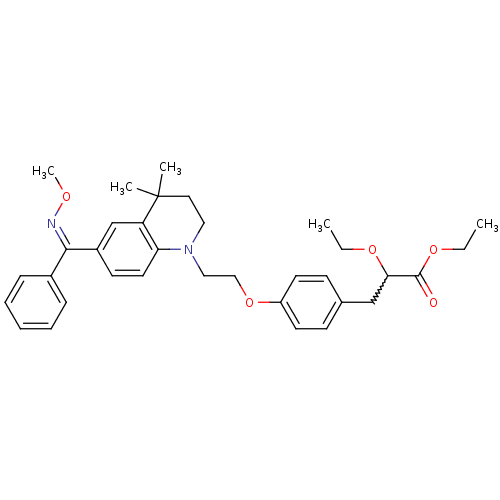
(CHEMBL399629 | ethyl 2-ethoxy-3-(4-(2-(6-((methoxy...)Show SMILES CCOC(Cc1ccc(OCCN2CCC(C)(C)c3cc(ccc23)C(=N/OC)\c2ccccc2)cc1)C(=O)OCC |w:3.3| Show InChI InChI=1S/C34H42N2O5/c1-6-39-31(33(37)40-7-2)23-25-13-16-28(17-14-25)41-22-21-36-20-19-34(3,4)29-24-27(15-18-30(29)36)32(35-38-5)26-11-9-8-10-12-26/h8-18,24,31H,6-7,19-23H2,1-5H3/b35-32- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SPOT-EA3857
Curated by ChEMBL
| Assay Description
Displacement of [3H]Rosiglitazone from human PPARgamma |
Bioorg Med Chem Lett 18: 1617-22 (2008)
Article DOI: 10.1016/j.bmcl.2008.01.067
BindingDB Entry DOI: 10.7270/Q2222TGW |
More data for this
Ligand-Target Pair | |
Neprilysin
(Homo sapiens (Human)) | BDBM21641

(2-(2-benzyl-3-sulfanylpropanamido)acetic acid | CH...)Show InChI InChI=1S/C12H15NO3S/c14-11(15)7-13-12(16)10(8-17)6-9-4-2-1-3-5-9/h1-5,10,17H,6-8H2,(H,13,16)(H,14,15) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM
Curated by ChEMBL
| Assay Description
Inhibition of human NEP-mediated amyloid beta hydrolysis |
Eur J Med Chem 79: 184-93 (2014)
Article DOI: 10.1016/j.ejmech.2014.04.009
BindingDB Entry DOI: 10.7270/Q27H1M34 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Angiotensin-converting enzyme
(Homo sapiens (Human)) | BDBM21642

((2S)-1-[(2S)-2-methyl-3-sulfanylpropanoyl]pyrrolid...)Show InChI InChI=1S/C9H15NO3S/c1-6(5-14)8(11)10-4-2-3-7(10)9(12)13/h6-7,14H,2-5H2,1H3,(H,12,13)/t6-,7+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM
Curated by ChEMBL
| Assay Description
Inhibition of human ACE-mediated amyloid beta hydrolysis |
Eur J Med Chem 79: 184-93 (2014)
Article DOI: 10.1016/j.ejmech.2014.04.009
BindingDB Entry DOI: 10.7270/Q27H1M34 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Insulin-degrading enzyme
(Homo sapiens (Human)) | BDBM50005641

