 Found 466 hits with Last Name = 'stoy' and Initial = 'p'
Found 466 hits with Last Name = 'stoy' and Initial = 'p' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Serine/threonine-protein kinase TNNI3K
(Homo sapiens (Human)) | BDBM50578225
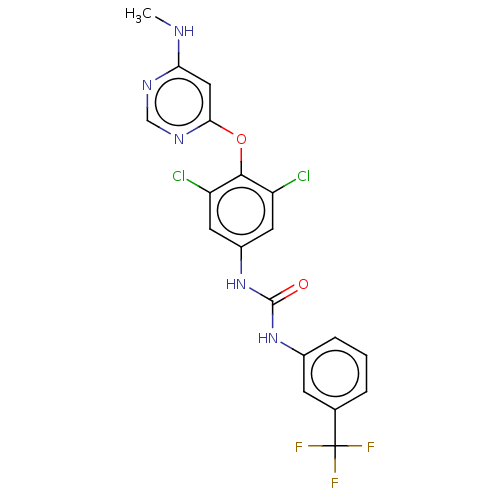
(CHEMBL4869303)Show SMILES CNc1cc(Oc2c(Cl)cc(NC(=O)Nc3cccc(c3)C(F)(F)F)cc2Cl)ncn1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| 0.540 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Binding affinity to full length human His-MBP-TNNI3K assessed as off-rate constant in presence of rhodamine green labeled GW805818X by fluorescence c... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00700
BindingDB Entry DOI: 10.7270/Q23X8BG9 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Serine/threonine-protein kinase TNNI3K
(Mus musculus) | BDBM50578225

(CHEMBL4869303)Show SMILES CNc1cc(Oc2c(Cl)cc(NC(=O)Nc3cccc(c3)C(F)(F)F)cc2Cl)ncn1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Binding affinity to full length mouse His-MBP-TNNI3K assessed as off-rate constant in presence of rhodamine green labeled GW805818X by fluorescence c... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00700
BindingDB Entry DOI: 10.7270/Q23X8BG9 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50141580
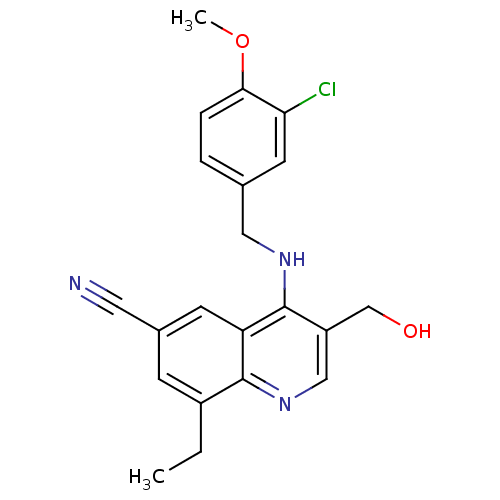
(4-(3-Chloro-4-methoxy-benzylamino)-8-ethyl-3-hydro...)Show SMILES CCc1cc(cc2c(NCc3ccc(OC)c(Cl)c3)c(CO)cnc12)C#N Show InChI InChI=1S/C21H20ClN3O2/c1-3-15-6-14(9-23)7-17-20(15)25-11-16(12-26)21(17)24-10-13-4-5-19(27-2)18(22)8-13/h4-8,11,26H,3,10,12H2,1-2H3,(H,24,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.0500 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of phosphodiesterase 5 from human platelets |
Bioorg Med Chem Lett 14: 1577-80 (2004)
Article DOI: 10.1016/j.bmcl.2003.12.090
BindingDB Entry DOI: 10.7270/Q2G1607Q |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50104208
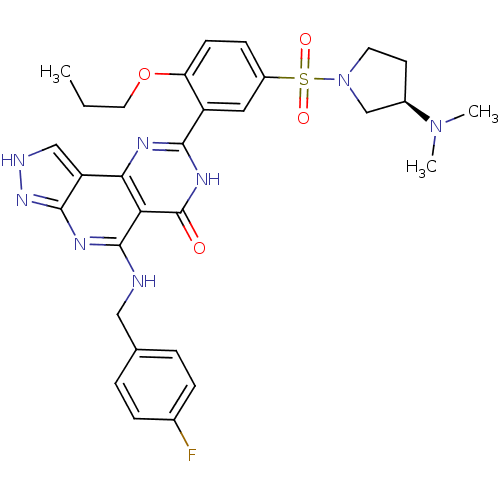
(8-[5-((R)-3-Dimethylamino-pyrrolidine-1-sulfonyl)-...)Show SMILES CCCOc1ccc(cc1-c1nc2c3c[nH]nc3nc(NCc3ccc(F)cc3)c2c(=O)[nH]1)S(=O)(=O)N1CC[C@H](C1)N(C)C Show InChI InChI=1S/C30H33FN8O4S/c1-4-13-43-24-10-9-21(44(41,42)39-12-11-20(17-39)38(2)3)14-22(24)27-34-26-23-16-33-37-28(23)35-29(25(26)30(40)36-27)32-15-18-5-7-19(31)8-6-18/h5-10,14,16,20H,4,11-13,15,17H2,1-3H3,(H,34,36,40)(H2,32,33,35,37)/t20-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 0.310 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of PDE5 from human platelets |
Bioorg Med Chem Lett 11: 2461-4 (2001)
BindingDB Entry DOI: 10.7270/Q2BK1BM5 |
More data for this
Ligand-Target Pair | |
Vascular endothelial growth factor receptor 2
(Homo sapiens (Human)) | BDBM50578208
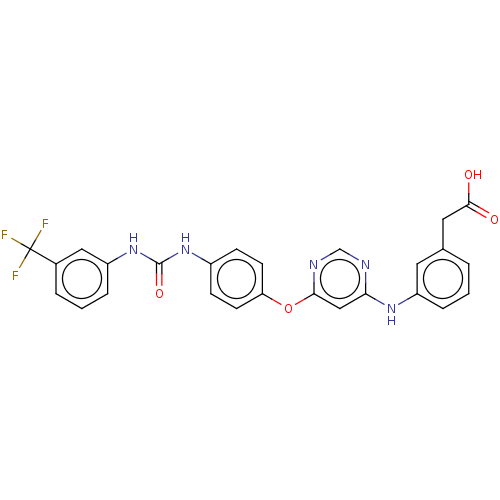
(CHEMBL4875220)Show SMILES OC(=O)Cc1cccc(Nc2cc(Oc3ccc(NC(=O)Nc4cccc(c4)C(F)(F)F)cc3)ncn2)c1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of GST-6His tagged VEGFR2 (unknown origin) using biotin-aminohexyl-EEEEYFELVAKKKK-NH2 peptide substrate incubated for 90 mins by HTRF assa... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00700
BindingDB Entry DOI: 10.7270/Q23X8BG9 |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50141584
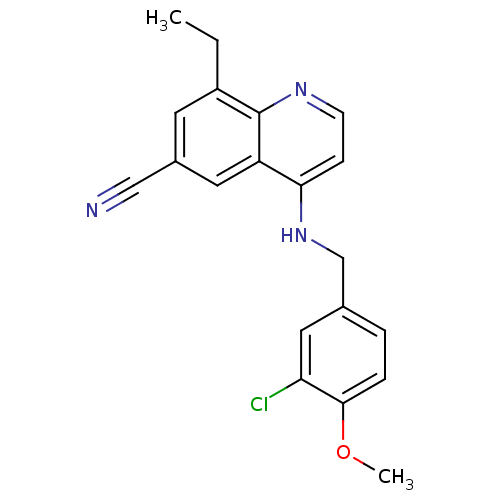
(4-(3-Chloro-4-methoxy-benzylamino)-8-ethyl-quinoli...)Show InChI InChI=1S/C20H18ClN3O/c1-3-15-8-14(11-22)9-16-18(6-7-23-20(15)16)24-12-13-4-5-19(25-2)17(21)10-13/h4-10H,3,12H2,1-2H3,(H,23,24) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of phosphodiesterase 5 from human platelets |
Bioorg Med Chem Lett 14: 1577-80 (2004)
Article DOI: 10.1016/j.bmcl.2003.12.090
BindingDB Entry DOI: 10.7270/Q2G1607Q |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50141588
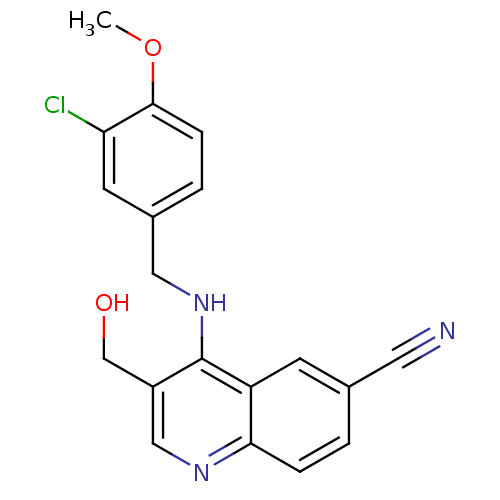
(4-(3-Chloro-4-methoxy-benzylamino)-3-hydroxymethyl...)Show InChI InChI=1S/C19H16ClN3O2/c1-25-18-5-3-13(7-16(18)20)9-23-19-14(11-24)10-22-17-4-2-12(8-21)6-15(17)19/h2-7,10,24H,9,11H2,1H3,(H,22,23) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.480 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of phosphodiesterase 5 from human platelets |
Bioorg Med Chem Lett 14: 1577-80 (2004)
Article DOI: 10.1016/j.bmcl.2003.12.090
BindingDB Entry DOI: 10.7270/Q2G1607Q |
More data for this
Ligand-Target Pair | |
Transient receptor potential cation channel subfamily V member 4
(Homo sapiens (Human)) | BDBM50502628

