

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Coagulation factor XI (Homo sapiens (Human)) | BDBM413625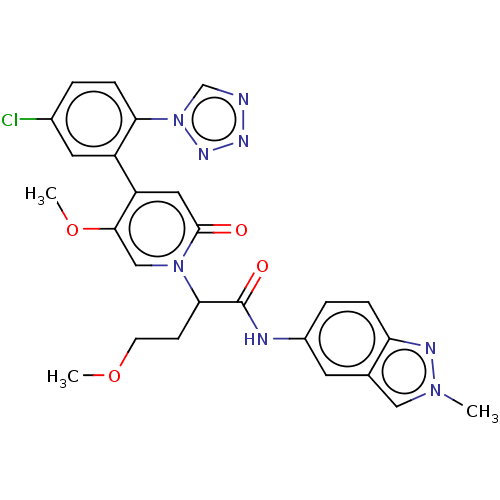 (2-{4-[5-Chloro-2-(1H-tetrazol-1-yl)phenyl]-5-metho...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.260 | n/a | n/a | n/a | n/a | n/a | n/a |
BAYER PHARMA AKTIENGESELLSCHAFT US Patent | Assay Description The factor XIa inhibition of the substances according to the invention is determined using a biochemical test system which utilizes the reaction of a... | US Patent US10421742 (2019) BindingDB Entry DOI: 10.7270/Q26M3967 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM413725 (N-(Quinoxalin-6-yl)-(2S)-2-{4-[5-chloro-2-(1H-tetr...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.260 | n/a | n/a | n/a | n/a | n/a | n/a |
BAYER PHARMA AKTIENGESELLSCHAFT US Patent | Assay Description The factor XIa inhibition of the substances according to the invention is determined using a biochemical test system which utilizes the reaction of a... | US Patent US10421742 (2019) BindingDB Entry DOI: 10.7270/Q26M3967 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM413721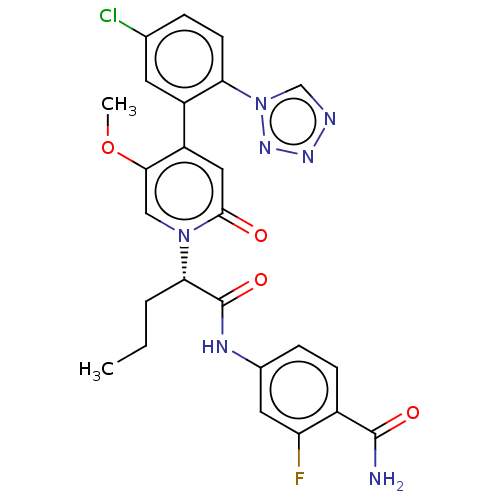 (4-{[(2S)-2-{4-[5-Chloro-2-(1H-tetrazol-1-yl)phenyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.310 | n/a | n/a | n/a | n/a | n/a | n/a |
BAYER PHARMA AKTIENGESELLSCHAFT US Patent | Assay Description The factor XIa inhibition of the substances according to the invention is determined using a biochemical test system which utilizes the reaction of a... | US Patent US10421742 (2019) BindingDB Entry DOI: 10.7270/Q26M3967 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM413727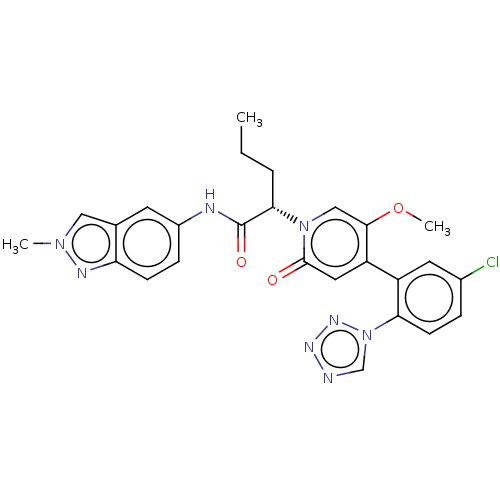 ((2S)-2-{4-[5-Chloro-2-(1H-tetrazol-1-yl)phenyl]-5-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.340 | n/a | n/a | n/a | n/a | n/a | n/a |
BAYER PHARMA AKTIENGESELLSCHAFT US Patent | Assay Description The factor XIa inhibition of the substances according to the invention is determined using a biochemical test system which utilizes the reaction of a... | US Patent US10421742 (2019) BindingDB Entry DOI: 10.7270/Q26M3967 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM413626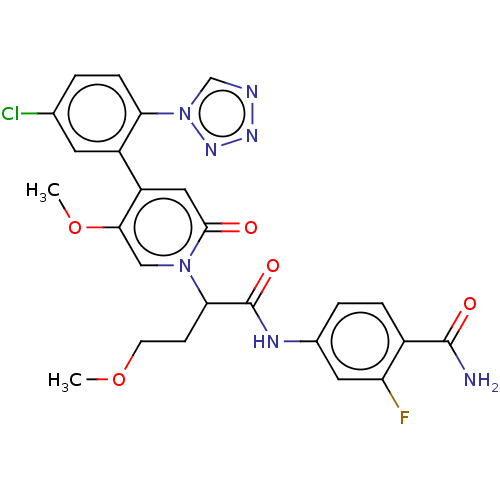 (4-[(2-{4-[5-Chloro-2-(1H-tetrazol-1-yl)phenyl]-5-m...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.340 | n/a | n/a | n/a | n/a | n/a | n/a |
BAYER PHARMA AKTIENGESELLSCHAFT US Patent | Assay Description The factor XIa inhibition of the substances according to the invention is determined using a biochemical test system which utilizes the reaction of a... | US Patent US10421742 (2019) BindingDB Entry DOI: 10.7270/Q26M3967 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM413745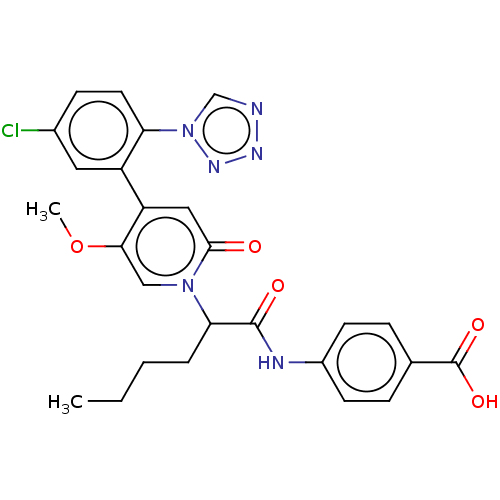 (4-{[2-{4-[5-Chloro-2-(1H-tetrazol-1-yl)phenyl]-5-m...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.360 | n/a | n/a | n/a | n/a | n/a | n/a |
BAYER PHARMA AKTIENGESELLSCHAFT US Patent | Assay Description The factor XIa inhibition of the substances according to the invention is determined using a biochemical test system which utilizes the reaction of a... | US Patent US10421742 (2019) BindingDB Entry DOI: 10.7270/Q26M3967 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM413654 (4-[(2-{4-[5-Chloro-2-(1,2-oxazol-3-yl)phenyl]-5-me...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.360 | n/a | n/a | n/a | n/a | n/a | n/a |