(CHEMBL3235416)Show SMILES CNC(=O)[C@H](Cc1c[nH]cn1)NC(=O)CN(CCCc1ccccc1)CC(O)=O |r| Show InChI InChI=1S/C20H27N5O4/c1-21-20(29)17(10-16-11-22-14-23-16)24-18(26)12-25(13-19(27)28)9-5-8-15-6-3-2-4-7-15/h2-4,6-7,11,14,17H,5,8-10,12-13H2,1H3,(H,21,29)(H,22,23)(H,24,26)(H,27,28)/t17-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant IDE-mediated amyloid beta (16 to 23) hydrolysis using ATTO 655-Cys-Lys-Leu-Val-Phe-Phe-Ala-Glu-Asp-Trp as substrate p... |
Eur J Med Chem 79: 184-93 (2014)
Article DOI: 10.1016/j.ejmech.2014.04.009
BindingDB Entry DOI: 10.7270/Q27H1M34 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Insulin-degrading enzyme
(Homo sapiens (Human)) | BDBM50005638
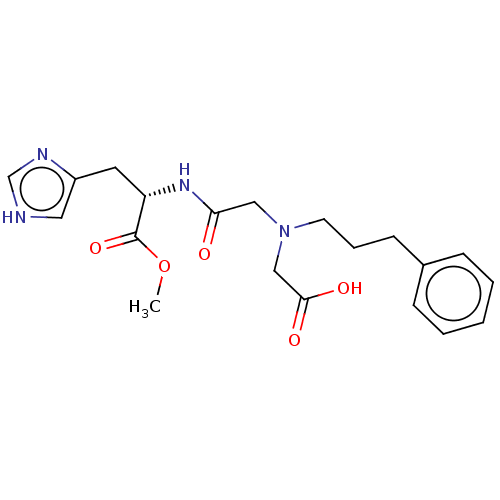
(CHEMBL3235414)Show SMILES COC(=O)[C@H](Cc1c[nH]cn1)NC(=O)CN(CCCc1ccccc1)CC(O)=O |r| Show InChI InChI=1S/C20H26N4O5/c1-29-20(28)17(10-16-11-21-14-22-16)23-18(25)12-24(13-19(26)27)9-5-8-15-6-3-2-4-7-15/h2-4,6-7,11,14,17H,5,8-10,12-13H2,1H3,(H,21,22)(H,23,25)(H,26,27)/t17-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 600 | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant IDE-mediated amyloid beta (16 to 23) hydrolysis using ATTO 655-Cys-Lys-Leu-Val-Phe-Phe-Ala-Glu-Asp-Trp as substrate p... |
Eur J Med Chem 79: 184-93 (2014)
Article DOI: 10.1016/j.ejmech.2014.04.009
BindingDB Entry DOI: 10.7270/Q27H1M34 |
More data for this
Ligand-Target Pair | |
Insulin-degrading enzyme
(Homo sapiens (Human)) | BDBM50005640
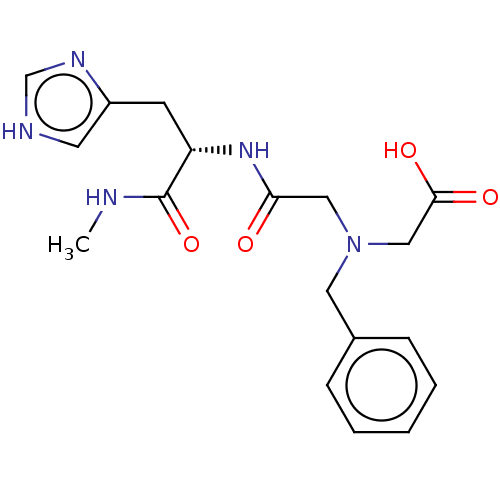
(CHEMBL3235415)Show SMILES CNC(=O)[C@H](Cc1c[nH]cn1)NC(=O)CN(CC(O)=O)Cc1ccccc1 |r| Show InChI InChI=1S/C18H23N5O4/c1-19-18(27)15(7-14-8-20-12-21-14)22-16(24)10-23(11-17(25)26)9-13-5-3-2-4-6-13/h2-6,8,12,15H,7,9-11H2,1H3,(H,19,27)(H,20,21)(H,22,24)(H,25,26)/t15-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 600 | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant IDE-mediated amyloid beta (16 to 23) hydrolysis using ATTO 655-Cys-Lys-Leu-Val-Phe-Phe-Ala-Glu-Asp-Trp as substrate p... |
Eur J Med Chem 79: 184-93 (2014)
Article DOI: 10.1016/j.ejmech.2014.04.009
BindingDB Entry DOI: 10.7270/Q27H1M34 |
More data for this
Ligand-Target Pair | |
Insulin-degrading enzyme
(Homo sapiens (Human)) | BDBM50005637
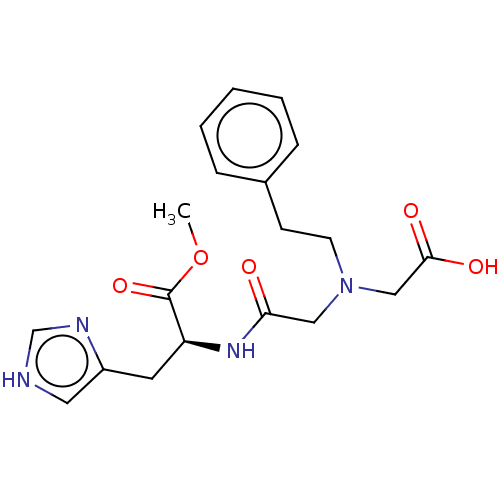
(CHEMBL3235413)Show SMILES COC(=O)[C@H](Cc1c[nH]cn1)NC(=O)CN(CCc1ccccc1)CC(O)=O |r| Show InChI InChI=1S/C19H24N4O5/c1-28-19(27)16(9-15-10-20-13-21-15)22-17(24)11-23(12-18(25)26)8-7-14-5-3-2-4-6-14/h2-6,10,13,16H,7-9,11-12H2,1H3,(H,20,21)(H,22,24)(H,25,26)/t16-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant IDE-mediated amyloid beta (16 to 23) hydrolysis using ATTO 655-Cys-Lys-Leu-Val-Phe-Phe-Ala-Glu-Asp-Trp as substrate p... |
Eur J Med Chem 79: 184-93 (2014)
Article DOI: 10.1016/j.ejmech.2014.04.009
BindingDB Entry DOI: 10.7270/Q27H1M34 |
More data for this
Ligand-Target Pair | |
Insulin-degrading enzyme
(Homo sapiens (Human)) | BDBM50005636
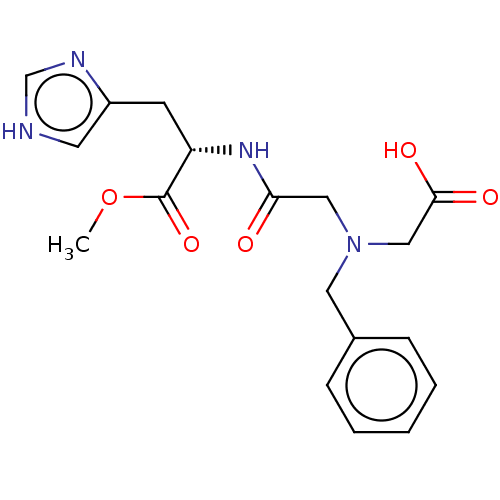
(CHEMBL3235412)Show SMILES COC(=O)[C@H](Cc1c[nH]cn1)NC(=O)CN(CC(O)=O)Cc1ccccc1 |r| Show InChI InChI=1S/C18H22N4O5/c1-27-18(26)15(7-14-8-19-12-20-14)21-16(23)10-22(11-17(24)25)9-13-5-3-2-4-6-13/h2-6,8,12,15H,7,9-11H2,1H3,(H,19,20)(H,21,23)(H,24,25)/t15-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant IDE-mediated amyloid beta (16 to 23) hydrolysis using ATTO 655-Cys-Lys-Leu-Val-Phe-Phe-Ala-Glu-Asp-Trp as substrate p... |
Eur J Med Chem 79: 184-93 (2014)
Article DOI: 10.1016/j.ejmech.2014.04.009
BindingDB Entry DOI: 10.7270/Q27H1M34 |
More data for this
Ligand-Target Pair | |
Insulin-degrading enzyme
(Homo sapiens (Human)) | BDBM50005641