(CHEMBL4469630)Show SMILES CC(C)(C)CN1C[C@@]2(CCC[C@](C)(Cn3cnc4ccc(cc34)C#N)C2)OC1=O |r| Show InChI InChI=1S/C23H30N4O2/c1-21(2,3)13-26-15-23(29-20(26)28)9-5-8-22(4,12-23)14-27-16-25-18-7-6-17(11-24)10-19(18)27/h6-7,10,16H,5,8-9,12-15H2,1-4H3/t22-,23-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human TRPV4 expressed in baculovirus infected HEK/MSR 2 cells pre-incubated for 10 mins followed by GSK634775A addition by Fura-2 dye b... |
ACS Med Chem Lett 10: 1228-1233 (2019)
Article DOI: 10.1021/acsmedchemlett.9b00274
BindingDB Entry DOI: 10.7270/Q2RX9GBF |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50141586
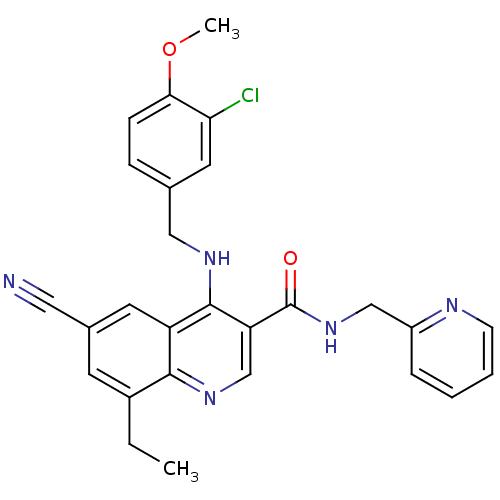
(4-(3-Chloro-4-methoxy-benzylamino)-6-cyano-8-ethyl...)Show SMILES CCc1cc(cc2c(NCc3ccc(OC)c(Cl)c3)c(cnc12)C(=O)NCc1ccccn1)C#N Show InChI InChI=1S/C27H24ClN5O2/c1-3-19-10-18(13-29)11-21-25(19)32-16-22(27(34)33-15-20-6-4-5-9-30-20)26(21)31-14-17-7-8-24(35-2)23(28)12-17/h4-12,16H,3,14-15H2,1-2H3,(H,31,32)(H,33,34) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.550 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of phosphodiesterase 5 from human platelets |
Bioorg Med Chem Lett 14: 1577-80 (2004)
Article DOI: 10.1016/j.bmcl.2003.12.090
BindingDB Entry DOI: 10.7270/Q2G1607Q |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50141583
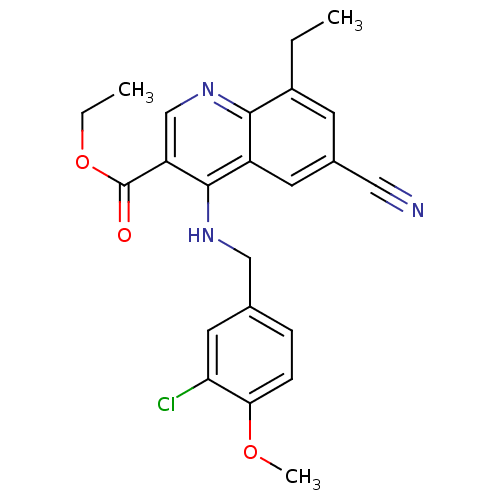
(4-(3-Chloro-4-methoxy-benzylamino)-6-cyano-8-ethyl...)Show SMILES CCOC(=O)c1cnc2c(CC)cc(cc2c1NCc1ccc(OC)c(Cl)c1)C#N Show InChI InChI=1S/C23H22ClN3O3/c1-4-16-8-15(11-25)9-17-21(16)27-13-18(23(28)30-5-2)22(17)26-12-14-6-7-20(29-3)19(24)10-14/h6-10,13H,4-5,12H2,1-3H3,(H,26,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of phosphodiesterase 5 from human platelets |
Bioorg Med Chem Lett 14: 1577-80 (2004)
Article DOI: 10.1016/j.bmcl.2003.12.090
BindingDB Entry DOI: 10.7270/Q2G1607Q |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50104206
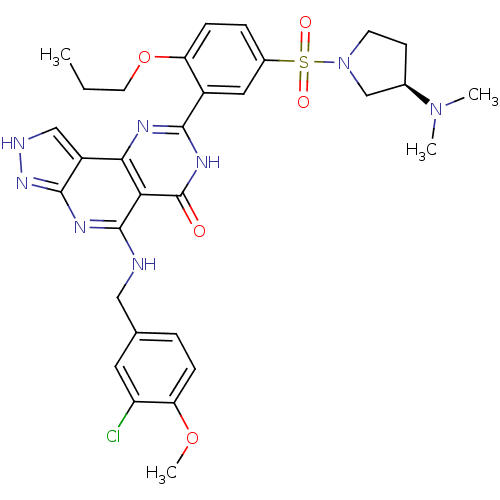
(5-(3-Chloro-4-methoxy-benzylamino)-8-[5-((R)-3-dim...)Show SMILES CCCOc1ccc(cc1-c1nc2c3c[nH]nc3nc(NCc3ccc(OC)c(Cl)c3)c2c(=O)[nH]1)S(=O)(=O)N1CC[C@H](C1)N(C)C Show InChI InChI=1S/C31H35ClN8O5S/c1-5-12-45-24-9-7-20(46(42,43)40-11-10-19(17-40)39(2)3)14-21(24)28-35-27-22-16-34-38-29(22)36-30(26(27)31(41)37-28)33-15-18-6-8-25(44-4)23(32)13-18/h6-9,13-14,16,19H,5,10-12,15,17H2,1-4H3,(H,35,37,41)(H2,33,34,36,38)/t19-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of PDE5 from human platelets |
Bioorg Med Chem Lett 11: 2461-4 (2001)
BindingDB Entry DOI: 10.7270/Q2BK1BM5 |
More data for this
Ligand-Target Pair | |
Transient receptor potential cation channel subfamily V member 4
(Homo sapiens (Human)) | BDBM50450513
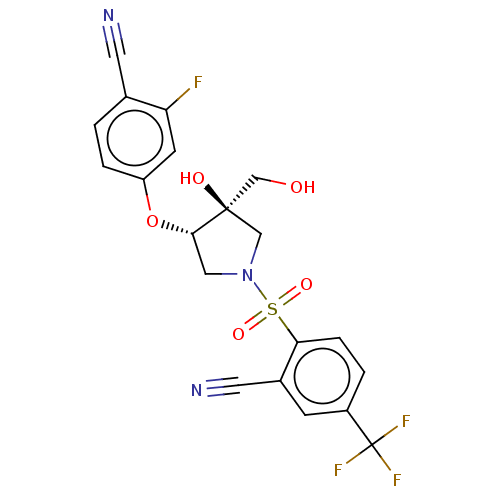
(CHEMBL4168219)Show SMILES OC[C@]1(O)CN(C[C@@H]1Oc1ccc(C#N)c(F)c1)S(=O)(=O)c1ccc(cc1C#N)C(F)(F)F |r| Show InChI InChI=1S/C20H15F4N3O5S/c21-16-6-15(3-1-12(16)7-25)32-18-9-27(10-19(18,29)11-28)33(30,31)17-4-2-14(20(22,23)24)5-13(17)8-26/h1-6,18,28-29H,9-11H2/t18-,19+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Antagonist activity at human TRPV4 expressed in BHK/AC9 or HEK MSR2 cells assessed as inhibition of PF-04674114-induced Ca2+ flux pre-incubated for 1... |