BAYER PHARMA AKTIENGESELLSCHAFT US Patent | Assay Description The factor XIa inhibition of the substances according to the invention is determined using a biochemical test system which utilizes the reaction of a... | US Patent US10421742 (2019) BindingDB Entry DOI: 10.7270/Q26M3967 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM413719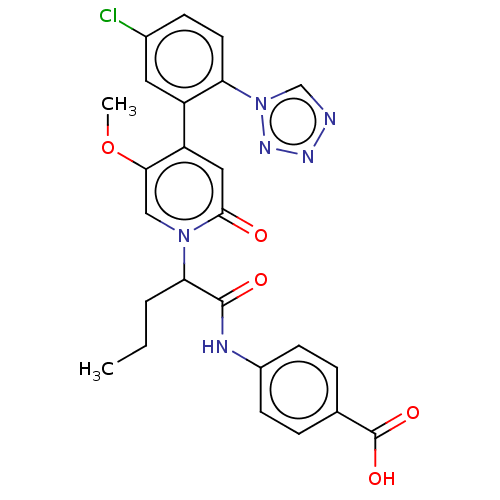 (4-{[2-{4-[5-Chloro-2-(1H-tetrazol-1-yl)phenyl]-5-m...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.360 | n/a | n/a | n/a | n/a | n/a | n/a |
BAYER PHARMA AKTIENGESELLSCHAFT US Patent | Assay Description The factor XIa inhibition of the substances according to the invention is determined using a biochemical test system which utilizes the reaction of a... | US Patent US10421742 (2019) BindingDB Entry DOI: 10.7270/Q26M3967 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM413610 (4-{[(2S)-2-{4-[5-Chloro-2-(1,3-oxazol-5-yl)phenyl]...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
BAYER PHARMA AKTIENGESELLSCHAFT US Patent | Assay Description The factor XIa inhibition of the substances according to the invention is determined using a biochemical test system which utilizes the reaction of a... | US Patent US10421742 (2019) BindingDB Entry DOI: 10.7270/Q26M3967 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM413722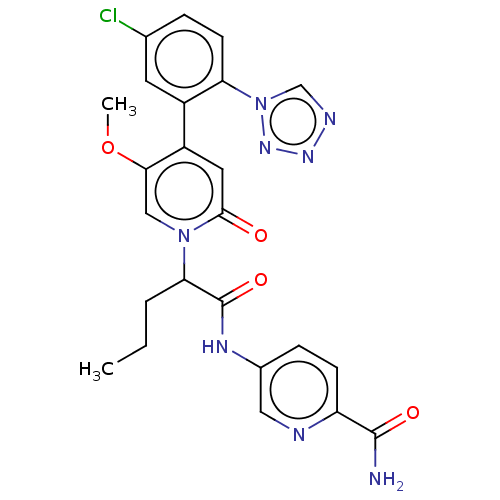 (5-{[2-{4-[5-Chloro-2-(1H-tetrazol-1-yl)phenyl]-5-m...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.460 | n/a | n/a | n/a | n/a | n/a | n/a |
BAYER PHARMA AKTIENGESELLSCHAFT US Patent | Assay Description The factor XIa inhibition of the substances according to the invention is determined using a biochemical test system which utilizes the reaction of a... | US Patent US10421742 (2019) BindingDB Entry DOI: 10.7270/Q26M3967 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasma kallikrein (Homo sapiens (Human)) | BDBM276779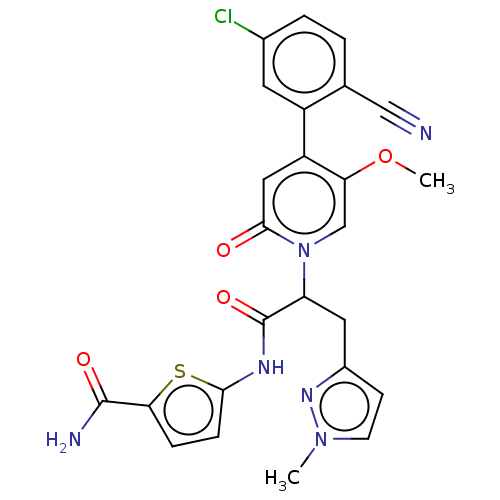 (5-({2-[4-(5-Chloro-2-cyanophenyl)-5-methoxy-2-oxop...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.490 | n/a | n/a | n/a | n/a | 7.4 | 25 |
BAYER PHARMA AKTIENGESELLSCHAFT US Patent | Assay Description Test substances are dissolved in dimethyl sulphoxide and serially diluted in dimethyl sulphoxide (3000 μM to 0.0078 μM; resulting final con... | US Patent US10071995 (2018) BindingDB Entry DOI: 10.7270/Q2H13429 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM413726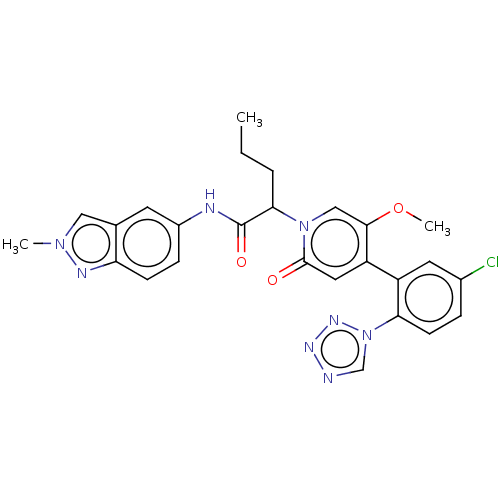 (2-{4-[5-Chloro-2-(1H-tetrazol-1-yl)phenyl]-5-metho...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
BAYER PHARMA AKTIENGESELLSCHAFT US Patent | Assay Description The factor XIa inhibition of the substances according to the invention is determined using a biochemical test system which utilizes the reaction of a... | US Patent US10421742 (2019) BindingDB Entry DOI: 10.7270/Q26M3967 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM413652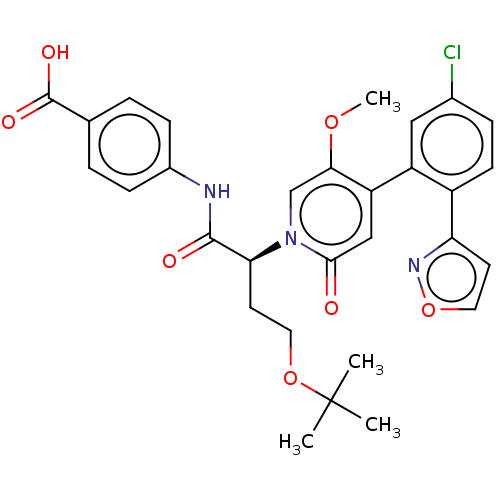 (4-{[(2S)-4-tert-Butoxy-2-{4-[5-chloro-2-(1,2-oxazo...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.530 | n/a | n/a | n/a | n/a | n/a | n/a |
