 Found 183 hits with Last Name = 'stamper' and Initial = 'ml'
Found 183 hits with Last Name = 'stamper' and Initial = 'ml' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Protein kinase C delta type
(Homo sapiens (Human)) | BDBM3153

(2-{[2,6-dihydroxy-4-({[(1R,2R)-2-[(4-hydroxybenzen...)Show SMILES OC(=O)c1cccc(O)c1C(=O)c1c(O)cc(cc1O)C(=O)O[C@@H]1CCC[C@H]1NC(=O)c1ccc(O)cc1 |r| Show InChI InChI=1S/C27H23NO10/c29-15-9-7-13(8-10-15)25(34)28-17-4-2-6-21(17)38-27(37)14-11-19(31)23(20(32)12-14)24(33)22-16(26(35)36)3-1-5-18(22)30/h1,3,5,7-12,17,21,29-32H,2,4,6H2,(H,28,34)(H,35,36)/t17-,21-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of Human Protein kinase C delta |
Bioorg Med Chem Lett 5: 1839-1842 (1995)
Article DOI: 10.1016/0960-894X(95)00303-B
BindingDB Entry DOI: 10.7270/Q2QN66R9 |
More data for this
Ligand-Target Pair | |
Protein kinase C epsilon type
(Homo sapiens (Human)) | BDBM3152
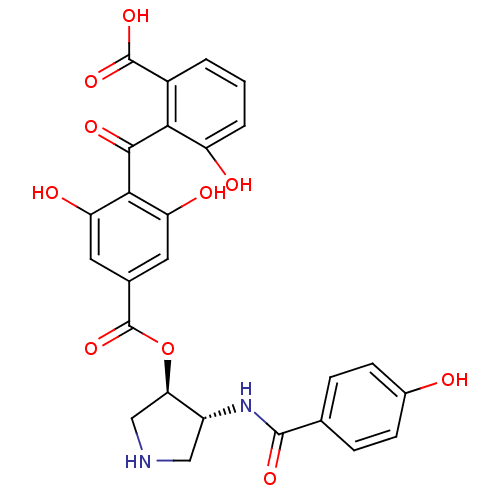
(2-{[2,6-dihydroxy-4-({[(3R,4R)-4-[(4-hydroxybenzen...)Show SMILES OC(=O)c1cccc(O)c1C(=O)c1c(O)cc(cc1O)C(=O)O[C@@H]1CNC[C@H]1NC(=O)c1ccc(O)cc1 |r| Show InChI InChI=1S/C26H22N2O10/c29-14-6-4-12(5-7-14)24(34)28-16-10-27-11-20(16)38-26(37)13-8-18(31)22(19(32)9-13)23(33)21-15(25(35)36)2-1-3-17(21)30/h1-9,16,20,27,29-32H,10-11H2,(H,28,34)(H,35,36)/t16-,20-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Sphinx Laboratories
| Assay Description
PKC was assayed by quantitating the incorporation of 32P from [gamma-32P]ATP into histone type IIIs. |
J Med Chem 45: 2624-43 (2002)
Article DOI: 10.1021/jm020018f
BindingDB Entry DOI: 10.7270/Q2BG2M50 |
More data for this
Ligand-Target Pair | |
Protein kinase C delta type
(Homo sapiens (Human)) | BDBM3239
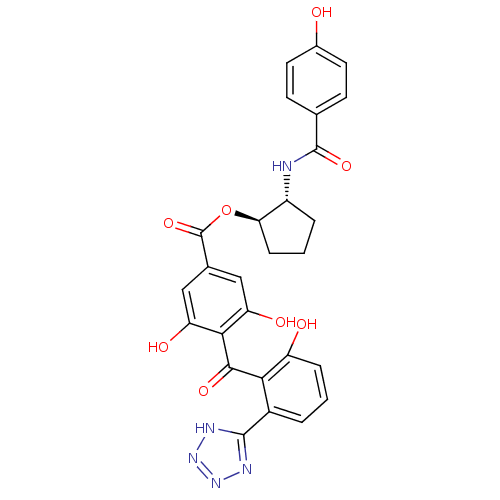
((1R,2R)-2-[(4-hydroxybenzene)amido]cyclopentyl 3,5...)Show SMILES Oc1ccc(cc1)C(=O)N[C@@H]1CCC[C@H]1OC(=O)c1cc(O)c(C(=O)c2c(O)cccc2-c2nnn[nH]2)c(O)c1 |r| Show InChI InChI=1S/C27H23N5O8/c33-15-9-7-13(8-10-15)26(38)28-17-4-2-6-21(17)40-27(39)14-11-19(35)23(20(36)12-14)24(37)22-16(3-1-5-18(22)34)25-29-31-32-30-25/h1,3,5,7-12,17,21,33-36H,2,4,6H2,(H,28,38)(H,29,30,31,32)/t17-,21-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of Human Protein kinase C delta |
Bioorg Med Chem Lett 5: 1839-1842 (1995)
Article DOI: 10.1016/0960-894X(95)00303-B
BindingDB Entry DOI: 10.7270/Q2QN66R9 |
More data for this
Ligand-Target Pair | |
cAMP-dependent protein kinase catalytic subunit alpha
(Bos taurus (bovine)) | BDBM3153

(2-{[2,6-dihydroxy-4-({[(1R,2R)-2-[(4-hydroxybenzen...)Show SMILES OC(=O)c1cccc(O)c1C(=O)c1c(O)cc(cc1O)C(=O)O[C@@H]1CCC[C@H]1NC(=O)c1ccc(O)cc1 |r| Show InChI InChI=1S/C27H23NO10/c29-15-9-7-13(8-10-15)25(34)28-17-4-2-6-21(17)38-27(37)14-11-19(31)23(20(32)12-14)24(33)22-16(26(35)36)3-1-5-18(22)30/h1,3,5,7-12,17,21,29-32H,2,4,6H2,(H,28,34)(H,35,36)/t17-,21-/m1/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Sphinx Laboratories
| Assay Description
The activity of PKA, activated by cAMP, is measured by its ability to transfer phosphate from [gamma-32P]ATP to histone. |
J Med Chem 45: 2624-43 (2002)
Article DOI: 10.1021/jm020018f
BindingDB Entry DOI: 10.7270/Q2BG2M50 |
More data for this
Ligand-Target Pair | |
Protein kinase C alpha type
(Homo sapiens (Human)) | BDBM3152

(2-{[2,6-dihydroxy-4-({[(3R,4R)-4-[(4-hydroxybenzen...)Show SMILES OC(=O)c1cccc(O)c1C(=O)c1c(O)cc(cc1O)C(=O)O[C@@H]1CNC[C@H]1NC(=O)c1ccc(O)cc1 |r| Show InChI InChI=1S/C26H22N2O10/c29-14-6-4-12(5-7-14)24(34)28-16-10-27-11-20(16)38-26(37)13-8-18(31)22(19(32)9-13)23(33)21-15(25(35)36)2-1-3-17(21)30/h1-9,16,20,27,29-32H,10-11H2,(H,28,34)(H,35,36)/t16-,20-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
Sphinx Laboratories
| Assay Description
PKC was assayed by quantitating the incorporation of 32P from [gamma-32P]ATP into histone type IIIs. |
J Med Chem 45: 2624-43 (2002)
Article DOI: 10.1021/jm020018f
BindingDB Entry DOI: 10.7270/Q2BG2M50 |
More data for this
Ligand-Target Pair | |
Protein kinase C beta type
(Homo sapiens (Human)) | BDBM3149