J Med Chem 61: 9738-9755 (2018)
Article DOI: 10.1021/acs.jmedchem.8b01317
BindingDB Entry DOI: 10.7270/Q2CJ8H2P |
More data for this
Ligand-Target Pair | |
Transient receptor potential cation channel subfamily V member 4
(Homo sapiens (Human)) | BDBM50502648
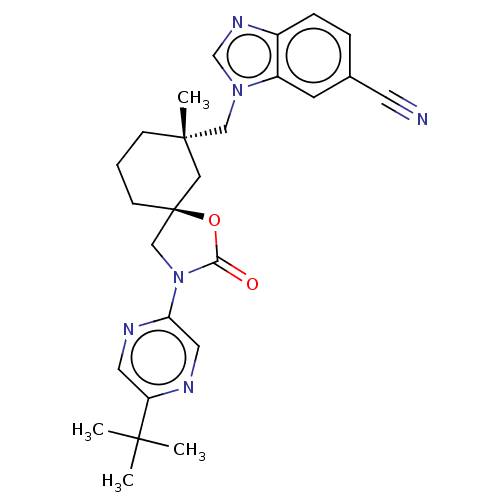
(CHEMBL4588831)Show SMILES CC(C)(C)c1cnc(cn1)N1C[C@@]2(CCC[C@](C)(Cn3cnc4ccc(cc34)C#N)C2)OC1=O |r| Show InChI InChI=1S/C26H30N6O2/c1-24(2,3)21-12-29-22(13-28-21)32-16-26(34-23(32)33)9-5-8-25(4,14-26)15-31-17-30-19-7-6-18(11-27)10-20(19)31/h6-7,10,12-13,17H,5,8-9,14-16H2,1-4H3/t25-,26-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human TRPV4 expressed in baculovirus infected HEK/MSR 2 cells pre-incubated for 10 mins followed by GSK634775A addition by Fura-2 dye b... |
ACS Med Chem Lett 10: 1228-1233 (2019)
Article DOI: 10.1021/acsmedchemlett.9b00274
BindingDB Entry DOI: 10.7270/Q2RX9GBF |
More data for this
Ligand-Target Pair | |
Transient receptor potential cation channel subfamily V member 4
(Homo sapiens (Human)) | BDBM50502635
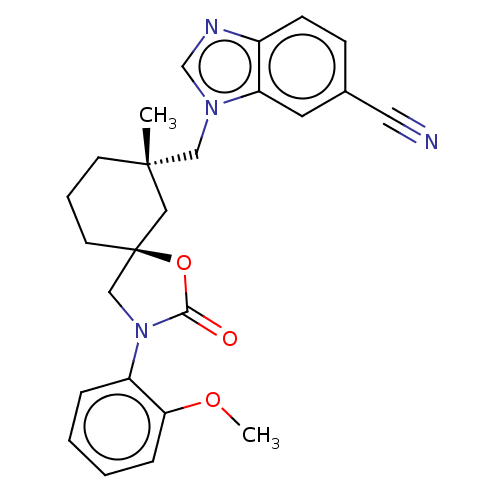
(CHEMBL4521512)Show SMILES COc1ccccc1N1C[C@@]2(CCC[C@](C)(Cn3cnc4ccc(cc34)C#N)C2)OC1=O |r| Show InChI InChI=1S/C25H26N4O3/c1-24(15-28-17-27-19-9-8-18(13-26)12-21(19)28)10-5-11-25(14-24)16-29(23(30)32-25)20-6-3-4-7-22(20)31-2/h3-4,6-9,12,17H,5,10-11,14-16H2,1-2H3/t24-,25-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human TRPV4 expressed in baculovirus infected HEK/MSR 2 cells pre-incubated for 10 mins followed by GSK634775A addition by Fura-2 dye b... |
ACS Med Chem Lett 10: 1228-1233 (2019)
Article DOI: 10.1021/acsmedchemlett.9b00274
BindingDB Entry DOI: 10.7270/Q2RX9GBF |
More data for this
Ligand-Target Pair | |
Vascular endothelial growth factor receptor 2
(Homo sapiens (Human)) | BDBM50578207
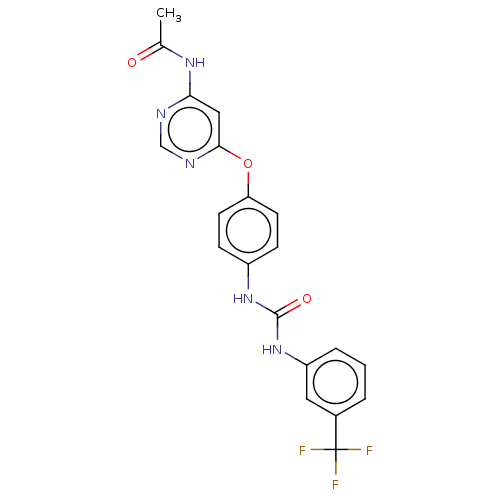
(CHEMBL4845883)Show SMILES CC(=O)Nc1cc(Oc2ccc(NC(=O)Nc3cccc(c3)C(F)(F)F)cc2)ncn1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of GST-6His tagged VEGFR2 (unknown origin) using biotin-aminohexyl-EEEEYFELVAKKKK-NH2 peptide substrate incubated for 90 mins by HTRF assa... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00700
BindingDB Entry DOI: 10.7270/Q23X8BG9 |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50141587
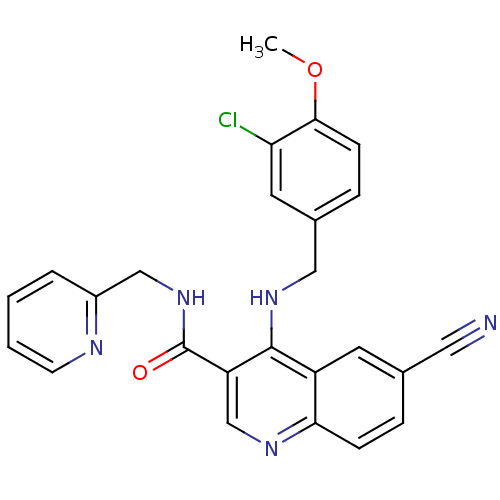
(4-(3-Chloro-4-methoxy-benzylamino)-6-cyano-quinoli...)Show SMILES COc1ccc(CNc2c(cnc3ccc(cc23)C#N)C(=O)NCc2ccccn2)cc1Cl Show InChI InChI=1S/C25H20ClN5O2/c1-33-23-8-6-17(11-21(23)26)13-30-24-19-10-16(12-27)5-7-22(19)29-15-20(24)25(32)31-14-18-4-2-3-9-28-18/h2-11,15H,13-14H2,1H3,(H,29,30)(H,31,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of phosphodiesterase 5 from human platelets |
Bioorg Med Chem Lett 14: 1577-80 (2004)
Article DOI: 10.1016/j.bmcl.2003.12.090
BindingDB Entry DOI: 10.7270/Q2G1607Q |
More data for this
Ligand-Target Pair | |
Transient receptor potential cation channel subfamily V member 4
(Homo sapiens (Human)) | BDBM50554960