BAYER PHARMA AKTIENGESELLSCHAFT US Patent | Assay Description The factor XIa inhibition of the substances according to the invention is determined using a biochemical test system which utilizes the reaction of a... | US Patent US10421742 (2019) BindingDB Entry DOI: 10.7270/Q26M3967 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM413720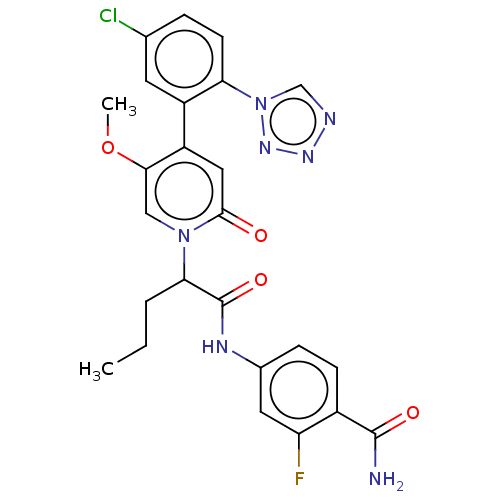 (4-{[2-{4-[5-Chloro-2-(1H-tetrazol-1-yl)phenyl]-5-m...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.550 | n/a | n/a | n/a | n/a | n/a | n/a |
BAYER PHARMA AKTIENGESELLSCHAFT US Patent | Assay Description The factor XIa inhibition of the substances according to the invention is determined using a biochemical test system which utilizes the reaction of a... | US Patent US10421742 (2019) BindingDB Entry DOI: 10.7270/Q26M3967 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM413777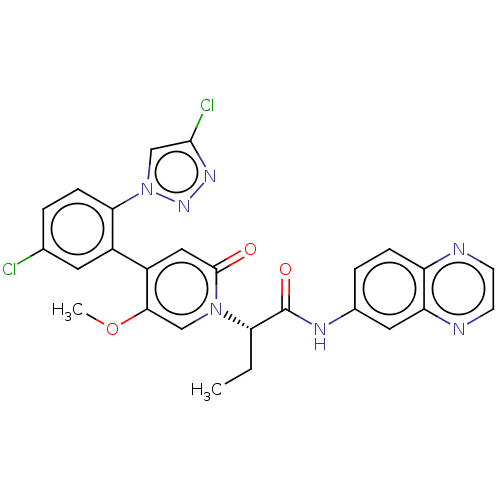 (N-(Quinoxalin-6-yl)-(2S)-2-{4-[5-chloro-2-(4-chlor...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.550 | n/a | n/a | n/a | n/a | n/a | n/a |
BAYER PHARMA AKTIENGESELLSCHAFT US Patent | Assay Description The factor XIa inhibition of the substances according to the invention is determined using a biochemical test system which utilizes the reaction of a... | US Patent US10421742 (2019) BindingDB Entry DOI: 10.7270/Q26M3967 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM413724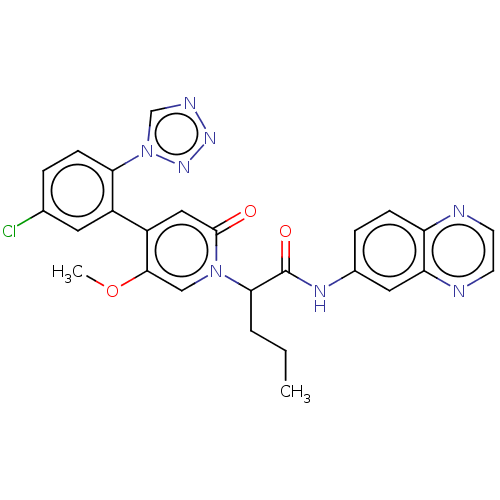 (N-(Quinoxalin-6-yl)-2-{4-[5-chloro-2-(1H-tetrazol-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.560 | n/a | n/a | n/a | n/a | n/a | n/a |
BAYER PHARMA AKTIENGESELLSCHAFT US Patent | Assay Description The factor XIa inhibition of the substances according to the invention is determined using a biochemical test system which utilizes the reaction of a... | US Patent US10421742 (2019) BindingDB Entry DOI: 10.7270/Q26M3967 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasma kallikrein (Homo sapiens (Human)) | BDBM413725 (N-(Quinoxalin-6-yl)-(2S)-2-{4-[5-chloro-2-(1H-tetr...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.570 | n/a | n/a | n/a | n/a | n/a | n/a |
BAYER PHARMA AKTIENGESELLSCHAFT US Patent | Assay Description To determine the plasma kallikrein inhibition of the substances according to the invention, a biochemical test system is used which utilizes the reac... | US Patent US10421742 (2019) BindingDB Entry DOI: 10.7270/Q26M3967 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM413746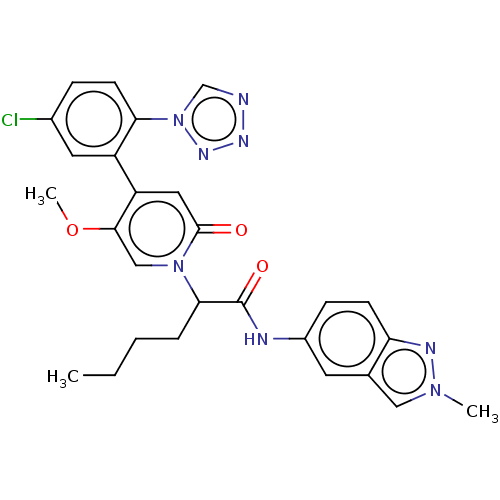 (2-{4-[5-Chloro-2-(1H-tetrazol-1-yl)phenyl]-5-metho...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.590 | n/a | n/a | n/a | n/a | n/a | n/a |
BAYER PHARMA AKTIENGESELLSCHAFT US Patent | Assay Description The factor XIa inhibition of the substances according to the invention is determined using a biochemical test system which utilizes the reaction of a... | US Patent US10421742 (2019) BindingDB Entry DOI: 10.7270/Q26M3967 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM413773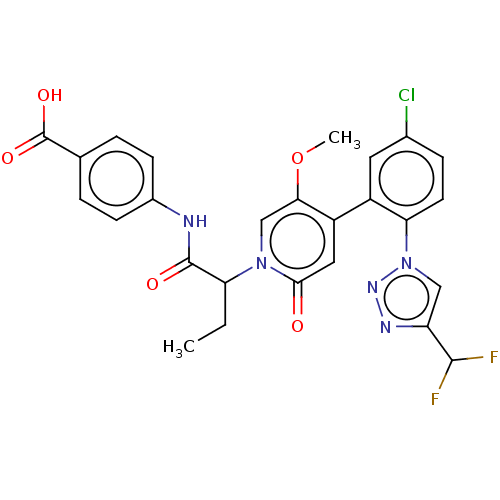 (4-({2-[4-{5-Chloro-2-[4-(difluoromethyl)-1H-1,2,3-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.620 | n/a | n/a | n/a | n/a | n/a | n/a |