(CHEMBL3235416)Show SMILES CNC(=O)[C@H](Cc1c[nH]cn1)NC(=O)CN(CCCc1ccccc1)CC(O)=O |r| Show InChI InChI=1S/C20H27N5O4/c1-21-20(29)17(10-16-11-22-14-23-16)24-18(26)12-25(13-19(27)28)9-5-8-15-6-3-2-4-7-15/h2-4,6-7,11,14,17H,5,8-10,12-13H2,1H3,(H,21,29)(H,22,23)(H,24,26)(H,27,28)/t17-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 3.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant IDE-mediated amyloid beta (1 to 40) hydrolysis preincubated for 10 mins measured after 30 mins by spectrophotometer a... |
Eur J Med Chem 79: 184-93 (2014)
Article DOI: 10.1016/j.ejmech.2014.04.009
BindingDB Entry DOI: 10.7270/Q27H1M34 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Insulin-degrading enzyme
(Homo sapiens (Human)) | BDBM50005646
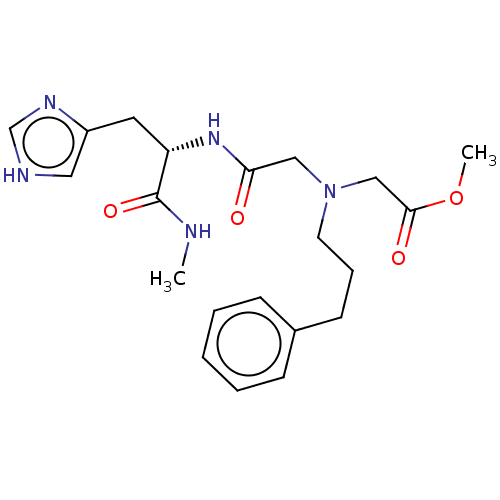
(CHEMBL3235419)Show SMILES CNC(=O)[C@H](Cc1c[nH]cn1)NC(=O)CN(CCCc1ccccc1)CC(=O)OC |r| Show InChI InChI=1S/C21H29N5O4/c1-22-21(29)18(11-17-12-23-15-24-17)25-19(27)13-26(14-20(28)30-2)10-6-9-16-7-4-3-5-8-16/h3-5,7-8,12,15,18H,6,9-11,13-14H2,1-2H3,(H,22,29)(H,23,24)(H,25,27)/t18-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant IDE-mediated amyloid beta (16 to 23) hydrolysis using ATTO 655-Cys-Lys-Leu-Val-Phe-Phe-Ala-Glu-Asp-Trp as substrate p... |
Eur J Med Chem 79: 184-93 (2014)
Article DOI: 10.1016/j.ejmech.2014.04.009
BindingDB Entry DOI: 10.7270/Q27H1M34 |
More data for this
Ligand-Target Pair | |
Insulin-degrading enzyme
(Homo sapiens (Human)) | BDBM50005645