(CHEMBL4749555)Show SMILES N[C@@H](CCNS(=O)(=O)c1ccc(cc1C(F)(F)F)C#N)COc1ccc(C#N)c(F)c1 |r| | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Antagonist activity at human TRPV4 expressed in BHK or HEK MSR2 cells assessed as inhibition of TRPV4 agonist GSK634775 (EC80)-induced response incub... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01303
BindingDB Entry DOI: 10.7270/Q2XW4PFK |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50141582
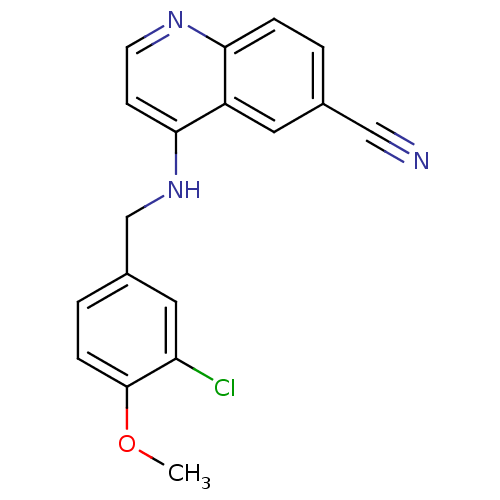
(4-(3-Chloro-4-methoxy-benzylamino)-quinoline-6-car...)Show InChI InChI=1S/C18H14ClN3O/c1-23-18-5-3-13(9-15(18)19)11-22-17-6-7-21-16-4-2-12(10-20)8-14(16)17/h2-9H,11H2,1H3,(H,21,22) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of phosphodiesterase 5 from human platelets |
Bioorg Med Chem Lett 14: 1577-80 (2004)
Article DOI: 10.1016/j.bmcl.2003.12.090
BindingDB Entry DOI: 10.7270/Q2G1607Q |
More data for this
Ligand-Target Pair | |
Transient receptor potential cation channel subfamily V member 4
(Homo sapiens (Human)) | BDBM50502626
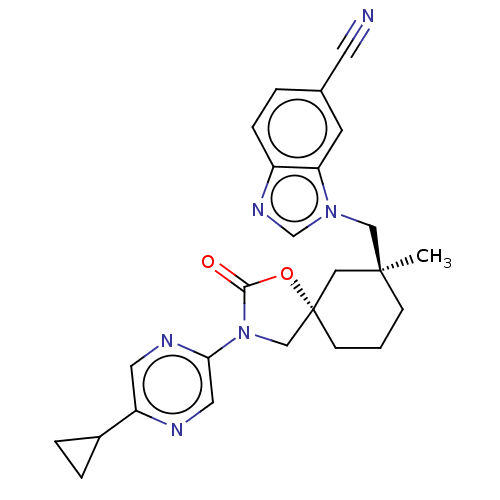
(CHEMBL4536058)Show SMILES C[C@]1(Cn2cnc3ccc(cc23)C#N)CCC[C@@]2(CN(C(=O)O2)c2cnc(cn2)C2CC2)C1 |r| Show InChI InChI=1S/C25H26N6O2/c1-24(14-30-16-29-19-6-3-17(10-26)9-21(19)30)7-2-8-25(13-24)15-31(23(32)33-25)22-12-27-20(11-28-22)18-4-5-18/h3,6,9,11-12,16,18H,2,4-5,7-8,13-15H2,1H3/t24-,25-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human TRPV4 expressed in baculovirus infected HEK/MSR 2 cells pre-incubated for 10 mins followed by GSK634775A addition by Fura-2 dye b... |
ACS Med Chem Lett 10: 1228-1233 (2019)
Article DOI: 10.1021/acsmedchemlett.9b00274
BindingDB Entry DOI: 10.7270/Q2RX9GBF |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM14390