BAYER PHARMA AKTIENGESELLSCHAFT US Patent | Assay Description The factor XIa inhibition of the substances according to the invention is determined using a biochemical test system which utilizes the reaction of a... | US Patent US10421742 (2019) BindingDB Entry DOI: 10.7270/Q26M3967 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM413847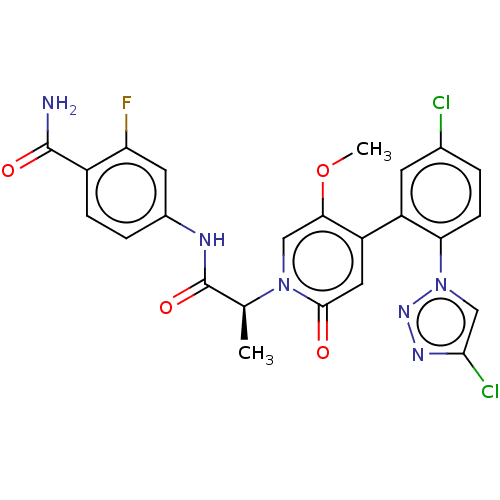 (4-{[(2S)-2-{4-[5-Chloro-2-(4-chloro-1H-1,2,3-triaz...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.640 | n/a | n/a | n/a | n/a | n/a | n/a |
BAYER PHARMA AKTIENGESELLSCHAFT US Patent | Assay Description The factor XIa inhibition of the substances according to the invention is determined using a biochemical test system which utilizes the reaction of a... | US Patent US10421742 (2019) BindingDB Entry DOI: 10.7270/Q26M3967 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM413797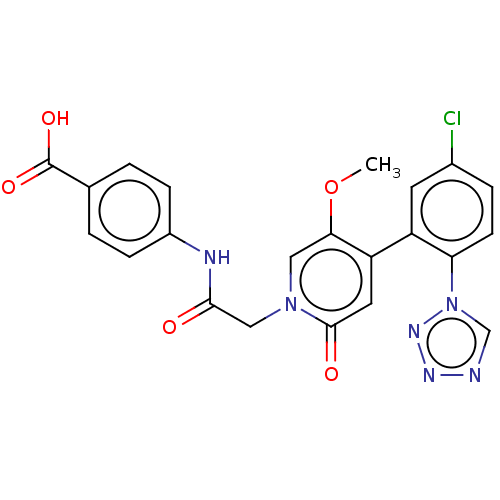 (4-[({4-[5-Chloro-2-(1H-tetrazol-1-yl)phenyl]-5-met...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.650 | n/a | n/a | n/a | n/a | n/a | n/a |
BAYER PHARMA AKTIENGESELLSCHAFT US Patent | Assay Description The factor XIa inhibition of the substances according to the invention is determined using a biochemical test system which utilizes the reaction of a... | US Patent US10421742 (2019) BindingDB Entry DOI: 10.7270/Q26M3967 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM413851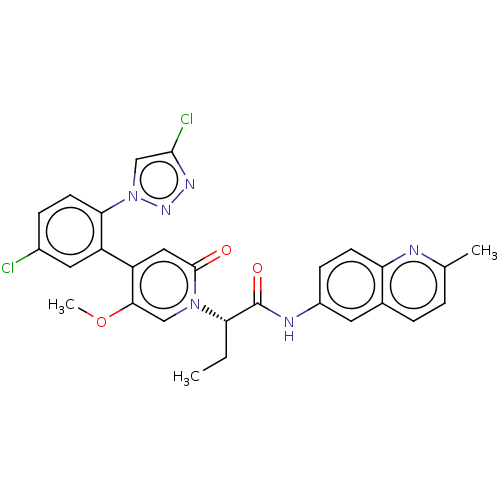 ((2S)-2-{4-[5-Chloro-2-(4-chloro-1H-1,2,3-triazol-1...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.650 | n/a | n/a | n/a | n/a | n/a | n/a |
BAYER PHARMA AKTIENGESELLSCHAFT US Patent | Assay Description The factor XIa inhibition of the substances according to the invention is determined using a biochemical test system which utilizes the reaction of a... | US Patent US10421742 (2019) BindingDB Entry DOI: 10.7270/Q26M3967 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM413793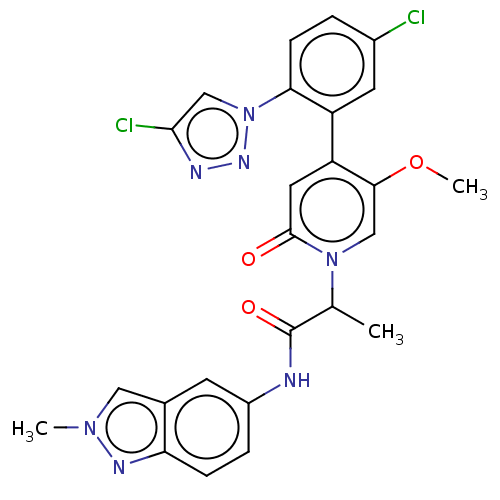 (2-{4-[5-Chloro-2-(4-chloro-1H-1,2,3-triazol-1-yl)p...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.650 | n/a | n/a | n/a | n/a | n/a | n/a |
BAYER PHARMA AKTIENGESELLSCHAFT US Patent | Assay Description The factor XIa inhibition of the substances according to the invention is determined using a biochemical test system which utilizes the reaction of a... | US Patent US10421742 (2019) BindingDB Entry DOI: 10.7270/Q26M3967 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM413723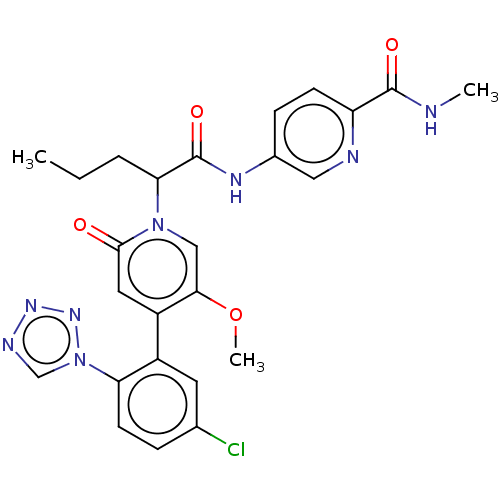 (5-{[2-{4-[5-Chloro-2-(1H-tetrazol-1-yl)phenyl]-5-m...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.660 | n/a | n/a | n/a | n/a | n/a | n/a |
BAYER PHARMA AKTIENGESELLSCHAFT US Patent | Assay Description The factor XIa inhibition of the substances according to the invention is determined using a biochemical test system which utilizes the reaction of a... | US Patent US10421742 (2019) BindingDB Entry DOI: 10.7270/Q26M3967 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM413633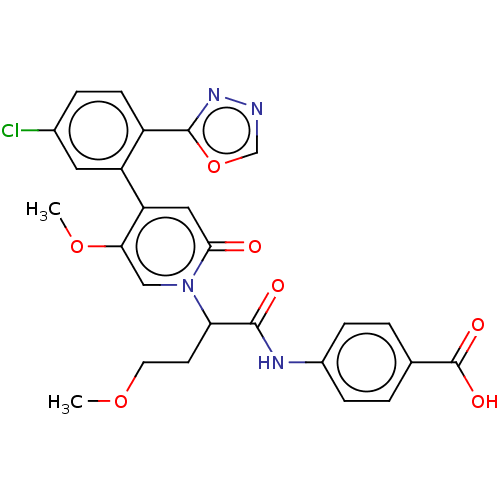 (4-[(2-{4-[5-Chloro-2-(1,3,4-oxadiazol-2-yl)phenyl]...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
BAYER PHARMA AKTIENGESELLSCHAFT US Patent | Assay Description The factor XIa inhibition of the substances according to the invention is determined using a biochemical test system which utilizes the reaction of a... | US Patent US10421742 (2019) BindingDB Entry DOI: 10.7270/Q26M3967 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM413770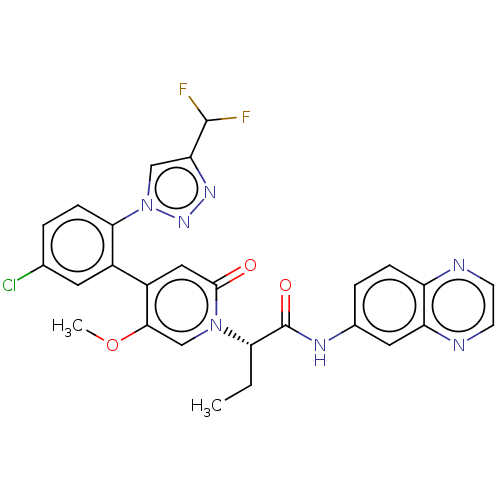 (N-(Quinoxalin-6-yl)-(2S)-2-[4-{5-chloro-2-[4-(difl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