(CHEMBL3235418)Show SMILES COC(=O)[C@H](Cc1c[nH]cn1)NC(=O)CN(CC(O)=O)C(=O)c1ccccc1 |r| Show InChI InChI=1S/C18H20N4O6/c1-28-18(27)14(7-13-8-19-11-20-13)21-15(23)9-22(10-16(24)25)17(26)12-5-3-2-4-6-12/h2-6,8,11,14H,7,9-10H2,1H3,(H,19,20)(H,21,23)(H,24,25)/t14-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant IDE-mediated amyloid beta (16 to 23) hydrolysis using ATTO 655-Cys-Lys-Leu-Val-Phe-Phe-Ala-Glu-Asp-Trp as substrate p... |
Eur J Med Chem 79: 184-93 (2014)
Article DOI: 10.1016/j.ejmech.2014.04.009
BindingDB Entry DOI: 10.7270/Q27H1M34 |
More data for this
Ligand-Target Pair | |
Insulin-degrading enzyme
(Homo sapiens (Human)) | BDBM50005643

(CHEMBL3235417)Show SMILES COC(=O)[C@H](Cc1ccccc1)NC(=O)CN(CC(O)=O)Cc1ccccc1 |r| Show InChI InChI=1S/C21H24N2O5/c1-28-21(27)18(12-16-8-4-2-5-9-16)22-19(24)14-23(15-20(25)26)13-17-10-6-3-7-11-17/h2-11,18H,12-15H2,1H3,(H,22,24)(H,25,26)/t18-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant IDE-mediated amyloid beta (16 to 23) hydrolysis using ATTO 655-Cys-Lys-Leu-Val-Phe-Phe-Ala-Glu-Asp-Trp as substrate p... |
Eur J Med Chem 79: 184-93 (2014)
Article DOI: 10.1016/j.ejmech.2014.04.009
BindingDB Entry DOI: 10.7270/Q27H1M34 |
More data for this
Ligand-Target Pair | |
Neprilysin
(Homo sapiens (Human)) | BDBM50005641

(CHEMBL3235416)Show SMILES CNC(=O)[C@H](Cc1c[nH]cn1)NC(=O)CN(CCCc1ccccc1)CC(O)=O |r| Show InChI InChI=1S/C20H27N5O4/c1-21-20(29)17(10-16-11-22-14-23-16)24-18(26)12-25(13-19(27)28)9-5-8-15-6-3-2-4-7-15/h2-4,6-7,11,14,17H,5,8-10,12-13H2,1H3,(H,21,29)(H,22,23)(H,24,26)(H,27,28)/t17-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM
Curated by ChEMBL
| Assay Description
Inhibition of human NEP-mediated amyloid beta hydrolysis |
Eur J Med Chem 79: 184-93 (2014)
Article DOI: 10.1016/j.ejmech.2014.04.009
BindingDB Entry DOI: 10.7270/Q27H1M34 |
More data for this
Ligand-Target Pair | |
Endothelin-converting enzyme 1
(Homo sapiens (Human)) | BDBM50005641

(CHEMBL3235416)Show SMILES CNC(=O)[C@H](Cc1c[nH]cn1)NC(=O)CN(CCCc1ccccc1)CC(O)=O |r| Show InChI InChI=1S/C20H27N5O4/c1-21-20(29)17(10-16-11-22-14-23-16)24-18(26)12-25(13-19(27)28)9-5-8-15-6-3-2-4-7-15/h2-4,6-7,11,14,17H,5,8-10,12-13H2,1H3,(H,21,29)(H,22,23)(H,24,26)(H,27,28)/t17-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant ECE1-mediated amyloid beta hydrolysis after 45 mins by fluorimetry assay |
Eur J Med Chem 79: 184-93 (2014)
Article DOI: 10.1016/j.ejmech.2014.04.009
BindingDB Entry DOI: 10.7270/Q27H1M34 |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme
(Homo sapiens (Human)) | BDBM50005641

(CHEMBL3235416)Show SMILES CNC(=O)[C@H](Cc1c[nH]cn1)NC(=O)CN(CCCc1ccccc1)CC(O)=O |r| Show InChI InChI=1S/C20H27N5O4/c1-21-20(29)17(10-16-11-22-14-23-16)24-18(26)12-25(13-19(27)28)9-5-8-15-6-3-2-4-7-15/h2-4,6-7,11,14,17H,5,8-10,12-13H2,1H3,(H,21,29)(H,22,23)(H,24,26)(H,27,28)/t17-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM
Curated by ChEMBL
| Assay Description
Inhibition of human ACE-mediated amyloid beta hydrolysis |
Eur J Med Chem 79: 184-93 (2014)
Article DOI: 10.1016/j.ejmech.2014.04.009
BindingDB Entry DOI: 10.7270/Q27H1M34 |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme 2
(Homo sapiens (Human)) | BDBM50005641