(5-[2-ethoxy-5-(4-methyl-1-piperazinylsulfonyl)phen...)Show SMILES CCCc1nn(C)c2c1nc([nH]c2=O)-c1cc(ccc1OCC)S(=O)(=O)N1CCN(C)CC1 Show InChI InChI=1S/C22H30N6O4S/c1-5-7-17-19-20(27(4)25-17)22(29)24-21(23-19)16-14-15(8-9-18(16)32-6-2)33(30,31)28-12-10-26(3)11-13-28/h8-9,14H,5-7,10-13H2,1-4H3,(H,23,24,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
Article
PubMed
| n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of phosphodiesterase 5 from human platelets |
Bioorg Med Chem Lett 14: 1577-80 (2004)
Article DOI: 10.1016/j.bmcl.2003.12.090
BindingDB Entry DOI: 10.7270/Q2G1607Q |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Transient receptor potential cation channel subfamily V member 4
(Rattus norvegicus) | BDBM50502640

(CHEMBL4470585)Show SMILES CC(C)(O)c1cnc(cn1)N1C[C@@]2(CCC[C@](C)(Cn3cnc4ccc(cc34)C#N)C2)OC1=O |r| Show InChI InChI=1S/C25H28N6O3/c1-23(2,33)20-11-28-21(12-27-20)31-15-25(34-22(31)32)8-4-7-24(3,13-25)14-30-16-29-18-6-5-17(10-26)9-19(18)30/h5-6,9,11-12,16,33H,4,7-8,13-15H2,1-3H3/t24-,25-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of rat TRPV4 expressed in baculovirus infected HEK/MSR 2 cells pre-incubated for 10 mins followed by GSK634775A addition by Fura-2 dye bas... |
ACS Med Chem Lett 10: 1228-1233 (2019)
Article DOI: 10.1021/acsmedchemlett.9b00274
BindingDB Entry DOI: 10.7270/Q2RX9GBF |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Transient receptor potential cation channel subfamily V member 4
(Homo sapiens (Human)) | BDBM50502649
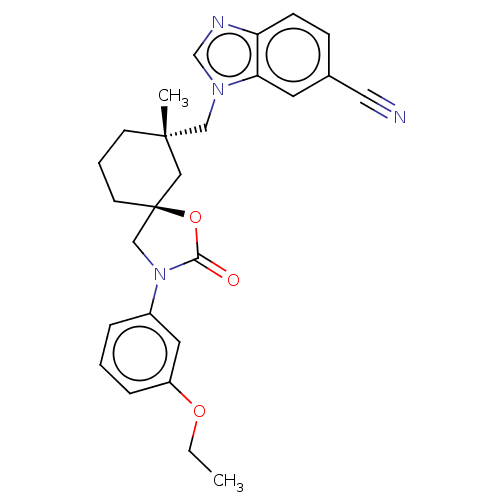
(CHEMBL4532369)Show SMILES CCOc1cccc(c1)N1C[C@@]2(CCC[C@](C)(Cn3cnc4ccc(cc34)C#N)C2)OC1=O |r| Show InChI InChI=1S/C26H28N4O3/c1-3-32-21-7-4-6-20(13-21)30-17-26(33-24(30)31)11-5-10-25(2,15-26)16-29-18-28-22-9-8-19(14-27)12-23(22)29/h4,6-9,12-13,18H,3,5,10-11,15-17H2,1-2H3/t25-,26-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human TRPV4 expressed in baculovirus infected HEK/MSR 2 cells pre-incubated for 10 mins followed by GSK634775A addition by Fura-2 dye b... |
ACS Med Chem Lett 10: 1228-1233 (2019)
Article DOI: 10.1021/acsmedchemlett.9b00274
BindingDB Entry DOI: 10.7270/Q2RX9GBF |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM14390