BAYER PHARMA AKTIENGESELLSCHAFT US Patent | Assay Description The factor XIa inhibition of the substances according to the invention is determined using a biochemical test system which utilizes the reaction of a... | US Patent US10421742 (2019) BindingDB Entry DOI: 10.7270/Q26M3967 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM413749 (4-({2-[4-{5-Chloro-2-[4-(difluoromethyl)-1H-1,2,3-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.710 | n/a | n/a | n/a | n/a | n/a | n/a |
BAYER PHARMA AKTIENGESELLSCHAFT US Patent | Assay Description The factor XIa inhibition of the substances according to the invention is determined using a biochemical test system which utilizes the reaction of a... | US Patent US10421742 (2019) BindingDB Entry DOI: 10.7270/Q26M3967 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM413748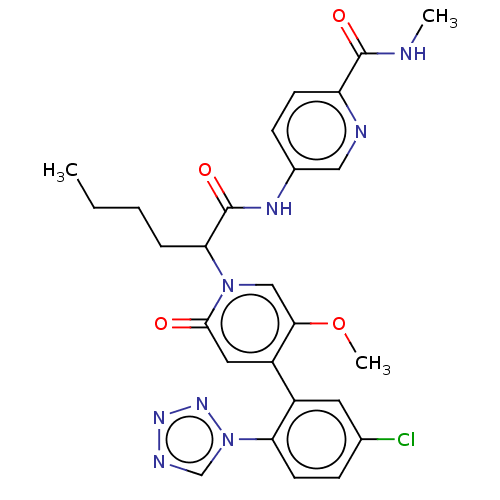 (5-{[2-{4-[5-Chloro-2-(1H-tetrazol-1-yl)phenyl]-5-m...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.710 | n/a | n/a | n/a | n/a | n/a | n/a |
BAYER PHARMA AKTIENGESELLSCHAFT US Patent | Assay Description The factor XIa inhibition of the substances according to the invention is determined using a biochemical test system which utilizes the reaction of a... | US Patent US10421742 (2019) BindingDB Entry DOI: 10.7270/Q26M3967 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM413743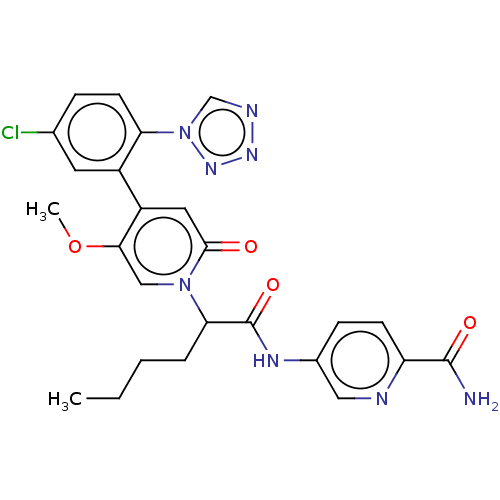 (5-{[2-{4-[5-Chloro-2-(1H-tetrazol-1-yl)phenyl]-5-m...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.720 | n/a | n/a | n/a | n/a | n/a | n/a |
BAYER PHARMA AKTIENGESELLSCHAFT US Patent | Assay Description The factor XIa inhibition of the substances according to the invention is determined using a biochemical test system which utilizes the reaction of a... | US Patent US10421742 (2019) BindingDB Entry DOI: 10.7270/Q26M3967 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM413796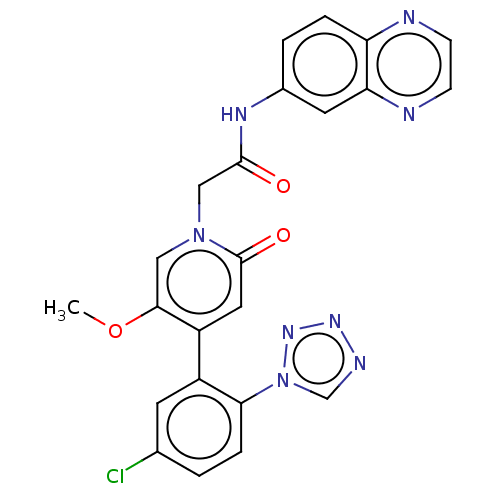 (N-(Quinoxalin-6-yl)-2-{4-[5-chloro-2-(1H-tetrazol-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.730 | n/a | n/a | n/a | n/a | n/a | n/a |
BAYER PHARMA AKTIENGESELLSCHAFT US Patent | Assay Description The factor XIa inhibition of the substances according to the invention is determined using a biochemical test system which utilizes the reaction of a... | US Patent US10421742 (2019) BindingDB Entry DOI: 10.7270/Q26M3967 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM413858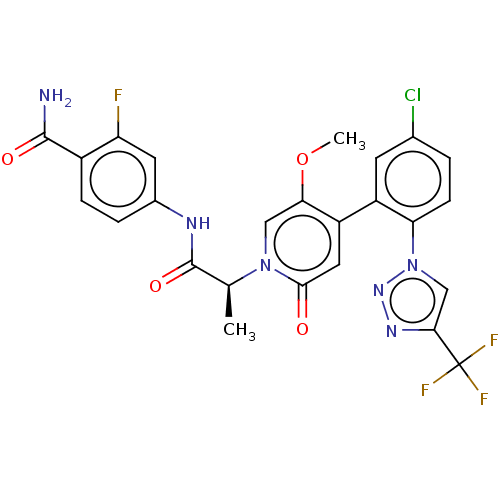 (4-({(2S)-2-[4-{5-Chloro-2-[4-(trifluoromethyl)-1H-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.740 | n/a | n/a | n/a | n/a | n/a | n/a |
BAYER PHARMA AKTIENGESELLSCHAFT US Patent | Assay Description The factor XIa inhibition of the substances according to the invention is determined using a biochemical test system which utilizes the reaction of a... | US Patent US10421742 (2019) BindingDB Entry DOI: 10.7270/Q26M3967 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM413792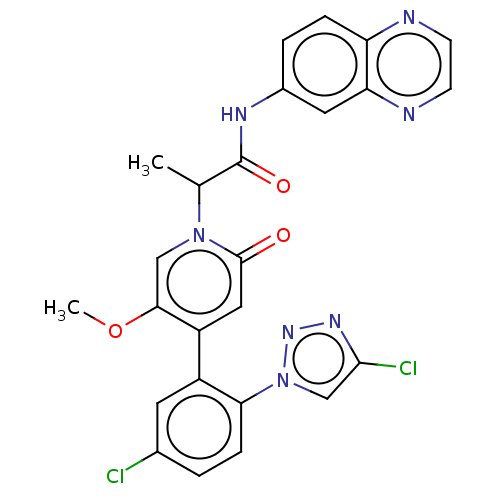 (N-(Quinoxalin-6-yl)-2-{4-[5-chloro-2-(4-chloro-1H-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.75 | n/a | n/a | n/a | n/a | n/a | n/a |