(2-{[2,6-dihydroxy-4-({[(3R,4R)-3-[(4-hydroxybenzen...)Show SMILES OC(=O)c1cccc(O)c1C(=O)c1c(O)cc(cc1O)C(=O)O[C@@H]1CCCNC[C@H]1NC(=O)c1ccc(O)cc1 |r| Show InChI InChI=1S/C28H26N2O10/c31-16-8-6-14(7-9-16)26(36)30-18-13-29-10-2-5-22(18)40-28(39)15-11-20(33)24(21(34)12-15)25(35)23-17(27(37)38)3-1-4-19(23)32/h1,3-4,6-9,11-12,18,22,29,31-34H,2,5,10,13H2,(H,30,36)(H,37,38)/t18-,22-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
Sphinx Laboratories
| Assay Description
PKC was assayed by quantitating the incorporation of 32P from [gamma-32P]ATP into histone type IIIs. |
J Med Chem 45: 2624-43 (2002)
Article DOI: 10.1021/jm020018f
BindingDB Entry DOI: 10.7270/Q2BG2M50 |
More data for this
Ligand-Target Pair | |
Protein kinase C delta type
(Homo sapiens (Human)) | BDBM3236
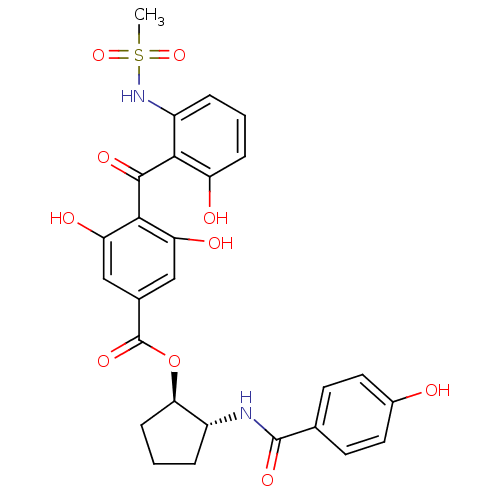
((1R,2R)-2-[(4-hydroxybenzene)amido]cyclopentyl 3,5...)Show SMILES CS(=O)(=O)Nc1cccc(O)c1C(=O)c1c(O)cc(cc1O)C(=O)O[C@@H]1CCC[C@H]1NC(=O)c1ccc(O)cc1 |r| Show InChI InChI=1S/C27H26N2O10S/c1-40(37,38)29-18-5-2-6-19(31)23(18)25(34)24-20(32)12-15(13-21(24)33)27(36)39-22-7-3-4-17(22)28-26(35)14-8-10-16(30)11-9-14/h2,5-6,8-13,17,22,29-33H,3-4,7H2,1H3,(H,28,35)/t17-,22-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of Human Protein kinase C delta |
Bioorg Med Chem Lett 5: 1839-1842 (1995)
Article DOI: 10.1016/0960-894X(95)00303-B
BindingDB Entry DOI: 10.7270/Q2QN66R9 |
More data for this
Ligand-Target Pair | |
Protein kinase C beta type
(Homo sapiens (Human)) | BDBM3152

(2-{[2,6-dihydroxy-4-({[(3R,4R)-4-[(4-hydroxybenzen...)Show SMILES OC(=O)c1cccc(O)c1C(=O)c1c(O)cc(cc1O)C(=O)O[C@@H]1CNC[C@H]1NC(=O)c1ccc(O)cc1 |r| Show InChI InChI=1S/C26H22N2O10/c29-14-6-4-12(5-7-14)24(34)28-16-10-27-11-20(16)38-26(37)13-8-18(31)22(19(32)9-13)23(33)21-15(25(35)36)2-1-3-17(21)30/h1-9,16,20,27,29-32H,10-11H2,(H,28,34)(H,35,36)/t16-,20-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 33 | n/a | n/a | n/a | n/a | n/a | n/a |
Sphinx Laboratories
| Assay Description
PKC was assayed by quantitating the incorporation of 32P from [gamma-32P]ATP into histone type IIIs. |
J Med Chem 45: 2624-43 (2002)
Article DOI: 10.1021/jm020018f
BindingDB Entry DOI: 10.7270/Q2BG2M50 |
More data for this
Ligand-Target Pair | |
Protein kinase C epsilon type
(Homo sapiens (Human)) | BDBM3149

(2-{[2,6-dihydroxy-4-({[(3R,4R)-3-[(4-hydroxybenzen...)Show SMILES OC(=O)c1cccc(O)c1C(=O)c1c(O)cc(cc1O)C(=O)O[C@@H]1CCCNC[C@H]1NC(=O)c1ccc(O)cc1 |r| Show InChI InChI=1S/C28H26N2O10/c31-16-8-6-14(7-9-16)26(36)30-18-13-29-10-2-5-22(18)40-28(39)15-11-20(33)24(21(34)12-15)25(35)23-17(27(37)38)3-1-4-19(23)32/h1,3-4,6-9,11-12,18,22,29,31-34H,2,5,10,13H2,(H,30,36)(H,37,38)/t18-,22-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 38 | n/a | n/a | n/a | n/a | n/a | n/a |
Sphinx Laboratories
| Assay Description
PKC was assayed by quantitating the incorporation of 32P from [gamma-32P]ATP into histone type IIIs. |
J Med Chem 45: 2624-43 (2002)
Article DOI: 10.1021/jm020018f
BindingDB Entry DOI: 10.7270/Q2BG2M50 |
More data for this
Ligand-Target Pair | |
Protein kinase C alpha type
(Homo sapiens (Human)) | BDBM3153

(2-{[2,6-dihydroxy-4-({[(1R,2R)-2-[(4-hydroxybenzen...)Show SMILES OC(=O)c1cccc(O)c1C(=O)c1c(O)cc(cc1O)C(=O)O[C@@H]1CCC[C@H]1NC(=O)c1ccc(O)cc1 |r| Show InChI InChI=1S/C27H23NO10/c29-15-9-7-13(8-10-15)25(34)28-17-4-2-6-21(17)38-27(37)14-11-19(31)23(20(32)12-14)24(33)22-16(26(35)36)3-1-5-18(22)30/h1,3,5,7-12,17,21,29-32H,2,4,6H2,(H,28,34)(H,35,36)/t17-,21-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of Human Protein kinase C alpha |
Bioorg Med Chem Lett 5: 1839-1842 (1995)
Article DOI: 10.1016/0960-894X(95)00303-B
BindingDB Entry DOI: 10.7270/Q2QN66R9 |
More data for this
Ligand-Target Pair | |
Protein kinase C alpha type
(Homo sapiens (Human)) | BDBM3153

(2-{[2,6-dihydroxy-4-({[(1R,2R)-2-[(4-hydroxybenzen...)Show SMILES OC(=O)c1cccc(O)c1C(=O)c1c(O)cc(cc1O)C(=O)O[C@@H]1CCC[C@H]1NC(=O)c1ccc(O)cc1 |r| Show InChI InChI=1S/C27H23NO10/c29-15-9-7-13(8-10-15)25(34)28-17-4-2-6-21(17)38-27(37)14-11-19(31)23(20(32)12-14)24(33)22-16(26(35)36)3-1-5-18(22)30/h1,3,5,7-12,17,21,29-32H,2,4,6H2,(H,28,34)(H,35,36)/t17-,21-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
Sphinx Laboratories
| Assay Description
PKC was assayed by quantitating the incorporation of 32P from [gamma-32P]ATP into histone type IIIs. |
J Med Chem 45: 2624-43 (2002)
Article DOI: 10.1021/jm020018f
BindingDB Entry DOI: 10.7270/Q2BG2M50 |
More data for this
Ligand-Target Pair | |
Protein kinase C epsilon type
(Homo sapiens (Human)) | BDBM3153

(2-{[2,6-dihydroxy-4-({[(1R,2R)-2-[(4-hydroxybenzen...)Show SMILES OC(=O)c1cccc(O)c1C(=O)c1c(O)cc(cc1O)C(=O)O[C@@H]1CCC[C@H]1NC(=O)c1ccc(O)cc1 |r| Show InChI InChI=1S/C27H23NO10/c29-15-9-7-13(8-10-15)25(34)28-17-4-2-6-21(17)38-27(37)14-11-19(31)23(20(32)12-14)24(33)22-16(26(35)36)3-1-5-18(22)30/h1,3,5,7-12,17,21,29-32H,2,4,6H2,(H,28,34)(H,35,36)/t17-,21-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of Human Protein kinase C epsilon |
Bioorg Med Chem Lett 5: 1839-1842 (1995)
Article DOI: 10.1016/0960-894X(95)00303-B
BindingDB Entry DOI: 10.7270/Q2QN66R9 |
More data for this
Ligand-Target Pair | |
cAMP-dependent protein kinase catalytic subunit alpha
(Bos taurus (bovine)) | BDBM3230