(5-[2-ethoxy-5-(4-methyl-1-piperazinylsulfonyl)phen...)Show SMILES CCCc1nn(C)c2c1nc([nH]c2=O)-c1cc(ccc1OCC)S(=O)(=O)N1CCN(C)CC1 Show InChI InChI=1S/C22H30N6O4S/c1-5-7-17-19-20(27(4)25-17)22(29)24-21(23-19)16-14-15(8-9-18(16)32-6-2)33(30,31)28-12-10-26(3)11-13-28/h8-9,14H,5-7,10-13H2,1-4H3,(H,23,24,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
PubMed
| n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of PDE5 from human platelets |
Bioorg Med Chem Lett 11: 2461-4 (2001)
BindingDB Entry DOI: 10.7270/Q2BK1BM5 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50104204
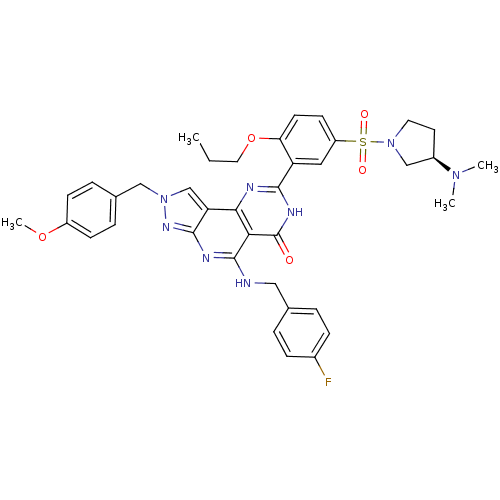
(8-[5-((R)-3-Dimethylamino-pyrrolidine-1-sulfonyl)-...)Show SMILES CCCOc1ccc(cc1-c1nc2c3cn(Cc4ccc(OC)cc4)nc3nc(NCc3ccc(F)cc3)c2c(=O)[nH]1)S(=O)(=O)N1CC[C@H](C1)N(C)C Show InChI InChI=1S/C38H41FN8O5S/c1-5-18-52-32-15-14-29(53(49,50)47-17-16-27(22-47)45(2)3)19-30(32)35-41-34-31-23-46(21-25-8-12-28(51-4)13-9-25)44-36(31)42-37(33(34)38(48)43-35)40-20-24-6-10-26(39)11-7-24/h6-15,19,23,27H,5,16-18,20-22H2,1-4H3,(H,40,42,44)(H,41,43,48)/t27-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of PDE5 from human platelets |
Bioorg Med Chem Lett 11: 2461-4 (2001)
BindingDB Entry DOI: 10.7270/Q2BK1BM5 |
More data for this
Ligand-Target Pair | |
Transient receptor potential cation channel subfamily V member 4
(Homo sapiens (Human)) | BDBM50502643
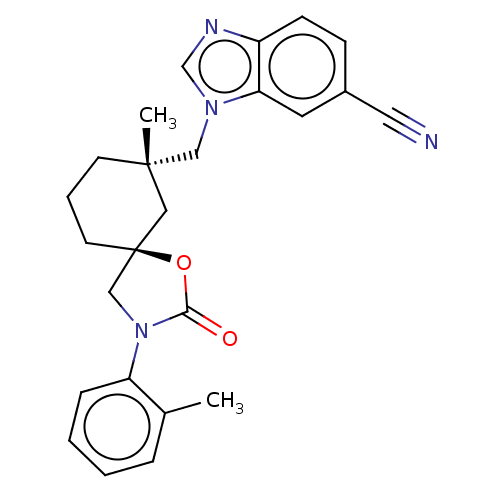
(CHEMBL4541827)Show SMILES Cc1ccccc1N1C[C@@]2(CCC[C@](C)(Cn3cnc4ccc(cc34)C#N)C2)OC1=O |r| Show InChI InChI=1S/C25H26N4O2/c1-18-6-3-4-7-21(18)29-16-25(31-23(29)30)11-5-10-24(2,14-25)15-28-17-27-20-9-8-19(13-26)12-22(20)28/h3-4,6-9,12,17H,5,10-11,14-16H2,1-2H3/t24-,25-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human TRPV4 expressed in baculovirus infected HEK/MSR 2 cells pre-incubated for 10 mins followed by GSK634775A addition by Fura-2 dye b... |
ACS Med Chem Lett 10: 1228-1233 (2019)
Article DOI: 10.1021/acsmedchemlett.9b00274
BindingDB Entry DOI: 10.7270/Q2RX9GBF |
More data for this
Ligand-Target Pair | |
Transient receptor potential cation channel subfamily V member 4
(Homo sapiens (Human)) | BDBM50502640

(CHEMBL4470585)Show SMILES CC(C)(O)c1cnc(cn1)N1C[C@@]2(CCC[C@](C)(Cn3cnc4ccc(cc34)C#N)C2)OC1=O |r| Show InChI InChI=1S/C25H28N6O3/c1-23(2,33)20-11-28-21(12-27-20)31-15-25(34-22(31)32)8-4-7-24(3,13-25)14-30-16-29-18-6-5-17(10-26)9-19(18)30/h5-6,9,11-12,16,33H,4,7-8,13-15H2,1-3H3/t24-,25-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human TRPV4 expressed in baculovirus infected HEK/MSR 2 cells pre-incubated for 10 mins followed by GSK634775A addition by Fura-2 dye b... |
ACS Med Chem Lett 10: 1228-1233 (2019)
Article DOI: 10.1021/acsmedchemlett.9b00274
BindingDB Entry DOI: 10.7270/Q2RX9GBF |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Vascular endothelial growth factor receptor 2
(Homo sapiens (Human)) | BDBM50578189

(CHEMBL4856992)Show SMILES COc1cc2ncnc(Oc3ccc(NC(=O)Nc4cccc(c4)C(F)(F)F)cc3)c2cc1OC | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of GST-6His tagged VEGFR2 (unknown origin) using biotin-aminohexyl-EEEEYFELVAKKKK-NH2 peptide substrate incubated for 90 mins by HTRF assa... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00700
BindingDB Entry DOI: 10.7270/Q23X8BG9 |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50104207

(8-[5-((R)-3-Dimethylamino-pyrrolidine-1-sulfonyl)-...)Show SMILES CCCOc1ccc(cc1-c1nc2c3c[nH]nc3ncc2c(=O)[nH]1)S(=O)(=O)N1CC[C@H](C1)N(C)C Show InChI InChI=1S/C23H27N7O4S/c1-4-9-34-19-6-5-15(35(32,33)30-8-7-14(13-30)29(2)3)10-16(19)22-26-20-17-12-25-28-21(17)24-11-18(20)23(31)27-22/h5-6,10-12,14H,4,7-9,13H2,1-3H3,(H,24,25,28)(H,26,27,31)/t14-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of PDE5 from human platelets |
Bioorg Med Chem Lett 11: 2461-4 (2001)
BindingDB Entry DOI: 10.7270/Q2BK1BM5 |
More data for this
Ligand-Target Pair | |
Transient receptor potential cation channel subfamily V member 4
(Homo sapiens (Human)) | BDBM50450500
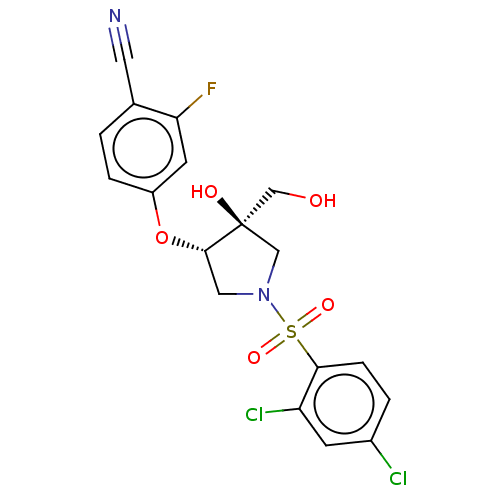
(CHEMBL4164465)Show SMILES OC[C@]1(O)CN(C[C@@H]1Oc1ccc(C#N)c(F)c1)S(=O)(=O)c1ccc(Cl)cc1Cl |r| Show InChI InChI=1S/C18H15Cl2FN2O5S/c19-12-2-4-16(14(20)5-12)29(26,27)23-8-17(18(25,9-23)10-24)28-13-3-1-11(7-22)15(21)6-13/h1-6,17,24-25H,8-10H2/t17-,18+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Antagonist activity at human TRPV4 expressed in BHK/AC9 or HEK MSR2 cells assessed as inhibition of PF-04674114-induced Ca2+ flux pre-incubated for 1... |
J Med Chem 61: 9738-9755 (2018)
Article DOI: 10.1021/acs.jmedchem.8b01317
BindingDB Entry DOI: 10.7270/Q2CJ8H2P |
More data for this
Ligand-Target Pair | |
Transient receptor potential cation channel subfamily V member 4
(Homo sapiens (Human)) | BDBM50502653