BAYER PHARMA AKTIENGESELLSCHAFT US Patent | Assay Description The factor XIa inhibition of the substances according to the invention is determined using a biochemical test system which utilizes the reaction of a... | US Patent US10421742 (2019) BindingDB Entry DOI: 10.7270/Q26M3967 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM413747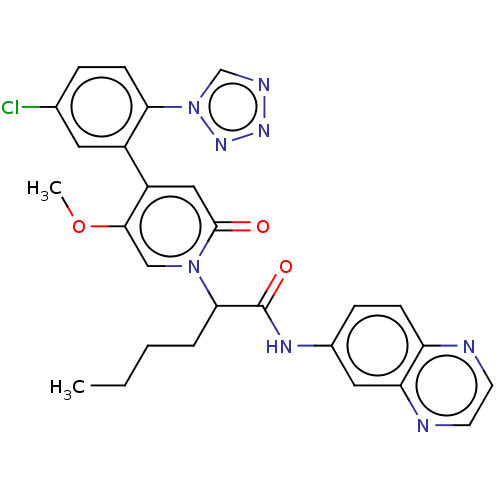 (N-(Quinoxalin-6-yl)-2-{4-[5-chloro-2-(1H-tetrazol-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.760 | n/a | n/a | n/a | n/a | n/a | n/a |
BAYER PHARMA AKTIENGESELLSCHAFT US Patent | Assay Description The factor XIa inhibition of the substances according to the invention is determined using a biochemical test system which utilizes the reaction of a... | US Patent US10421742 (2019) BindingDB Entry DOI: 10.7270/Q26M3967 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM413775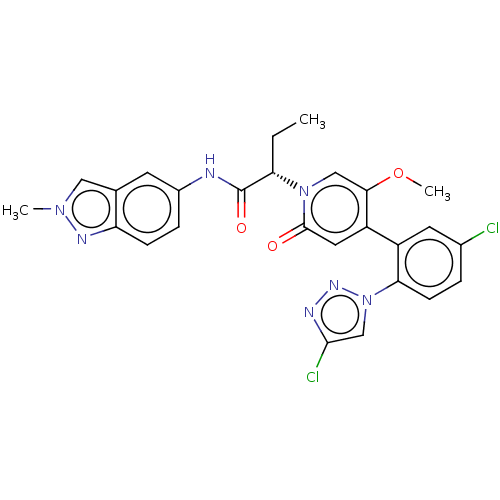 ((2S)-2-{4-[5-Chloro-2-(4-chloro-1H-1,2,3-triazol-1...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.780 | n/a | n/a | n/a | n/a | n/a | n/a |
BAYER PHARMA AKTIENGESELLSCHAFT US Patent | Assay Description The factor XIa inhibition of the substances according to the invention is determined using a biochemical test system which utilizes the reaction of a... | US Patent US10421742 (2019) BindingDB Entry DOI: 10.7270/Q26M3967 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasma kallikrein (Homo sapiens (Human)) | BDBM413727 ((2S)-2-{4-[5-Chloro-2-(1H-tetrazol-1-yl)phenyl]-5-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.780 | n/a | n/a | n/a | n/a | n/a | n/a |
BAYER PHARMA AKTIENGESELLSCHAFT US Patent | Assay Description To determine the plasma kallikrein inhibition of the substances according to the invention, a biochemical test system is used which utilizes the reac... | US Patent US10421742 (2019) BindingDB Entry DOI: 10.7270/Q26M3967 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM413817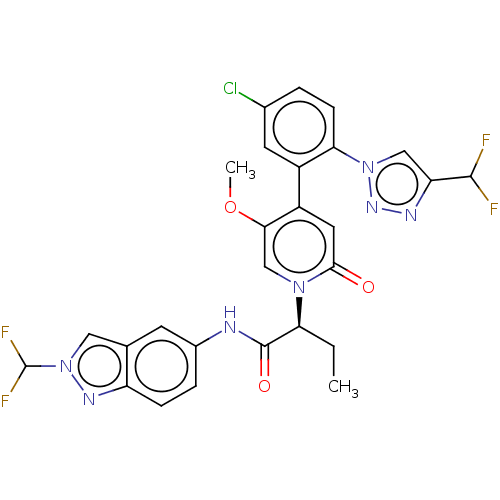 ((2S)-2-[4-{5-Chloro-2-[4-(difluoromethyl)-1H-1,2,3...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.780 | n/a | n/a | n/a | n/a | n/a | n/a |
BAYER PHARMA AKTIENGESELLSCHAFT US Patent | Assay Description The factor XIa inhibition of the substances according to the invention is determined using a biochemical test system which utilizes the reaction of a... | US Patent US10421742 (2019) BindingDB Entry DOI: 10.7270/Q26M3967 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM413840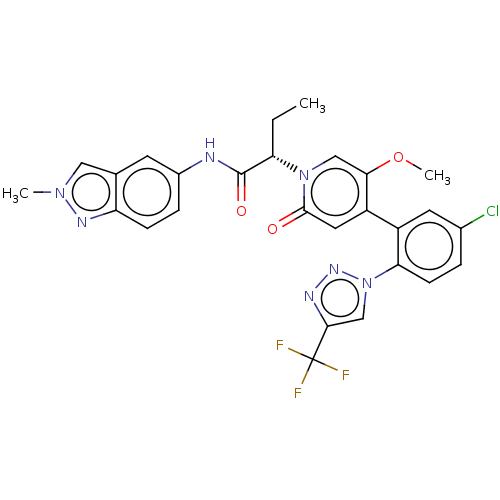 ((2S)-2-[4-{5-Chloro-2-[4-(trifluoromethyl)-1H-1,2,...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.790 | n/a | n/a | n/a | n/a | n/a | n/a |
BAYER PHARMA AKTIENGESELLSCHAFT US Patent | Assay Description The factor XIa inhibition of the substances according to the invention is determined using a biochemical test system which utilizes the reaction of a... | US Patent US10421742 (2019) BindingDB Entry DOI: 10.7270/Q26M3967 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM413861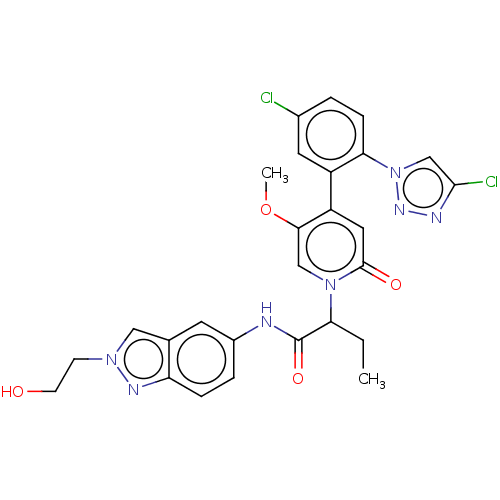 (2-{4-[5-Chloro-2-(4-chloro-1H-1,2,3-triazol-1-yl)p...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.810 | n/a | n/a | n/a | n/a | n/a | n/a |