(CHEMBL3235416)Show SMILES CNC(=O)[C@H](Cc1c[nH]cn1)NC(=O)CN(CCCc1ccccc1)CC(O)=O |r| Show InChI InChI=1S/C20H27N5O4/c1-21-20(29)17(10-16-11-22-14-23-16)24-18(26)12-25(13-19(27)28)9-5-8-15-6-3-2-4-7-15/h2-4,6-7,11,14,17H,5,8-10,12-13H2,1H3,(H,21,29)(H,22,23)(H,24,26)(H,27,28)/t17-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant ACE2-mediated amyloid beta hydrolysis using Mca-Tyr-Val-Ala-Asp-Pro-Ala-Lys-(DNP)-OH as substrate after 20 mins by fl... |
Eur J Med Chem 79: 184-93 (2014)
Article DOI: 10.1016/j.ejmech.2014.04.009
BindingDB Entry DOI: 10.7270/Q27H1M34 |
More data for this
Ligand-Target Pair | |
Collagenase 3
(Homo sapiens (Human)) | BDBM50005641

(CHEMBL3235416)Show SMILES CNC(=O)[C@H](Cc1c[nH]cn1)NC(=O)CN(CCCc1ccccc1)CC(O)=O |r| Show InChI InChI=1S/C20H27N5O4/c1-21-20(29)17(10-16-11-22-14-23-16)24-18(26)12-25(13-19(27)28)9-5-8-15-6-3-2-4-7-15/h2-4,6-7,11,14,17H,5,8-10,12-13H2,1H3,(H,21,29)(H,22,23)(H,24,26)(H,27,28)/t17-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant MMP13-mediated amyloid beta hydrolysis after 10 mins by fluorimetry assay |
Eur J Med Chem 79: 184-93 (2014)
Article DOI: 10.1016/j.ejmech.2014.04.009
BindingDB Entry DOI: 10.7270/Q27H1M34 |
More data for this
Ligand-Target Pair | |
Interstitial collagenase
(Homo sapiens (Human)) | BDBM50005641

(CHEMBL3235416)Show SMILES CNC(=O)[C@H](Cc1c[nH]cn1)NC(=O)CN(CCCc1ccccc1)CC(O)=O |r| Show InChI InChI=1S/C20H27N5O4/c1-21-20(29)17(10-16-11-22-14-23-16)24-18(26)12-25(13-19(27)28)9-5-8-15-6-3-2-4-7-15/h2-4,6-7,11,14,17H,5,8-10,12-13H2,1H3,(H,21,29)(H,22,23)(H,24,26)(H,27,28)/t17-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant MMP1-mediated amyloid beta hydrolysis using DNP-Pro-Cha-Gly- Cys(Me)-His-Ala- Lys(n-Me-Abz)-NH2 as substrate after 40... |
Eur J Med Chem 79: 184-93 (2014)
Article DOI: 10.1016/j.ejmech.2014.04.009
BindingDB Entry DOI: 10.7270/Q27H1M34 |
More data for this
Ligand-Target Pair | |
Disintegrin and metalloproteinase domain-containing protein 17
(Homo sapiens (Human)) | BDBM50005641

(CHEMBL3235416)Show SMILES CNC(=O)[C@H](Cc1c[nH]cn1)NC(=O)CN(CCCc1ccccc1)CC(O)=O |r| Show InChI InChI=1S/C20H27N5O4/c1-21-20(29)17(10-16-11-22-14-23-16)24-18(26)12-25(13-19(27)28)9-5-8-15-6-3-2-4-7-15/h2-4,6-7,11,14,17H,5,8-10,12-13H2,1H3,(H,21,29)(H,22,23)(H,24,26)(H,27,28)/t17-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant TACE-mediated amyloid beta hydrolysis after 5 mins by fluorimetry assay |
Eur J Med Chem 79: 184-93 (2014)
Article DOI: 10.1016/j.ejmech.2014.04.009
BindingDB Entry DOI: 10.7270/Q27H1M34 |
More data for this
Ligand-Target Pair | |
Insulin-degrading enzyme
(Homo sapiens (Human)) | BDBM50005641