((3R,4R)-4-[(4-hydroxybenzene)amido]pyrrolidin-3-yl...)Show SMILES Oc1ccc(cc1)C(=O)N[C@@H]1CNC[C@H]1OC(=O)c1cc(O)c(C(=O)c2c(O)ccc3CCCCc23)c(O)c1 |r| Show InChI InChI=1S/C29H28N2O8/c32-18-8-5-16(6-9-18)28(37)31-20-13-30-14-24(20)39-29(38)17-11-22(34)26(23(35)12-17)27(36)25-19-4-2-1-3-15(19)7-10-21(25)33/h5-12,20,24,30,32-35H,1-4,13-14H2,(H,31,37)/t20-,24-/m1/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
Sphinx Laboratories
| Assay Description
The activity of PKA, activated by cAMP, is measured by its ability to transfer phosphate from [gamma-32P]ATP to histone. |
J Med Chem 45: 2624-43 (2002)
Article DOI: 10.1021/jm020018f
BindingDB Entry DOI: 10.7270/Q2BG2M50 |
More data for this
Ligand-Target Pair | |
Protein kinase C beta type
(Homo sapiens (Human)) | BDBM3235

((1R,2R)-2-[(4-hydroxybenzene)amido]cyclopentyl 3,5...)Show SMILES Oc1ccc(cc1)C(=O)N[C@@H]1CCC[C@H]1OC(=O)c1cc(O)c(C(=O)c2c(O)cccc2NS(=O)(=O)C(F)(F)F)c(O)c1 |r| Show InChI InChI=1S/C27H23F3N2O10S/c28-27(29,30)43(40,41)32-17-4-1-5-18(34)22(17)24(37)23-19(35)11-14(12-20(23)36)26(39)42-21-6-2-3-16(21)31-25(38)13-7-9-15(33)10-8-13/h1,4-5,7-12,16,21,32-36H,2-3,6H2,(H,31,38)/t16-,21-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
Sphinx Laboratories
| Assay Description
PKC was assayed by quantitating the incorporation of 32P from [gamma-32P]ATP into histone type IIIs. |
J Med Chem 45: 2624-43 (2002)
Article DOI: 10.1021/jm020018f
BindingDB Entry DOI: 10.7270/Q2BG2M50 |
More data for this
Ligand-Target Pair | |
Protein kinase C delta type
(Homo sapiens (Human)) | BDBM3235

((1R,2R)-2-[(4-hydroxybenzene)amido]cyclopentyl 3,5...)Show SMILES Oc1ccc(cc1)C(=O)N[C@@H]1CCC[C@H]1OC(=O)c1cc(O)c(C(=O)c2c(O)cccc2NS(=O)(=O)C(F)(F)F)c(O)c1 |r| Show InChI InChI=1S/C27H23F3N2O10S/c28-27(29,30)43(40,41)32-17-4-1-5-18(34)22(17)24(37)23-19(35)11-14(12-20(23)36)26(39)42-21-6-2-3-16(21)31-25(38)13-7-9-15(33)10-8-13/h1,4-5,7-12,16,21,32-36H,2-3,6H2,(H,31,38)/t16-,21-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | <50 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of Human Protein kinase C delta |
Bioorg Med Chem Lett 5: 1839-1842 (1995)
Article DOI: 10.1016/0960-894X(95)00303-B
BindingDB Entry DOI: 10.7270/Q2QN66R9 |
More data for this
Ligand-Target Pair | |
Protein kinase C epsilon type
(Homo sapiens (Human)) | BDBM3153

(2-{[2,6-dihydroxy-4-({[(1R,2R)-2-[(4-hydroxybenzen...)Show SMILES OC(=O)c1cccc(O)c1C(=O)c1c(O)cc(cc1O)C(=O)O[C@@H]1CCC[C@H]1NC(=O)c1ccc(O)cc1 |r| Show InChI InChI=1S/C27H23NO10/c29-15-9-7-13(8-10-15)25(34)28-17-4-2-6-21(17)38-27(37)14-11-19(31)23(20(32)12-14)24(33)22-16(26(35)36)3-1-5-18(22)30/h1,3,5,7-12,17,21,29-32H,2,4,6H2,(H,28,34)(H,35,36)/t17-,21-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
Sphinx Laboratories
| Assay Description
PKC was assayed by quantitating the incorporation of 32P from [gamma-32P]ATP into histone type IIIs. |
J Med Chem 45: 2624-43 (2002)
Article DOI: 10.1021/jm020018f
BindingDB Entry DOI: 10.7270/Q2BG2M50 |
More data for this
Ligand-Target Pair | |
Protein kinase C beta type
(Homo sapiens (Human)) | BDBM3153

(2-{[2,6-dihydroxy-4-({[(1R,2R)-2-[(4-hydroxybenzen...)Show SMILES OC(=O)c1cccc(O)c1C(=O)c1c(O)cc(cc1O)C(=O)O[C@@H]1CCC[C@H]1NC(=O)c1ccc(O)cc1 |r| Show InChI InChI=1S/C27H23NO10/c29-15-9-7-13(8-10-15)25(34)28-17-4-2-6-21(17)38-27(37)14-11-19(31)23(20(32)12-14)24(33)22-16(26(35)36)3-1-5-18(22)30/h1,3,5,7-12,17,21,29-32H,2,4,6H2,(H,28,34)(H,35,36)/t17-,21-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
Sphinx Laboratories
| Assay Description
PKC was assayed by quantitating the incorporation of 32P from [gamma-32P]ATP into histone type IIIs. |
J Med Chem 45: 2624-43 (2002)
Article DOI: 10.1021/jm020018f
BindingDB Entry DOI: 10.7270/Q2BG2M50 |
More data for this
Ligand-Target Pair | |
Protein kinase C beta type
(Homo sapiens (Human)) | BDBM3153

(2-{[2,6-dihydroxy-4-({[(1R,2R)-2-[(4-hydroxybenzen...)Show SMILES OC(=O)c1cccc(O)c1C(=O)c1c(O)cc(cc1O)C(=O)O[C@@H]1CCC[C@H]1NC(=O)c1ccc(O)cc1 |r| Show InChI InChI=1S/C27H23NO10/c29-15-9-7-13(8-10-15)25(34)28-17-4-2-6-21(17)38-27(37)14-11-19(31)23(20(32)12-14)24(33)22-16(26(35)36)3-1-5-18(22)30/h1,3,5,7-12,17,21,29-32H,2,4,6H2,(H,28,34)(H,35,36)/t17-,21-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of Human Protein kinase C beta 2 |
Bioorg Med Chem Lett 5: 1839-1842 (1995)
Article DOI: 10.1016/0960-894X(95)00303-B
BindingDB Entry DOI: 10.7270/Q2QN66R9 |
More data for this
Ligand-Target Pair | |
Protein kinase C beta type
(Homo sapiens (Human)) | BDBM3235

((1R,2R)-2-[(4-hydroxybenzene)amido]cyclopentyl 3,5...)Show SMILES Oc1ccc(cc1)C(=O)N[C@@H]1CCC[C@H]1OC(=O)c1cc(O)c(C(=O)c2c(O)cccc2NS(=O)(=O)C(F)(F)F)c(O)c1 |r| Show InChI InChI=1S/C27H23F3N2O10S/c28-27(29,30)43(40,41)32-17-4-1-5-18(34)22(17)24(37)23-19(35)11-14(12-20(23)36)26(39)42-21-6-2-3-16(21)31-25(38)13-7-9-15(33)10-8-13/h1,4-5,7-12,16,21,32-36H,2-3,6H2,(H,31,38)/t16-,21-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of Human Protein kinase C beta 2 |
Bioorg Med Chem Lett 5: 1839-1842 (1995)
Article DOI: 10.1016/0960-894X(95)00303-B
BindingDB Entry DOI: 10.7270/Q2QN66R9 |
More data for this
Ligand-Target Pair | |
cAMP-dependent protein kinase catalytic subunit alpha
(Bos taurus (bovine)) | BDBM3152

(2-{[2,6-dihydroxy-4-({[(3R,4R)-4-[(4-hydroxybenzen...)Show SMILES OC(=O)c1cccc(O)c1C(=O)c1c(O)cc(cc1O)C(=O)O[C@@H]1CNC[C@H]1NC(=O)c1ccc(O)cc1 |r| Show InChI InChI=1S/C26H22N2O10/c29-14-6-4-12(5-7-14)24(34)28-16-10-27-11-20(16)38-26(37)13-8-18(31)22(19(32)9-13)23(33)21-15(25(35)36)2-1-3-17(21)30/h1-9,16,20,27,29-32H,10-11H2,(H,28,34)(H,35,36)/t16-,20-/m1/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 60 | n/a | n/a | n/a | n/a | n/a | n/a |
Sphinx Laboratories
| Assay Description
The activity of PKA, activated by cAMP, is measured by its ability to transfer phosphate from [gamma-32P]ATP to histone. |
J Med Chem 45: 2624-43 (2002)
Article DOI: 10.1021/jm020018f
BindingDB Entry DOI: 10.7270/Q2BG2M50 |
More data for this
Ligand-Target Pair | |
Protein kinase C alpha type
(Homo sapiens (Human)) | BDBM3149