BAYER PHARMA AKTIENGESELLSCHAFT US Patent | Assay Description The factor XIa inhibition of the substances according to the invention is determined using a biochemical test system which utilizes the reaction of a... | US Patent US10421742 (2019) BindingDB Entry DOI: 10.7270/Q26M3967 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM413779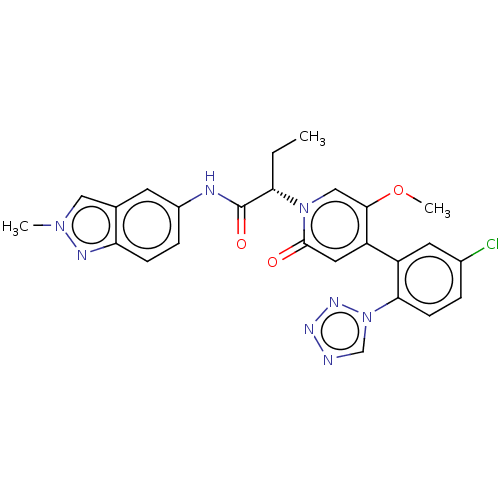 (2-{4-[5-Chloro-2-(1H-tetrazol-1-yl)phenyl]-5-metho...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.820 | n/a | n/a | n/a | n/a | n/a | n/a |
BAYER PHARMA AKTIENGESELLSCHAFT US Patent | Assay Description The factor XIa inhibition of the substances according to the invention is determined using a biochemical test system which utilizes the reaction of a... | US Patent US10421742 (2019) BindingDB Entry DOI: 10.7270/Q26M3967 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM413641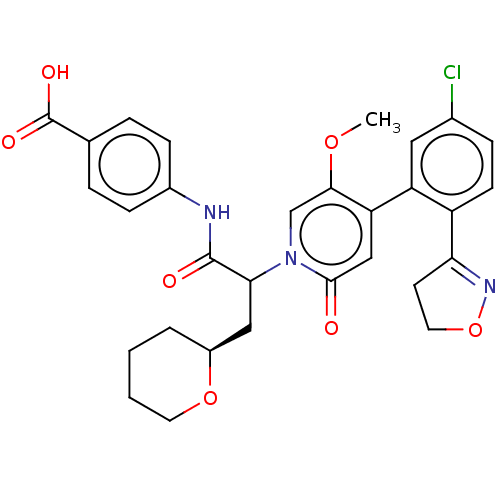 (4-[(2-{4-[5-Chloro-2-(4,5-dihydro-1,2-oxazol-3-yl)...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.830 | n/a | n/a | n/a | n/a | n/a | n/a |
BAYER PHARMA AKTIENGESELLSCHAFT US Patent | Assay Description The factor XIa inhibition of the substances according to the invention is determined using a biochemical test system which utilizes the reaction of a... | US Patent US10421742 (2019) BindingDB Entry DOI: 10.7270/Q26M3967 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM413737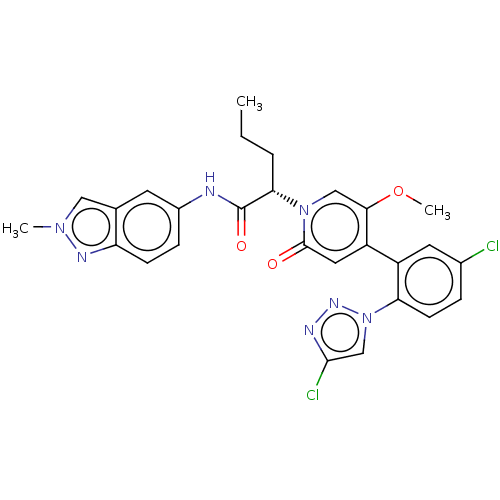 ((2S)-2-{4-[5-Chloro-2-(4-chloro-1H-1,2,3-triazol-1...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.840 | n/a | n/a | n/a | n/a | n/a | n/a |
BAYER PHARMA AKTIENGESELLSCHAFT US Patent | Assay Description The factor XIa inhibition of the substances according to the invention is determined using a biochemical test system which utilizes the reaction of a... | US Patent US10421742 (2019) BindingDB Entry DOI: 10.7270/Q26M3967 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM413744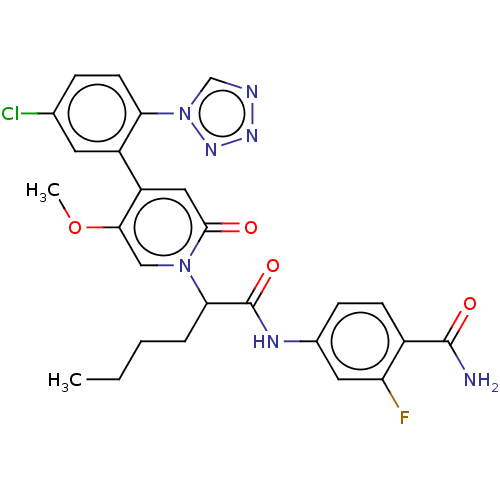 (4-{[2-{4-[5-Chloro-2-(1H-tetrazol-1-yl)phenyl]-5-m...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.840 | n/a | n/a | n/a | n/a | n/a | n/a |
BAYER PHARMA AKTIENGESELLSCHAFT US Patent | Assay Description The factor XIa inhibition of the substances according to the invention is determined using a biochemical test system which utilizes the reaction of a... | US Patent US10421742 (2019) BindingDB Entry DOI: 10.7270/Q26M3967 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM413864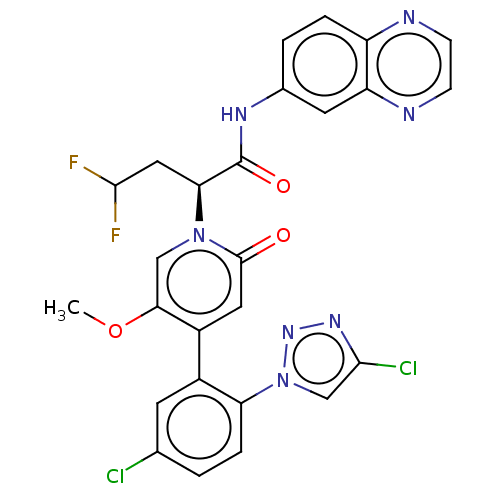 ((2S)-2-{4-[5-Chloro-2-(4-chloro-1H-1,2,3-triazol-1...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.850 | n/a | n/a | n/a | n/a | n/a | n/a |
BAYER PHARMA AKTIENGESELLSCHAFT US Patent | Assay Description The factor XIa inhibition of the substances according to the invention is determined using a biochemical test system which utilizes the reaction of a... | US Patent US10421742 (2019) BindingDB Entry DOI: 10.7270/Q26M3967 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM413772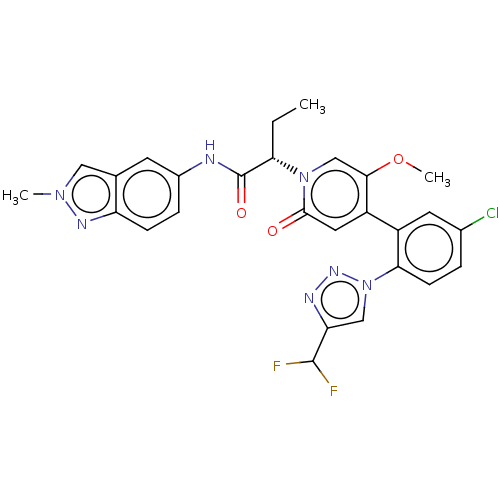 ((2S)-2-[4-{5-Chloro-2-[4-(difluoromethyl)-1H-1,2,3...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.860 | n/a | n/a | n/a | n/a | n/a | n/a |