(CHEMBL3235416)Show SMILES CNC(=O)[C@H](Cc1c[nH]cn1)NC(=O)CN(CCCc1ccccc1)CC(O)=O |r| Show InChI InChI=1S/C20H27N5O4/c1-21-20(29)17(10-16-11-22-14-23-16)24-18(26)12-25(13-19(27)28)9-5-8-15-6-3-2-4-7-15/h2-4,6-7,11,14,17H,5,8-10,12-13H2,1H3,(H,21,29)(H,22,23)(H,24,26)(H,27,28)/t17-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | n/a | n/a | 530 | n/a | n/a | n/a | n/a |
INSERM
Curated by ChEMBL
| Assay Description
Induction of human recombinant IDE-mediated insulin hydrolysis preincubated for 10 mins measured after 30 mins by spectrophotometer analysis |
Eur J Med Chem 79: 184-93 (2014)
Article DOI: 10.1016/j.ejmech.2014.04.009
BindingDB Entry DOI: 10.7270/Q27H1M34 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Peroxisome proliferator-activated receptor alpha
(Homo sapiens (Human)) | BDBM50171895
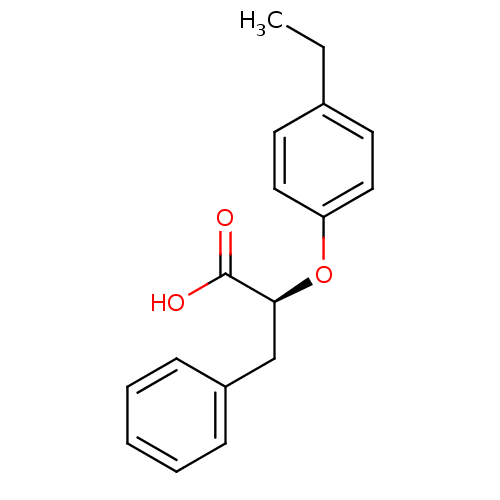
((2S)-2-(4-ethylphenoxy)-3-phenylpropanoic acid | (...)Show InChI InChI=1S/C17H18O3/c1-2-13-8-10-15(11-9-13)20-16(17(18)19)12-14-6-4-3-5-7-14/h3-11,16H,2,12H2,1H3,(H,18,19)/t16-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 700 | n/a | n/a | n/a | n/a |
Università degli Studi di Milano
Curated by ChEMBL
| Assay Description
Effective concentration against human PPARalpha expressed in HepG2 cells |
J Med Chem 48: 5509-19 (2005)
Article DOI: 10.1021/jm0502844
BindingDB Entry DOI: 10.7270/Q2PV6JXF |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor alpha
(Mus musculus) | BDBM28759

((2S)-3-phenyl-2-(4-phenylphenoxy)propanoic acid | ...)Show SMILES OC(=O)[C@H](Cc1ccccc1)Oc1ccc(cc1)-c1ccccc1 |r| Show InChI InChI=1S/C21H18O3/c22-21(23)20(15-16-7-3-1-4-8-16)24-19-13-11-18(12-14-19)17-9-5-2-6-10-17/h1-14,20H,15H2,(H,22,23)/t20-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a |
Università degli Studi di Milano
Curated by ChEMBL
| Assay Description
Effective concentration against murine PPARalpha in transactivation assay |
J Med Chem 48: 5509-19 (2005)
Article DOI: 10.1021/jm0502844
BindingDB Entry DOI: 10.7270/Q2PV6JXF |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50171896
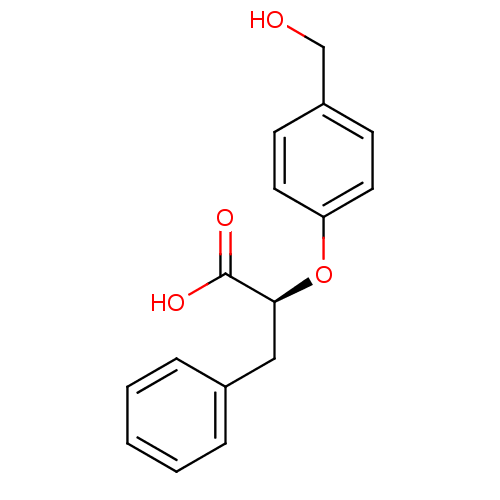
((S)-2-(4-Hydroxymethyl-phenoxy)-3-phenyl-propionic...)Show InChI InChI=1S/C16H16O4/c17-11-13-6-8-14(9-7-13)20-15(16(18)19)10-12-4-2-1-3-5-12/h1-9,15,17H,10-11H2,(H,18,19)/t15-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 2.57E+4 | n/a | n/a | n/a | n/a |
Università degli Studi di Milano
Curated by ChEMBL
| Assay Description
Effective concentration against human PPARgamma expressed in HepG2 cells |
J Med Chem 48: 5509-19 (2005)
Article DOI: 10.1021/jm0502844
BindingDB Entry DOI: 10.7270/Q2PV6JXF |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor alpha
(Homo sapiens (Human)) | BDBM28759