(CHEMBL4514491)Show SMILES C[C@]1(Cn2cnc3ccc(cc23)C#N)CCC[C@@]2(CN(C(=O)O2)c2ccccc2Cl)C1 |r| Show InChI InChI=1S/C24H23ClN4O2/c1-23(14-28-16-27-19-8-7-17(12-26)11-21(19)28)9-4-10-24(13-23)15-29(22(30)31-24)20-6-3-2-5-18(20)25/h2-3,5-8,11,16H,4,9-10,13-15H2,1H3/t23-,24-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human TRPV4 expressed in baculovirus infected HEK/MSR 2 cells pre-incubated for 10 mins followed by GSK634775A addition by Fura-2 dye b... |
ACS Med Chem Lett 10: 1228-1233 (2019)
Article DOI: 10.1021/acsmedchemlett.9b00274
BindingDB Entry DOI: 10.7270/Q2RX9GBF |
More data for this
Ligand-Target Pair | |
Vascular endothelial growth factor receptor 2
(Homo sapiens (Human)) | BDBM68211

(Type II inhibitor, 5)Show SMILES FC(F)(F)c1cccc(NC(=O)Nc2ccc(Oc3ncnc4[nH]ccc34)cc2)c1 Show InChI InChI=1S/C20H14F3N5O2/c21-20(22,23)12-2-1-3-14(10-12)28-19(29)27-13-4-6-15(7-5-13)30-18-16-8-9-24-17(16)25-11-26-18/h1-11H,(H,24,25,26)(H2,27,28,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of GST-6His tagged VEGFR2 (unknown origin) using biotin-aminohexyl-EEEEYFELVAKKKK-NH2 peptide substrate incubated for 90 mins by HTRF assa... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00700
BindingDB Entry DOI: 10.7270/Q23X8BG9 |
More data for this
Ligand-Target Pair | |
Transient receptor potential cation channel subfamily V member 4
(Homo sapiens (Human)) | BDBM50450500

(CHEMBL4164465)Show SMILES OC[C@]1(O)CN(C[C@@H]1Oc1ccc(C#N)c(F)c1)S(=O)(=O)c1ccc(Cl)cc1Cl |r| Show InChI InChI=1S/C18H15Cl2FN2O5S/c19-12-2-4-16(14(20)5-12)29(26,27)23-8-17(18(25,9-23)10-24)28-13-3-1-11(7-22)15(21)6-13/h1-6,17,24-25H,8-10H2/t17-,18+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Antagonist activity at human TRPV4 expressed in BHK/AC9 or HEK MSR2 cells assessed as inhibition of PF-04674114-induced Ca2+ flux pre-incubated for 1... |
J Med Chem 61: 9738-9755 (2018)
Article DOI: 10.1021/acs.jmedchem.8b01317
BindingDB Entry DOI: 10.7270/Q2CJ8H2P |
More data for this
Ligand-Target Pair | |
Vascular endothelial growth factor receptor 2
(Homo sapiens (Human)) | BDBM50578219
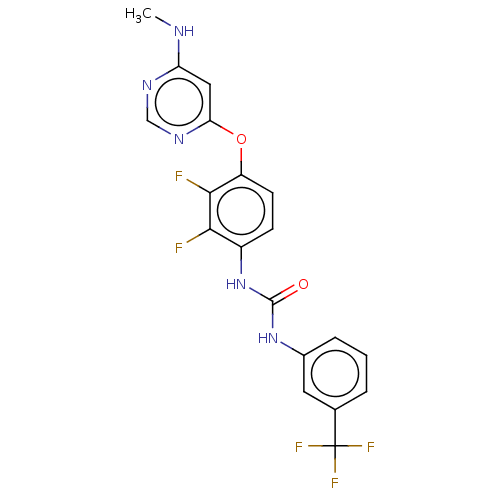
(CHEMBL4857556)Show SMILES CNc1cc(Oc2ccc(NC(=O)Nc3cccc(c3)C(F)(F)F)c(F)c2F)ncn1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of GST-6His tagged VEGFR2 (unknown origin) using biotin-aminohexyl-EEEEYFELVAKKKK-NH2 peptide substrate incubated for 90 mins by HTRF assa... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00700
BindingDB Entry DOI: 10.7270/Q23X8BG9 |
More data for this
Ligand-Target Pair | |
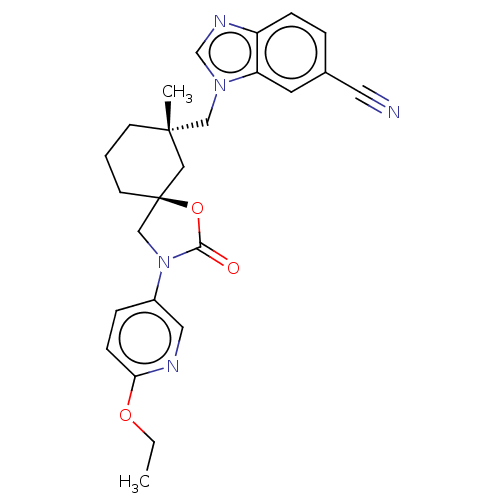
Transient receptor potential cation channel subfamily V member 4
(Homo sapiens (Human)) | BDBM50502636
(CHEMBL4588158)Show SMILES CCOc1ccc(cn1)N1C[C@@]2(CCC[C@](C)(Cn3cnc4ccc(cc34)C#N)C2)OC1=O |r| Show InChI InChI=1S/C25H27N5O3/c1-3-32-22-8-6-19(13-27-22)30-16-25(33-23(30)31)10-4-9-24(2,14-25)15-29-17-28-20-7-5-18(12-26)11-21(20)29/h5-8,11,13,17H,3-4,9-10,14-16H2,1-2H3/t24-,25-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.10 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human TRPV4 expressed in baculovirus infected HEK/MSR 2 cells pre-incubated for 10 mins followed by GSK634775A addition by Fura-2 dye b... |
ACS Med Chem Lett 10: 1228-1233 (2019)
Article DOI: 10.1021/acsmedchemlett.9b00274
BindingDB Entry DOI: 10.7270/Q2RX9GBF |
More data for this
Ligand-Target Pair | |
Vascular endothelial growth factor receptor 2
(Homo sapiens (Human)) | BDBM50578191
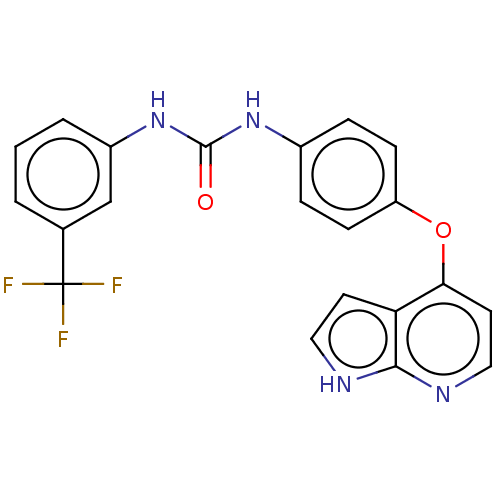
(CHEMBL4852364)Show SMILES FC(F)(F)c1cccc(NC(=O)Nc2ccc(Oc3ccnc4[nH]ccc34)cc2)c1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of GST-6His tagged VEGFR2 (unknown origin) using biotin-aminohexyl-EEEEYFELVAKKKK-NH2 peptide substrate incubated for 90 mins by HTRF assa... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00700
BindingDB Entry DOI: 10.7270/Q23X8BG9 |
More data for this
Ligand-Target Pair | |
Vascular endothelial growth factor receptor 2
(Homo sapiens (Human)) | BDBM50578209