BAYER PHARMA AKTIENGESELLSCHAFT US Patent | Assay Description The factor XIa inhibition of the substances according to the invention is determined using a biochemical test system which utilizes the reaction of a... | US Patent US10421742 (2019) BindingDB Entry DOI: 10.7270/Q26M3967 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM413850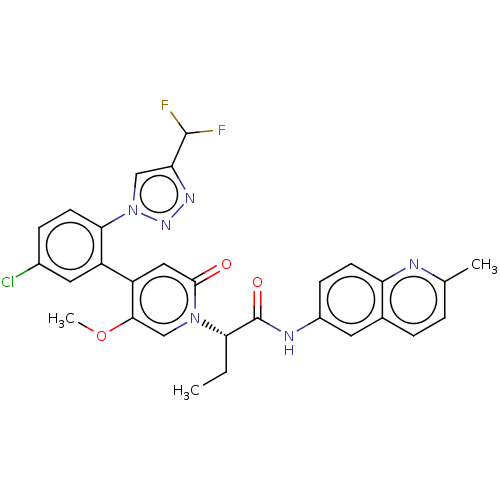 ((2S)-2-[4-{5-Chloro-2-[4-(difluoromethyl)-1H-1,2,3...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.870 | n/a | n/a | n/a | n/a | n/a | n/a |
BAYER PHARMA AKTIENGESELLSCHAFT US Patent | Assay Description The factor XIa inhibition of the substances according to the invention is determined using a biochemical test system which utilizes the reaction of a... | US Patent US10421742 (2019) BindingDB Entry DOI: 10.7270/Q26M3967 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM413844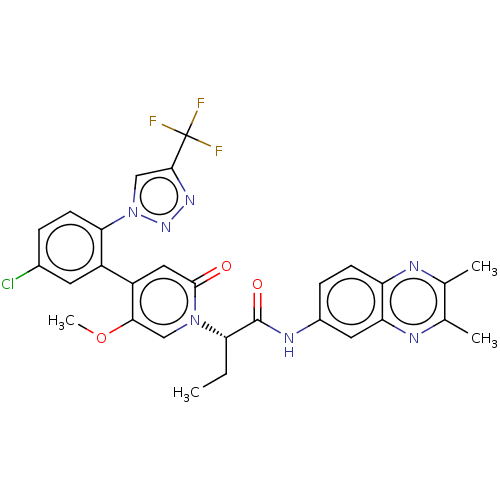 ((2S)-2-[4-{5-Chloro-2-[4-(trifluoromethyl)-1H-1,2,...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.890 | n/a | n/a | n/a | n/a | n/a | n/a |
BAYER PHARMA AKTIENGESELLSCHAFT US Patent | Assay Description The factor XIa inhibition of the substances according to the invention is determined using a biochemical test system which utilizes the reaction of a... | US Patent US10421742 (2019) BindingDB Entry DOI: 10.7270/Q26M3967 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM413846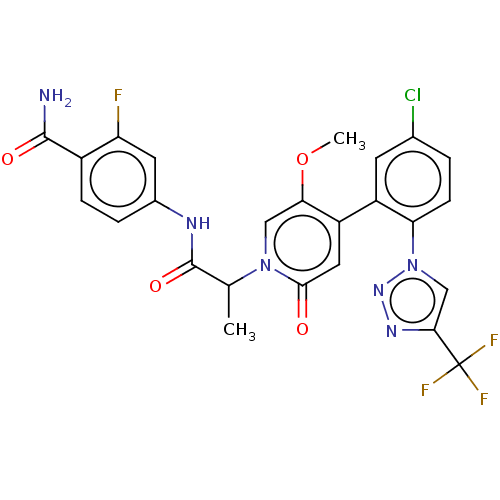 (4-({2-[4-{5-Chloro-2-[4-(trifluoromethyl)-1H-1,2,3...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.890 | n/a | n/a | n/a | n/a | n/a | n/a |
BAYER PHARMA AKTIENGESELLSCHAFT US Patent | Assay Description The factor XIa inhibition of the substances according to the invention is determined using a biochemical test system which utilizes the reaction of a... | US Patent US10421742 (2019) BindingDB Entry DOI: 10.7270/Q26M3967 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM413849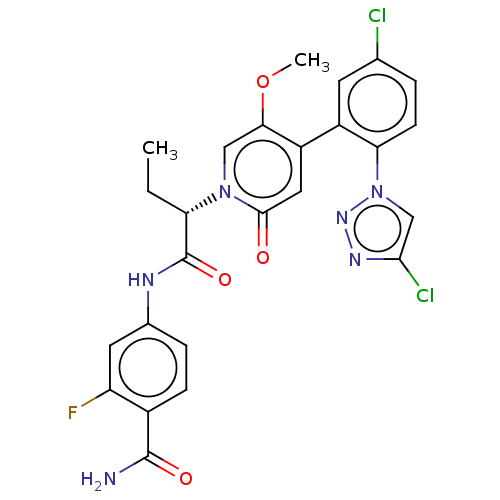 (4-{[(2S)-2-{4-[5-Chloro-2-(4-chloro-1H-1,2,3-triaz...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.890 | n/a | n/a | n/a | n/a | n/a | n/a |
BAYER PHARMA AKTIENGESELLSCHAFT US Patent | Assay Description The factor XIa inhibition of the substances according to the invention is determined using a biochemical test system which utilizes the reaction of a... | US Patent US10421742 (2019) BindingDB Entry DOI: 10.7270/Q26M3967 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM413842 (4-({(2S)-2-[4-{5-Chloro-2-[4-(trifluoromethyl)-1H-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | US Patent | n/a | n/a | 0.920 | n/a | n/a | n/a | n/a | n/a | n/a |
BAYER PHARMA AKTIENGESELLSCHAFT US Patent | Assay Description The factor XIa inhibition of the substances according to the invention is determined using a biochemical test system which utilizes the reaction of a... | US Patent US10421742 (2019) BindingDB Entry DOI: 10.7270/Q26M3967 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM413696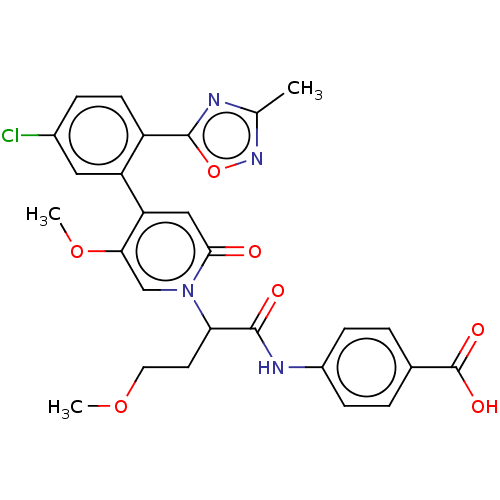 (4-[(2-{4-[5-Chloro-2-(3-methyl-1,2,4-oxadiazol-5-y...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.920 | n/a | n/a | n/a | n/a | n/a | n/a |
BAYER PHARMA AKTIENGESELLSCHAFT US Patent | Assay Description The factor XIa inhibition of the substances according to the invention is determined using a biochemical test system which utilizes the reaction of a... | US Patent US10421742 (2019) BindingDB Entry DOI: 10.7270/Q26M3967 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 1127 total ) | Next | Last >> |