((2S)-3-phenyl-2-(4-phenylphenoxy)propanoic acid | ...)Show SMILES OC(=O)[C@H](Cc1ccccc1)Oc1ccc(cc1)-c1ccccc1 |r| Show InChI InChI=1S/C21H18O3/c22-21(23)20(15-16-7-3-1-4-8-16)24-19-13-11-18(12-14-19)17-9-5-2-6-10-17/h1-14,20H,15H2,(H,22,23)/t20-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 500 | n/a | n/a | n/a | n/a |
Università degli Studi di Milano
Curated by ChEMBL
| Assay Description
Effective concentration against human PPARalpha expressed in HepG2 cells |
J Med Chem 48: 5509-19 (2005)
Article DOI: 10.1021/jm0502844
BindingDB Entry DOI: 10.7270/Q2PV6JXF |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor alpha
(Homo sapiens (Human)) | BDBM50171897

((2S)-2-(4-chlorophenoxy)-3-phenylpropanoic acid | ...)Show InChI InChI=1S/C15H13ClO3/c16-12-6-8-13(9-7-12)19-14(15(17)18)10-11-4-2-1-3-5-11/h1-9,14H,10H2,(H,17,18)/t14-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 3.90E+3 | n/a | n/a | n/a | n/a |
Università degli Studi di Milano
Curated by ChEMBL
| Assay Description
Effective concentration against human PPARalpha expressed in HepG2 cells |
J Med Chem 48: 5509-19 (2005)
Article DOI: 10.1021/jm0502844
BindingDB Entry DOI: 10.7270/Q2PV6JXF |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor alpha
(Homo sapiens (Human)) | BDBM50171899

((S)-2-(4'-Chloro-biphenyl-4-ylmethoxy)-pentanoic a...)Show InChI InChI=1S/C18H19ClO3/c1-2-3-17(18(20)21)22-12-13-4-6-14(7-5-13)15-8-10-16(19)11-9-15/h4-11,17H,2-3,12H2,1H3,(H,20,21)/t17-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 3.90E+3 | n/a | n/a | n/a | n/a |
Università degli Studi di Milano
Curated by ChEMBL
| Assay Description
Effective concentration against human PPARalpha expressed in HepG2 cells |
J Med Chem 48: 5509-19 (2005)
Article DOI: 10.1021/jm0502844
BindingDB Entry DOI: 10.7270/Q2PV6JXF |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50171898

((S)-3-Phenyl-2-(4-trifluoromethyl-phenoxy)-propion...)Show InChI InChI=1S/C16H13F3O3/c17-16(18,19)12-6-8-13(9-7-12)22-14(15(20)21)10-11-4-2-1-3-5-11/h1-9,14H,10H2,(H,20,21)/t14-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 900 | n/a | n/a | n/a | n/a |
Università degli Studi di Milano
Curated by ChEMBL
| Assay Description
Effective concentration against human PPARgamma expressed in HepG2 cells |
J Med Chem 48: 5509-19 (2005)
Article DOI: 10.1021/jm0502844
BindingDB Entry DOI: 10.7270/Q2PV6JXF |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50171900

((S)-3-Phenyl-2-(4-thiophen-2-yl-phenoxy)-propionic...)Show InChI InChI=1S/C19H16O3S/c20-19(21)17(13-14-5-2-1-3-6-14)22-16-10-8-15(9-11-16)18-7-4-12-23-18/h1-12,17H,13H2,(H,20,21)/t17-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 1.60E+3 | n/a | n/a | n/a | n/a |
Università degli Studi di Milano
Curated by ChEMBL
| Assay Description
Effective concentration against human PPARgamma expressed in HepG2 cells |
J Med Chem 48: 5509-19 (2005)
Article DOI: 10.1021/jm0502844
BindingDB Entry DOI: 10.7270/Q2PV6JXF |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM28699
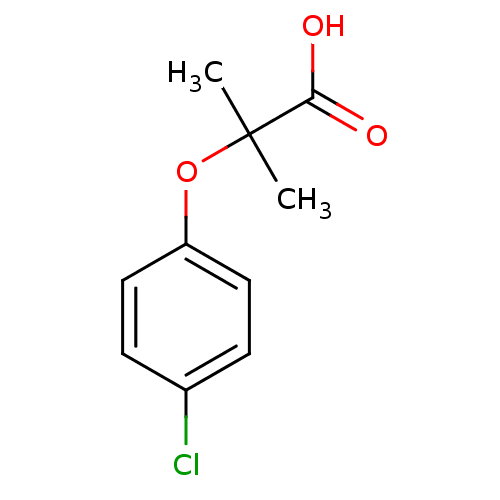
(2-(4-chlorophenoxy)-2-methylpropanoic acid | CHEMB...)Show InChI InChI=1S/C10H11ClO3/c1-10(2,9(12)13)14-8-5-3-7(11)4-6-8/h3-6H,1-2H3,(H,12,13) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 3.09E+5 | n/a | n/a | n/a | n/a |
Università degli Studi di Milano
Curated by ChEMBL
| Assay Description
Effective concentration against human PPARgamma expressed in HepG2 cells |
J Med Chem 48: 5509-19 (2005)
Article DOI: 10.1021/jm0502844
BindingDB Entry DOI: 10.7270/Q2PV6JXF |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50171901