(2-{[2,6-dihydroxy-4-({[(3R,4R)-3-[(4-hydroxybenzen...)Show SMILES OC(=O)c1cccc(O)c1C(=O)c1c(O)cc(cc1O)C(=O)O[C@@H]1CCCNC[C@H]1NC(=O)c1ccc(O)cc1 |r| Show InChI InChI=1S/C28H26N2O10/c31-16-8-6-14(7-9-16)26(36)30-18-13-29-10-2-5-22(18)40-28(39)15-11-20(33)24(21(34)12-15)25(35)23-17(27(37)38)3-1-4-19(23)32/h1,3-4,6-9,11-12,18,22,29,31-34H,2,5,10,13H2,(H,30,36)(H,37,38)/t18-,22-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 67 | n/a | n/a | n/a | n/a | n/a | n/a |
Sphinx Laboratories
| Assay Description
PKC was assayed by quantitating the incorporation of 32P from [gamma-32P]ATP into histone type IIIs. |
J Med Chem 45: 2624-43 (2002)
Article DOI: 10.1021/jm020018f
BindingDB Entry DOI: 10.7270/Q2BG2M50 |
More data for this
Ligand-Target Pair | |
Protein kinase C delta type
(Homo sapiens (Human)) | BDBM3240
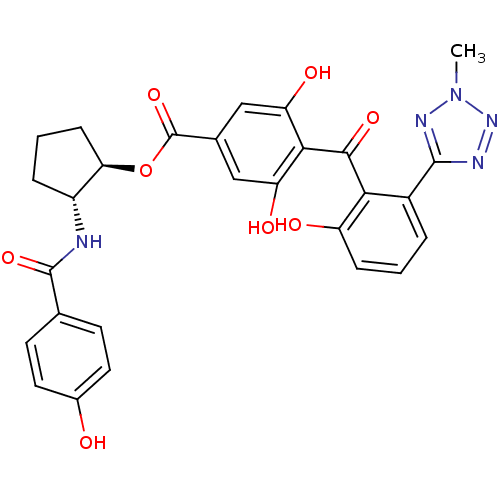
((1R,2R)-2-[(4-hydroxybenzene)amido]cyclopentyl 3,5...)Show SMILES Cn1nnc(n1)-c1cccc(O)c1C(=O)c1c(O)cc(cc1O)C(=O)O[C@@H]1CCC[C@H]1NC(=O)c1ccc(O)cc1 |r| Show InChI InChI=1S/C28H25N5O8/c1-33-31-26(30-32-33)17-4-2-6-19(35)23(17)25(38)24-20(36)12-15(13-21(24)37)28(40)41-22-7-3-5-18(22)29-27(39)14-8-10-16(34)11-9-14/h2,4,6,8-13,18,22,34-37H,3,5,7H2,1H3,(H,29,39)/t18-,22-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 90 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of Human Protein kinase C delta |
Bioorg Med Chem Lett 5: 1839-1842 (1995)
Article DOI: 10.1016/0960-894X(95)00303-B
BindingDB Entry DOI: 10.7270/Q2QN66R9 |
More data for this
Ligand-Target Pair | |
Protein kinase C alpha type
(Homo sapiens (Human)) | BDBM3235

((1R,2R)-2-[(4-hydroxybenzene)amido]cyclopentyl 3,5...)Show SMILES Oc1ccc(cc1)C(=O)N[C@@H]1CCC[C@H]1OC(=O)c1cc(O)c(C(=O)c2c(O)cccc2NS(=O)(=O)C(F)(F)F)c(O)c1 |r| Show InChI InChI=1S/C27H23F3N2O10S/c28-27(29,30)43(40,41)32-17-4-1-5-18(34)22(17)24(37)23-19(35)11-14(12-20(23)36)26(39)42-21-6-2-3-16(21)31-25(38)13-7-9-15(33)10-8-13/h1,4-5,7-12,16,21,32-36H,2-3,6H2,(H,31,38)/t16-,21-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
Sphinx Laboratories
| Assay Description
PKC was assayed by quantitating the incorporation of 32P from [gamma-32P]ATP into histone type IIIs. |
J Med Chem 45: 2624-43 (2002)
Article DOI: 10.1021/jm020018f
BindingDB Entry DOI: 10.7270/Q2BG2M50 |
More data for this
Ligand-Target Pair | |
Protein kinase C alpha type
(Homo sapiens (Human)) | BDBM3235

((1R,2R)-2-[(4-hydroxybenzene)amido]cyclopentyl 3,5...)Show SMILES Oc1ccc(cc1)C(=O)N[C@@H]1CCC[C@H]1OC(=O)c1cc(O)c(C(=O)c2c(O)cccc2NS(=O)(=O)C(F)(F)F)c(O)c1 |r| Show InChI InChI=1S/C27H23F3N2O10S/c28-27(29,30)43(40,41)32-17-4-1-5-18(34)22(17)24(37)23-19(35)11-14(12-20(23)36)26(39)42-21-6-2-3-16(21)31-25(38)13-7-9-15(33)10-8-13/h1,4-5,7-12,16,21,32-36H,2-3,6H2,(H,31,38)/t16-,21-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of Human Protein kinase C alpha |
Bioorg Med Chem Lett 5: 1839-1842 (1995)
Article DOI: 10.1016/0960-894X(95)00303-B
BindingDB Entry DOI: 10.7270/Q2QN66R9 |
More data for this
Ligand-Target Pair | |
Protein kinase C beta type
(Homo sapiens (Human)) | BDBM3236

((1R,2R)-2-[(4-hydroxybenzene)amido]cyclopentyl 3,5...)Show SMILES CS(=O)(=O)Nc1cccc(O)c1C(=O)c1c(O)cc(cc1O)C(=O)O[C@@H]1CCC[C@H]1NC(=O)c1ccc(O)cc1 |r| Show InChI InChI=1S/C27H26N2O10S/c1-40(37,38)29-18-5-2-6-19(31)23(18)25(34)24-20(32)12-15(13-21(24)33)27(36)39-22-7-3-4-17(22)28-26(35)14-8-10-16(30)11-9-14/h2,5-6,8-13,17,22,29-33H,3-4,7H2,1H3,(H,28,35)/t17-,22-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 110 | n/a | n/a | n/a | n/a | n/a | n/a |
Sphinx Laboratories
| Assay Description
PKC was assayed by quantitating the incorporation of 32P from [gamma-32P]ATP into histone type IIIs. |
J Med Chem 45: 2624-43 (2002)
Article DOI: 10.1021/jm020018f
BindingDB Entry DOI: 10.7270/Q2BG2M50 |
More data for this
Ligand-Target Pair | |
Protein kinase C delta type
(Homo sapiens (Human)) | BDBM3241

((1R,2R)-2-[(4-hydroxybenzene)amido]cyclopentyl 3,5...)Show SMILES Cn1nnnc1-c1cccc(O)c1C(=O)c1c(O)cc(cc1O)C(=O)O[C@@H]1CCC[C@H]1NC(=O)c1ccc(O)cc1 |r| Show InChI InChI=1S/C28H25N5O8/c1-33-26(30-31-32-33)17-4-2-6-19(35)23(17)25(38)24-20(36)12-15(13-21(24)37)28(40)41-22-7-3-5-18(22)29-27(39)14-8-10-16(34)11-9-14/h2,4,6,8-13,18,22,34-37H,3,5,7H2,1H3,(H,29,39)/t18-,22-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 130 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of Human Protein kinase C delta |
Bioorg Med Chem Lett 5: 1839-1842 (1995)
Article DOI: 10.1016/0960-894X(95)00303-B
BindingDB Entry DOI: 10.7270/Q2QN66R9 |
More data for this
Ligand-Target Pair | |
Protein kinase C beta type
(Homo sapiens (Human)) | BDBM3236