((R)-2-(Naphthalen-2-yloxy)-pentanoic acid | CHEMBL...)Show InChI InChI=1S/C15H16O3/c1-2-5-14(15(16)17)18-13-9-8-11-6-3-4-7-12(11)10-13/h3-4,6-10,14H,2,5H2,1H3,(H,16,17)/t14-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 1.25E+4 | n/a | n/a | n/a | n/a |
Università degli Studi di Milano
Curated by ChEMBL
| Assay Description
Effective concentration against human PPARgamma expressed in HepG2 cells |
J Med Chem 48: 5509-19 (2005)
Article DOI: 10.1021/jm0502844
BindingDB Entry DOI: 10.7270/Q2PV6JXF |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50171902
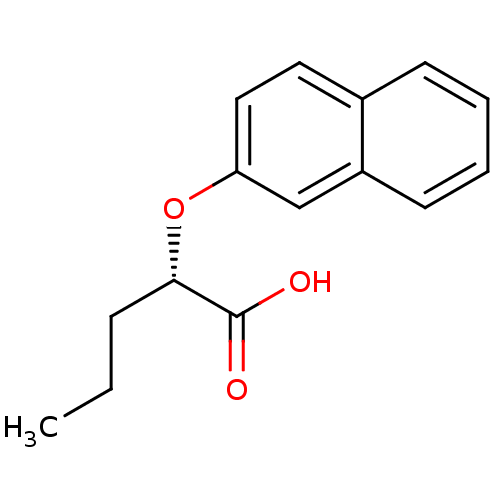
((S)-2-(Naphthalen-2-yloxy)-pentanoic acid | CHEMBL...)Show InChI InChI=1S/C15H16O3/c1-2-5-14(15(16)17)18-13-9-8-11-6-3-4-7-12(11)10-13/h3-4,6-10,14H,2,5H2,1H3,(H,16,17)/t14-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 7.20E+3 | n/a | n/a | n/a | n/a |
Università degli Studi di Milano
Curated by ChEMBL
| Assay Description
Effective concentration against human PPARgamma expressed in HepG2 cells |
J Med Chem 48: 5509-19 (2005)
Article DOI: 10.1021/jm0502844
BindingDB Entry DOI: 10.7270/Q2PV6JXF |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor alpha
(Homo sapiens (Human)) | BDBM50171903
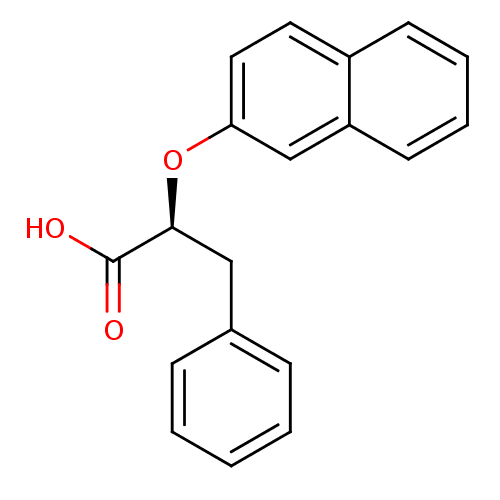
((S)-2-(Naphthalen-2-yloxy)-3-phenyl-propionic acid...)Show InChI InChI=1S/C19H16O3/c20-19(21)18(12-14-6-2-1-3-7-14)22-17-11-10-15-8-4-5-9-16(15)13-17/h1-11,13,18H,12H2,(H,20,21)/t18-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 1.40E+3 | n/a | n/a | n/a | n/a |
Università degli Studi di Milano
Curated by ChEMBL
| Assay Description
Effective concentration against human PPARalpha expressed in HepG2 cells |
J Med Chem 48: 5509-19 (2005)
Article DOI: 10.1021/jm0502844
BindingDB Entry DOI: 10.7270/Q2PV6JXF |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor alpha
(Homo sapiens (Human)) | BDBM28699

(2-(4-chlorophenoxy)-2-methylpropanoic acid | CHEMB...)Show InChI InChI=1S/C10H11ClO3/c1-10(2,9(12)13)14-8-5-3-7(11)4-6-8/h3-6H,1-2H3,(H,12,13) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | n/a | n/a | 3.96E+4 | n/a | n/a | n/a | n/a |
Università degli Studi di Milano
Curated by ChEMBL
| Assay Description
Effective concentration against human PPARalpha expressed in HepG2 cells |
J Med Chem 48: 5509-19 (2005)
Article DOI: 10.1021/jm0502844
BindingDB Entry DOI: 10.7270/Q2PV6JXF |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data