((1R,2R)-2-[(4-hydroxybenzene)amido]cyclopentyl 3,5...)Show SMILES CS(=O)(=O)Nc1cccc(O)c1C(=O)c1c(O)cc(cc1O)C(=O)O[C@@H]1CCC[C@H]1NC(=O)c1ccc(O)cc1 |r| Show InChI InChI=1S/C27H26N2O10S/c1-40(37,38)29-18-5-2-6-19(31)23(18)25(34)24-20(32)12-15(13-21(24)33)27(36)39-22-7-3-4-17(22)28-26(35)14-8-10-16(30)11-9-14/h2,5-6,8-13,17,22,29-33H,3-4,7H2,1H3,(H,28,35)/t17-,22-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 150 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of Human Protein kinase C beta 2 |
Bioorg Med Chem Lett 5: 1839-1842 (1995)
Article DOI: 10.1016/0960-894X(95)00303-B
BindingDB Entry DOI: 10.7270/Q2QN66R9 |
More data for this
Ligand-Target Pair | |
cAMP-dependent protein kinase catalytic subunit alpha
(Bos taurus (bovine)) | BDBM3239

((1R,2R)-2-[(4-hydroxybenzene)amido]cyclopentyl 3,5...)Show SMILES Oc1ccc(cc1)C(=O)N[C@@H]1CCC[C@H]1OC(=O)c1cc(O)c(C(=O)c2c(O)cccc2-c2nnn[nH]2)c(O)c1 |r| Show InChI InChI=1S/C27H23N5O8/c33-15-9-7-13(8-10-15)26(38)28-17-4-2-6-21(17)40-27(39)14-11-19(35)23(20(36)12-14)24(37)22-16(3-1-5-18(22)34)25-29-31-32-30-25/h1,3,5,7-12,17,21,33-36H,2,4,6H2,(H,28,38)(H,29,30,31,32)/t17-,21-/m1/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 210 | n/a | n/a | n/a | n/a | n/a | n/a |
Sphinx Laboratories
| Assay Description
The activity of PKA, activated by cAMP, is measured by its ability to transfer phosphate from [gamma-32P]ATP to histone. |
J Med Chem 45: 2624-43 (2002)
Article DOI: 10.1021/jm020018f
BindingDB Entry DOI: 10.7270/Q2BG2M50 |
More data for this
Ligand-Target Pair | |
cAMP-dependent protein kinase catalytic subunit alpha
(Bos taurus (bovine)) | BDBM3231

((3R,4R)-3-[(4-hydroxybenzene)amido]azepan-4-yl 3,5...)Show SMILES COc1cccc(O)c1C(=O)c1c(O)cc(cc1O)C(=O)O[C@@H]1CCCNC[C@H]1NC(=O)c1ccc(O)cc1 |r| Show InChI InChI=1S/C28H28N2O9/c1-38-23-5-2-4-19(32)25(23)26(35)24-20(33)12-16(13-21(24)34)28(37)39-22-6-3-11-29-14-18(22)30-27(36)15-7-9-17(31)10-8-15/h2,4-5,7-10,12-13,18,22,29,31-34H,3,6,11,14H2,1H3,(H,30,36)/t18-,22-/m1/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 250 | n/a | n/a | n/a | n/a | n/a | n/a |
Sphinx Laboratories
| Assay Description
The activity of PKA, activated by cAMP, is measured by its ability to transfer phosphate from [gamma-32P]ATP to histone. |
J Med Chem 45: 2624-43 (2002)
Article DOI: 10.1021/jm020018f
BindingDB Entry DOI: 10.7270/Q2BG2M50 |
More data for this
Ligand-Target Pair | |
Protein kinase C beta type
(Homo sapiens (Human)) | BDBM3239

((1R,2R)-2-[(4-hydroxybenzene)amido]cyclopentyl 3,5...)Show SMILES Oc1ccc(cc1)C(=O)N[C@@H]1CCC[C@H]1OC(=O)c1cc(O)c(C(=O)c2c(O)cccc2-c2nnn[nH]2)c(O)c1 |r| Show InChI InChI=1S/C27H23N5O8/c33-15-9-7-13(8-10-15)26(38)28-17-4-2-6-21(17)40-27(39)14-11-19(35)23(20(36)12-14)24(37)22-16(3-1-5-18(22)34)25-29-31-32-30-25/h1,3,5,7-12,17,21,33-36H,2,4,6H2,(H,28,38)(H,29,30,31,32)/t17-,21-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 260 | n/a | n/a | n/a | n/a | n/a | n/a |
Sphinx Laboratories
| Assay Description
PKC was assayed by quantitating the incorporation of 32P from [gamma-32P]ATP into histone type IIIs. |
J Med Chem 45: 2624-43 (2002)
Article DOI: 10.1021/jm020018f
BindingDB Entry DOI: 10.7270/Q2BG2M50 |
More data for this
Ligand-Target Pair | |
Protein kinase C beta type
(Homo sapiens (Human)) | BDBM3230

((3R,4R)-4-[(4-hydroxybenzene)amido]pyrrolidin-3-yl...)Show SMILES Oc1ccc(cc1)C(=O)N[C@@H]1CNC[C@H]1OC(=O)c1cc(O)c(C(=O)c2c(O)ccc3CCCCc23)c(O)c1 |r| Show InChI InChI=1S/C29H28N2O8/c32-18-8-5-16(6-9-18)28(37)31-20-13-30-14-24(20)39-29(38)17-11-22(34)26(23(35)12-17)27(36)25-19-4-2-1-3-15(19)7-10-21(25)33/h5-12,20,24,30,32-35H,1-4,13-14H2,(H,31,37)/t20-,24-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 260 | n/a | n/a | n/a | n/a | n/a | n/a |
Sphinx Laboratories
| Assay Description
PKC was assayed by quantitating the incorporation of 32P from [gamma-32P]ATP into histone type IIIs. |
J Med Chem 45: 2624-43 (2002)
Article DOI: 10.1021/jm020018f
BindingDB Entry DOI: 10.7270/Q2BG2M50 |
More data for this
Ligand-Target Pair | |
cAMP-dependent protein kinase catalytic subunit alpha
(Bos taurus (bovine)) | BDBM3228

((3R,4R)-4-[(4-hydroxybenzene)amido]pyrrolidin-3-yl...)Show SMILES Oc1ccc(cc1)C(=O)N[C@@H]1CNC[C@H]1OC(=O)c1cc(O)c(C(=O)c2c(O)ccc3ccccc23)c(O)c1 |r| Show InChI InChI=1S/C29H24N2O8/c32-18-8-5-16(6-9-18)28(37)31-20-13-30-14-24(20)39-29(38)17-11-22(34)26(23(35)12-17)27(36)25-19-4-2-1-3-15(19)7-10-21(25)33/h1-12,20,24,30,32-35H,13-14H2,(H,31,37)/t20-,24-/m1/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 290 | n/a | n/a | n/a | n/a | n/a | n/a |
Sphinx Laboratories
| Assay Description
The activity of PKA, activated by cAMP, is measured by its ability to transfer phosphate from [gamma-32P]ATP to histone. |
J Med Chem 45: 2624-43 (2002)
Article DOI: 10.1021/jm020018f
BindingDB Entry DOI: 10.7270/Q2BG2M50 |
More data for this
Ligand-Target Pair | |
Protein kinase C beta type
(Homo sapiens (Human)) | BDBM3239

((1R,2R)-2-[(4-hydroxybenzene)amido]cyclopentyl 3,5...)Show SMILES Oc1ccc(cc1)C(=O)N[C@@H]1CCC[C@H]1OC(=O)c1cc(O)c(C(=O)c2c(O)cccc2-c2nnn[nH]2)c(O)c1 |r| Show InChI InChI=1S/C27H23N5O8/c33-15-9-7-13(8-10-15)26(38)28-17-4-2-6-21(17)40-27(39)14-11-19(35)23(20(36)12-14)24(37)22-16(3-1-5-18(22)34)25-29-31-32-30-25/h1,3,5,7-12,17,21,33-36H,2,4,6H2,(H,28,38)(H,29,30,31,32)/t17-,21-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 290 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of Human Protein kinase C beta 2 |
Bioorg Med Chem Lett 5: 1839-1842 (1995)
Article DOI: 10.1016/0960-894X(95)00303-B
BindingDB Entry DOI: 10.7270/Q2QN66R9 |
More data for this
Ligand-Target Pair | |
Protein kinase C beta type
(Homo sapiens (Human)) | BDBM3220
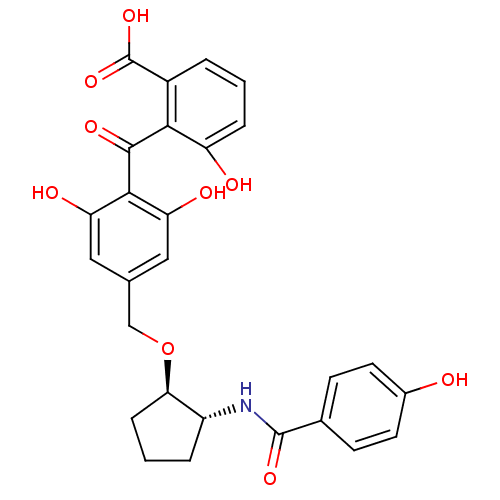
(2-{[2,6-dihydroxy-4-({[(1R,2R)-2-[(4-hydroxybenzen...)Show SMILES OC(=O)c1cccc(O)c1C(=O)c1c(O)cc(CO[C@@H]2CCC[C@H]2NC(=O)c2ccc(O)cc2)cc1O |r| Show InChI InChI=1S/C27H25NO9/c29-16-9-7-15(8-10-16)26(34)28-18-4-2-6-22(18)37-13-14-11-20(31)24(21(32)12-14)25(33)23-17(27(35)36)3-1-5-19(23)30/h1,3,5,7-12,18,22,29-32H,2,4,6,13H2,(H,28,34)(H,35,36)/t18-,22-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 290 | n/a | n/a | n/a | n/a | n/a | n/a |
Sphinx Laboratories
| Assay Description
PKC was assayed by quantitating the incorporation of 32P from [gamma-32P]ATP into histone type IIIs. |
J Med Chem 45: 2624-43 (2002)
Article DOI: 10.1021/jm020018f
BindingDB Entry DOI: 10.7270/Q2BG2M50 |
More data for this
Ligand-Target Pair | |
Protein kinase C delta type
(Homo sapiens (Human)) | BDBM50284982
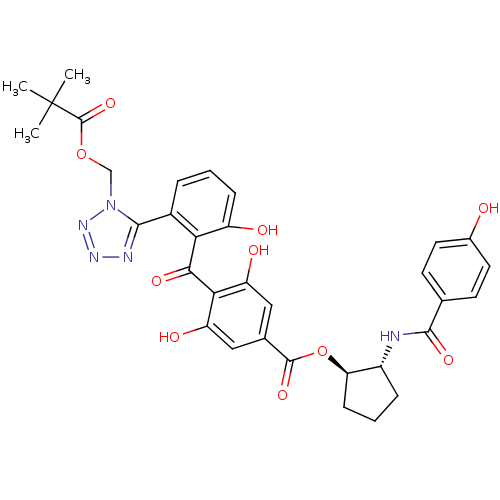
(4-{2-[1-(2,2-Dimethyl-propionyloxymethyl)-1H-tetra...)Show SMILES CC(C)(C)C(=O)OCn1nnnc1-c1cccc(O)c1C(=O)c1c(O)cc(cc1O)C(=O)O[C@@H]1CCC[C@H]1NC(=O)c1ccc(O)cc1 Show InChI InChI=1S/C33H33N5O10/c1-33(2,3)32(46)47-16-38-29(35-36-37-38)20-6-4-8-22(40)26(20)28(43)27-23(41)14-18(15-24(27)42)31(45)48-25-9-5-7-21(25)34-30(44)17-10-12-19(39)13-11-17/h4,6,8,10-15,21,25,39-42H,5,7,9,16H2,1-3H3,(H,34,44)/t21-,25-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 300 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of Human Protein kinase C delta |
Bioorg Med Chem Lett 5: 1839-1842 (1995)
Article DOI: 10.1016/0960-894X(95)00303-B
BindingDB Entry DOI: 10.7270/Q2QN66R9 |
More data for this
Ligand-Target Pair | |
Protein kinase C epsilon type
(Homo sapiens (Human)) | BDBM3230

((3R,4R)-4-[(4-hydroxybenzene)amido]pyrrolidin-3-yl...)Show SMILES Oc1ccc(cc1)C(=O)N[C@@H]1CNC[C@H]1OC(=O)c1cc(O)c(C(=O)c2c(O)ccc3CCCCc23)c(O)c1 |r| Show InChI InChI=1S/C29H28N2O8/c32-18-8-5-16(6-9-18)28(37)31-20-13-30-14-24(20)39-29(38)17-11-22(34)26(23(35)12-17)27(36)25-19-4-2-1-3-15(19)7-10-21(25)33/h5-12,20,24,30,32-35H,1-4,13-14H2,(H,31,37)/t20-,24-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 300 | n/a | n/a | n/a | n/a | n/a | n/a |
Sphinx Laboratories
| Assay Description
PKC was assayed by quantitating the incorporation of 32P from [gamma-32P]ATP into histone type IIIs. |
J Med Chem 45: 2624-43 (2002)
Article DOI: 10.1021/jm020018f
BindingDB Entry DOI: 10.7270/Q2BG2M50 |
More data for this
Ligand-Target Pair | |
cAMP-dependent protein kinase catalytic subunit alpha
(Bos taurus (bovine)) | BDBM3226
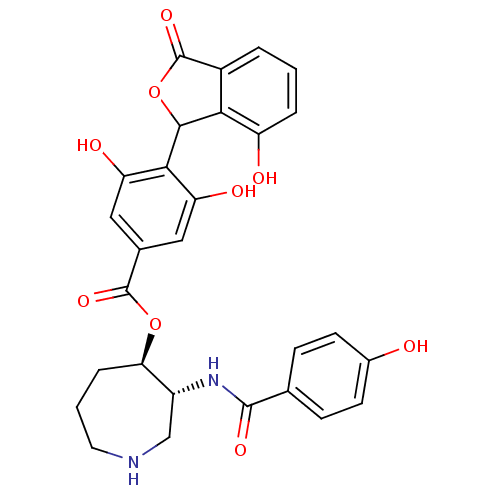
((3R,4R)-3-[(4-hydroxybenzene)amido]azepan-4-yl 3,5...)Show SMILES Oc1ccc(cc1)C(=O)N[C@@H]1CNCCC[C@H]1OC(=O)c1cc(O)c(C2OC(=O)c3cccc(O)c23)c(O)c1 |r| Show InChI InChI=1S/C28H26N2O9/c31-16-8-6-14(7-9-16)26(35)30-18-13-29-10-2-5-22(18)38-27(36)15-11-20(33)24(21(34)12-15)25-23-17(28(37)39-25)3-1-4-19(23)32/h1,3-4,6-9,11-12,18,22,25,29,31-34H,2,5,10,13H2,(H,30,35)/t18-,22-,25?/m1/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 300 | n/a | n/a | n/a | n/a | n/a | n/a |
Sphinx Laboratories
| Assay Description
The activity of PKA, activated by cAMP, is measured by its ability to transfer phosphate from [gamma-32P]ATP to histone. |
J Med Chem 45: 2624-43 (2002)
Article DOI: 10.1021/jm020018f
BindingDB Entry DOI: 10.7270/Q2BG2M50 |
More data for this
Ligand-Target Pair | |
Protein kinase C epsilon type
(Homo sapiens (Human)) | BDBM3235

((1R,2R)-2-[(4-hydroxybenzene)amido]cyclopentyl 3,5...)Show SMILES Oc1ccc(cc1)C(=O)N[C@@H]1CCC[C@H]1OC(=O)c1cc(O)c(C(=O)c2c(O)cccc2NS(=O)(=O)C(F)(F)F)c(O)c1 |r| Show InChI InChI=1S/C27H23F3N2O10S/c28-27(29,30)43(40,41)32-17-4-1-5-18(34)22(17)24(37)23-19(35)11-14(12-20(23)36)26(39)42-21-6-2-3-16(21)31-25(38)13-7-9-15(33)10-8-13/h1,4-5,7-12,16,21,32-36H,2-3,6H2,(H,31,38)/t16-,21-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 310 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of Human Protein kinase C epsilon |
Bioorg Med Chem Lett 5: 1839-1842 (1995)
Article DOI: 10.1016/0960-894X(95)00303-B
BindingDB Entry DOI: 10.7270/Q2QN66R9 |
More data for this
Ligand-Target Pair | |
Protein kinase C epsilon type
(Homo sapiens (Human)) | BDBM3235

((1R,2R)-2-[(4-hydroxybenzene)amido]cyclopentyl 3,5...)Show SMILES Oc1ccc(cc1)C(=O)N[C@@H]1CCC[C@H]1OC(=O)c1cc(O)c(C(=O)c2c(O)cccc2NS(=O)(=O)C(F)(F)F)c(O)c1 |r| Show InChI InChI=1S/C27H23F3N2O10S/c28-27(29,30)43(40,41)32-17-4-1-5-18(34)22(17)24(37)23-19(35)11-14(12-20(23)36)26(39)42-21-6-2-3-16(21)31-25(38)13-7-9-15(33)10-8-13/h1,4-5,7-12,16,21,32-36H,2-3,6H2,(H,31,38)/t16-,21-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 310 | n/a | n/a | n/a | n/a | n/a | n/a |
Sphinx Laboratories
| Assay Description
PKC was assayed by quantitating the incorporation of 32P from [gamma-32P]ATP into histone type IIIs. |
J Med Chem 45: 2624-43 (2002)
Article DOI: 10.1021/jm020018f
BindingDB Entry DOI: 10.7270/Q2BG2M50 |
More data for this
Ligand-Target Pair | |
cAMP-dependent protein kinase catalytic subunit alpha
(Bos taurus (bovine)) | BDBM3220

(2-{[2,6-dihydroxy-4-({[(1R,2R)-2-[(4-hydroxybenzen...)Show SMILES OC(=O)c1cccc(O)c1C(=O)c1c(O)cc(CO[C@@H]2CCC[C@H]2NC(=O)c2ccc(O)cc2)cc1O |r| Show InChI InChI=1S/C27H25NO9/c29-16-9-7-15(8-10-16)26(34)28-18-4-2-6-22(18)37-13-14-11-20(31)24(21(32)12-14)25(33)23-17(27(35)36)3-1-5-19(23)30/h1,3,5,7-12,18,22,29-32H,2,4,6,13H2,(H,28,34)(H,35,36)/t18-,22-/m1/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 320 | n/a | n/a | n/a | n/a | n/a | n/a |
Sphinx Laboratories
| Assay Description
The activity of PKA, activated by cAMP, is measured by its ability to transfer phosphate from [gamma-32P]ATP to histone. |
J Med Chem 45: 2624-43 (2002)
Article DOI: 10.1021/jm020018f
BindingDB Entry DOI: 10.7270/Q2BG2M50 |
More data for this
Ligand-Target Pair | |
cAMP-dependent protein kinase catalytic subunit alpha
(Bos taurus (bovine)) | BDBM3227
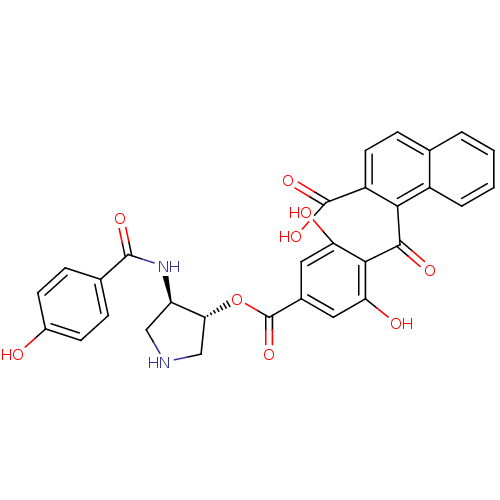
(1-{[2,6-dihydroxy-4-({[(3R,4R)-4-[(4-hydroxybenzen...)Show SMILES OC(=O)c1ccc2ccccc2c1C(=O)c1c(O)cc(cc1O)C(=O)O[C@@H]1CNC[C@H]1NC(=O)c1ccc(O)cc1 |r| Show InChI InChI=1S/C30H24N2O9/c33-18-8-5-16(6-9-18)28(37)32-21-13-31-14-24(21)41-30(40)17-11-22(34)26(23(35)12-17)27(36)25-19-4-2-1-3-15(19)7-10-20(25)29(38)39/h1-12,21,24,31,33-35H,13-14H2,(H,32,37)(H,38,39)/t21-,24-/m1/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 330 | n/a | n/a | n/a | n/a | n/a | n/a |
Sphinx Laboratories
| Assay Description
The activity of PKA, activated by cAMP, is measured by its ability to transfer phosphate from [gamma-32P]ATP to histone. |
J Med Chem 45: 2624-43 (2002)
Article DOI: 10.1021/jm020018f
BindingDB Entry DOI: 10.7270/Q2BG2M50 |
More data for this
Ligand-Target Pair | |
Protein kinase C beta type
(Homo sapiens (Human)) | BDBM3234
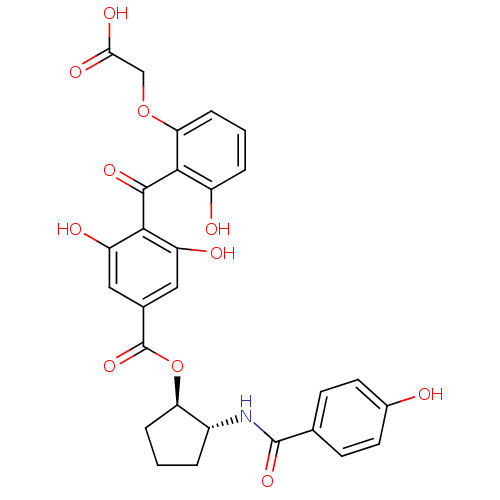
(1-trans-[4-(2-Carboxymethoxy-6-hydroxybenzoyl)-3,5...)Show SMILES OC(=O)COc1cccc(O)c1C(=O)c1c(O)cc(cc1O)C(=O)O[C@@H]1CCC[C@H]1NC(=O)c1ccc(O)cc1 |r| Show InChI InChI=1S/C28H25NO11/c30-16-9-7-14(8-10-16)27(37)29-17-3-1-5-21(17)40-28(38)15-11-19(32)24(20(33)12-15)26(36)25-18(31)4-2-6-22(25)39-13-23(34)35/h2,4,6-12,17,21,30-33H,1,3,5,13H2,(H,29,37)(H,34,35)/t17-,21-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 330 | n/a | n/a | n/a | n/a | n/a | n/a |
Sphinx Laboratories
| Assay Description
PKC was assayed by quantitating the incorporation of 32P from [gamma-32P]ATP into histone type IIIs. |
J Med Chem 45: 2624-43 (2002)
Article DOI: 10.1021/jm020018f
BindingDB Entry DOI: 10.7270/Q2BG2M50 |
More data for this
Ligand-Target Pair | |
Protein kinase C alpha type
(Homo sapiens (Human)) | BDBM3239

((1R,2R)-2-[(4-hydroxybenzene)amido]cyclopentyl 3,5...)Show SMILES Oc1ccc(cc1)C(=O)N[C@@H]1CCC[C@H]1OC(=O)c1cc(O)c(C(=O)c2c(O)cccc2-c2nnn[nH]2)c(O)c1 |r| Show InChI InChI=1S/C27H23N5O8/c33-15-9-7-13(8-10-15)26(38)28-17-4-2-6-21(17)40-27(39)14-11-19(35)23(20(36)12-14)24(37)22-16(3-1-5-18(22)34)25-29-31-32-30-25/h1,3,5,7-12,17,21,33-36H,2,4,6H2,(H,28,38)(H,29,30,31,32)/t17-,21-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 340 | n/a | n/a | n/a | n/a | n/a | n/a |
Sphinx Laboratories
| Assay Description
PKC was assayed by quantitating the incorporation of 32P from [gamma-32P]ATP into histone type IIIs. |
J Med Chem 45: 2624-43 (2002)
Article DOI: 10.1021/jm020018f
BindingDB Entry DOI: 10.7270/Q2BG2M50 |
More data for this
Ligand-Target Pair | |
Protein kinase C alpha type
(Homo sapiens (Human)) | BDBM3239

((1R,2R)-2-[(4-hydroxybenzene)amido]cyclopentyl 3,5...)Show SMILES Oc1ccc(cc1)C(=O)N[C@@H]1CCC[C@H]1OC(=O)c1cc(O)c(C(=O)c2c(O)cccc2-c2nnn[nH]2)c(O)c1 |r| Show InChI InChI=1S/C27H23N5O8/c33-15-9-7-13(8-10-15)26(38)28-17-4-2-6-21(17)40-27(39)14-11-19(35)23(20(36)12-14)24(37)22-16(3-1-5-18(22)34)25-29-31-32-30-25/h1,3,5,7-12,17,21,33-36H,2,4,6H2,(H,28,38)(H,29,30,31,32)/t17-,21-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 340 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of Human Protein kinase C alpha |
Bioorg Med Chem Lett 5: 1839-1842 (1995)
Article DOI: 10.1016/0960-894X(95)00303-B
BindingDB Entry DOI: 10.7270/Q2QN66R9 |
More data for this
Ligand-Target Pair | |
cAMP-dependent protein kinase catalytic subunit alpha
(Bos taurus (bovine)) | BDBM3216
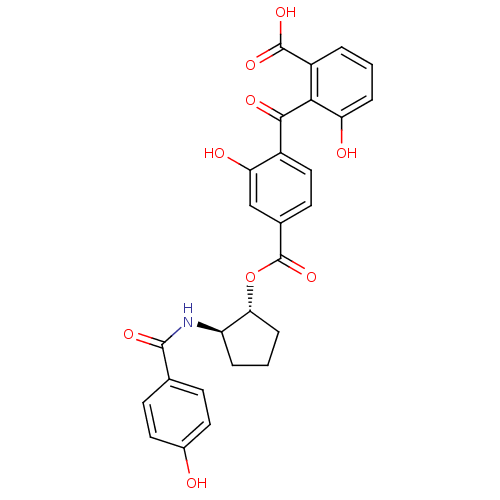
(3-hydroxy-2-{[2-hydroxy-4-({[(1R,2R)-2-[(4-hydroxy...)Show SMILES OC(=O)c1cccc(O)c1C(=O)c1ccc(cc1O)C(=O)O[C@@H]1CCC[C@H]1NC(=O)c1ccc(O)cc1 |r| Show InChI InChI=1S/C27H23NO9/c29-16-10-7-14(8-11-16)25(33)28-19-4-2-6-22(19)37-27(36)15-9-12-17(21(31)13-15)24(32)23-18(26(34)35)3-1-5-20(23)30/h1,3,5,7-13,19,22,29-31H,2,4,6H2,(H,28,33)(H,34,35)/t19-,22-/m1/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 350 | n/a | n/a | n/a | n/a | n/a | n/a |
Sphinx Laboratories
| Assay Description
The activity of PKA, activated by cAMP, is measured by its ability to transfer phosphate from [gamma-32P]ATP to histone. |
J Med Chem 45: 2624-43 (2002)
Article DOI: 10.1021/jm020018f
BindingDB Entry DOI: 10.7270/Q2BG2M50 |
More data for this
Ligand-Target Pair | |
cAMP-dependent protein kinase catalytic subunit alpha
(Bos taurus (bovine)) | BDBM3235

((1R,2R)-2-[(4-hydroxybenzene)amido]cyclopentyl 3,5...)Show SMILES Oc1ccc(cc1)C(=O)N[C@@H]1CCC[C@H]1OC(=O)c1cc(O)c(C(=O)c2c(O)cccc2NS(=O)(=O)C(F)(F)F)c(O)c1 |r| Show InChI InChI=1S/C27H23F3N2O10S/c28-27(29,30)43(40,41)32-17-4-1-5-18(34)22(17)24(37)23-19(35)11-14(12-20(23)36)26(39)42-21-6-2-3-16(21)31-25(38)13-7-9-15(33)10-8-13/h1,4-5,7-12,16,21,32-36H,2-3,6H2,(H,31,38)/t16-,21-/m1/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 350 | n/a | n/a | n/a | n/a | n/a | n/a |
Sphinx Laboratories
| Assay Description
The activity of PKA, activated by cAMP, is measured by its ability to transfer phosphate from [gamma-32P]ATP to histone. |
J Med Chem 45: 2624-43 (2002)
Article DOI: 10.1021/jm020018f
BindingDB Entry DOI: 10.7270/Q2BG2M50 |
More data for this
Ligand-Target Pair | |
cAMP-dependent protein kinase catalytic subunit alpha
(Bos taurus (bovine)) | BDBM3213

((3R,4R)-3-[(4-hydroxybenzene)amido]azepan-4-yl 4-[...)Show SMILES COc1cccc(OC)c1C(=O)c1c(O)cc(cc1O)C(=O)O[C@@H]1CCCNC[C@H]1NC(=O)c1ccc(O)cc1 |r| Show InChI InChI=1S/C29H30N2O9/c1-38-23-5-3-6-24(39-2)26(23)27(35)25-20(33)13-17(14-21(25)34)29(37)40-22-7-4-12-30-15-19(22)31-28(36)16-8-10-18(32)11-9-16/h3,5-6,8-11,13-14,19,22,30,32-34H,4,7,12,15H2,1-2H3,(H,31,36)/t19-,22-/m1/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 360 | n/a | n/a | n/a | n/a | n/a | n/a |
Sphinx Laboratories
| Assay Description
The activity of PKA, activated by cAMP, is measured by its ability to transfer phosphate from [gamma-32P]ATP to histone. |
J Med Chem 45: 2624-43 (2002)
Article DOI: 10.1021/jm020018f
BindingDB Entry DOI: 10.7270/Q2BG2M50 |
More data for this
Ligand-Target Pair | |
Protein kinase C epsilon type
(Homo sapiens (Human)) | BDBM3220

(2-{[2,6-dihydroxy-4-({[(1R,2R)-2-[(4-hydroxybenzen...)Show SMILES OC(=O)c1cccc(O)c1C(=O)c1c(O)cc(CO[C@@H]2CCC[C@H]2NC(=O)c2ccc(O)cc2)cc1O |r| Show InChI InChI=1S/C27H25NO9/c29-16-9-7-15(8-10-16)26(34)28-18-4-2-6-22(18)37-13-14-11-20(31)24(21(32)12-14)25(33)23-17(27(35)36)3-1-5-19(23)30/h1,3,5,7-12,18,22,29-32H,2,4,6,13H2,(H,28,34)(H,35,36)/t18-,22-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 370 | n/a | n/a | n/a | n/a | n/a | n/a |
Sphinx Laboratories
| Assay Description
PKC was assayed by quantitating the incorporation of 32P from [gamma-32P]ATP into histone type IIIs. |
J Med Chem 45: 2624-43 (2002)
Article DOI: 10.1021/jm020018f
BindingDB Entry DOI: 10.7270/Q2BG2M50 |
More data for this
Ligand-Target Pair | |
Protein kinase C epsilon type
(Homo sapiens (Human)) | BDBM3234

(1-trans-[4-(2-Carboxymethoxy-6-hydroxybenzoyl)-3,5...)Show SMILES OC(=O)COc1cccc(O)c1C(=O)c1c(O)cc(cc1O)C(=O)O[C@@H]1CCC[C@H]1NC(=O)c1ccc(O)cc1 |r| Show InChI InChI=1S/C28H25NO11/c30-16-9-7-14(8-10-16)27(37)29-17-3-1-5-21(17)40-28(38)15-11-19(32)24(20(33)12-15)26(36)25-18(31)4-2-6-22(25)39-13-23(34)35/h2,4,6-12,17,21,30-33H,1,3,5,13H2,(H,29,37)(H,34,35)/t17-,21-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 410 | n/a | n/a | n/a | n/a | n/a | n/a |
Sphinx Laboratories
| Assay Description
PKC was assayed by quantitating the incorporation of 32P from [gamma-32P]ATP into histone type IIIs. |
J Med Chem 45: 2624-43 (2002)
Article DOI: 10.1021/jm020018f
BindingDB Entry DOI: 10.7270/Q2BG2M50 |
More data for this
Ligand-Target Pair | |
Protein kinase C delta type
(Homo sapiens (Human)) | BDBM3238

((1R,2R)-2-[(4-hydroxybenzene)amido]cyclopentyl 4-[...)Show SMILES CC(=O)Nc1cccc(O)c1C(=O)c1c(O)cc(cc1O)C(=O)O[C@@H]1CCC[C@H]1NC(=O)c1ccc(O)cc1 |r| Show InChI InChI=1S/C28H26N2O9/c1-14(31)29-19-5-2-6-20(33)24(19)26(36)25-21(34)12-16(13-22(25)35)28(38)39-23-7-3-4-18(23)30-27(37)15-8-10-17(32)11-9-15/h2,5-6,8-13,18,23,32-35H,3-4,7H2,1H3,(H,29,31)(H,30,37)/t18-,23-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 420 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of Human Protein kinase C delta |
Bioorg Med Chem Lett 5: 1839-1842 (1995)
Article DOI: 10.1016/0960-894X(95)00303-B
BindingDB Entry DOI: 10.7270/Q2QN66R9 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data