(CHEMBL4867760)Show SMILES FC(F)(F)c1cccc(NC(=O)Nc2ccc(Oc3cc(Nc4cccc(CN5CCCC5)c4)ncn3)cc2)c1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of GST-6His tagged VEGFR2 (unknown origin) using biotin-aminohexyl-EEEEYFELVAKKKK-NH2 peptide substrate incubated for 90 mins by HTRF assa... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00700
BindingDB Entry DOI: 10.7270/Q23X8BG9 |
More data for this
Ligand-Target Pair | |
Vascular endothelial growth factor receptor 2
(Homo sapiens (Human)) | BDBM50578183
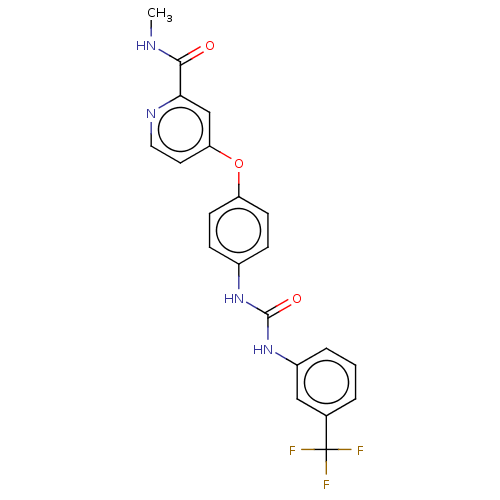
(CHEMBL1992306)Show SMILES CNC(=O)c1cc(Oc2ccc(NC(=O)Nc3cccc(c3)C(F)(F)F)cc2)ccn1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of GST-6His tagged VEGFR2 (unknown origin) using biotin-aminohexyl-EEEEYFELVAKKKK-NH2 peptide substrate incubated for 90 mins by HTRF assa... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00700
BindingDB Entry DOI: 10.7270/Q23X8BG9 |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50141579
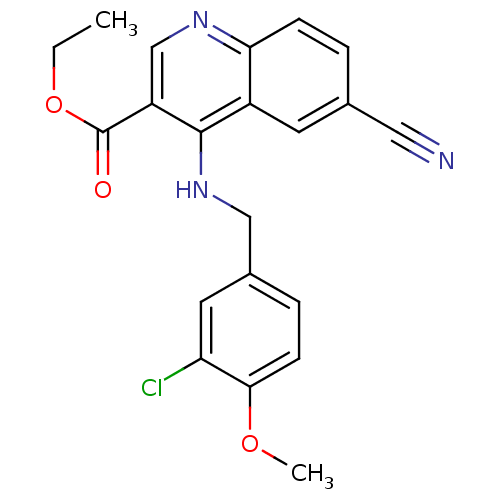
(4-(3-Chloro-4-methoxy-benzylamino)-6-cyano-quinoli...)Show SMILES CCOC(=O)c1cnc2ccc(cc2c1NCc1ccc(OC)c(Cl)c1)C#N Show InChI InChI=1S/C21H18ClN3O3/c1-3-28-21(26)16-12-24-18-6-4-13(10-23)8-15(18)20(16)25-11-14-5-7-19(27-2)17(22)9-14/h4-9,12H,3,11H2,1-2H3,(H,24,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of phosphodiesterase 5 from human platelets |
Bioorg Med Chem Lett 14: 1577-80 (2004)
Article DOI: 10.1016/j.bmcl.2003.12.090
BindingDB Entry DOI: 10.7270/Q2G1607Q |
More data for this
Ligand-Target Pair | |
Transient receptor potential cation channel subfamily V member 4
(Homo sapiens (Human)) | BDBM50502637

(CHEMBL4469982)Show SMILES CCOc1cnc(cn1)N1C[C@@]2(CCC[C@](C)(Cn3cnc4ccc(cc34)C#N)C2)OC1=O |r| Show InChI InChI=1S/C24H26N6O3/c1-3-32-21-12-26-20(11-27-21)30-15-24(33-22(30)31)8-4-7-23(2,13-24)14-29-16-28-18-6-5-17(10-25)9-19(18)29/h5-6,9,11-12,16H,3-4,7-8,13-15H2,1-2H3/t23-,24-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4.20 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human TRPV4 expressed in baculovirus infected HEK/MSR 2 cells pre-incubated for 10 mins followed by GSK634775A addition by Fura-2 dye b... |
ACS Med Chem Lett 10: 1228-1233 (2019)
Article DOI: 10.1021/acsmedchemlett.9b00274
BindingDB Entry DOI: 10.7270/Q2RX9GBF |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 14
(Homo sapiens (Human)) | BDBM50578221

(CHEMBL4870768)Show SMILES CNc1cc(Oc2ccc(NC(=O)Nc3cccc(c3)C(F)(F)F)c3ccccc23)ncn1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of p38alpha (unknown origin) |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00700
BindingDB Entry DOI: 10.7270/Q23X8BG9 |
More data for this
Ligand-Target Pair | |
Progesterone receptor
(Homo sapiens (Human)) | BDBM50304973

((S)-4-((2-bromobenzyl)(1-(methylsulfonyl)pyrrolidi...)Show SMILES CS(=O)(=O)N1CC[C@@H](C1)N(Cc1ccccc1Br)c1ccc(C#N)c(Cl)c1 |r| Show InChI InChI=1S/C19H19BrClN3O2S/c1-27(25,26)23-9-8-17(13-23)24(12-15-4-2-3-5-18(15)20)16-7-6-14(11-22)19(21)10-16/h2-7,10,17H,8-9,12-13H2,1H3/t17-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of PR |
Bioorg Med Chem Lett 20: 371-4 (2010)
Article DOI: 10.1016/j.bmcl.2009.10.092
BindingDB Entry DOI: 10.7270/Q2057G17 |
More data for this
Ligand-Target Pair | |
Vascular endothelial growth factor receptor 2
(Homo sapiens (Human)) | BDBM50578186
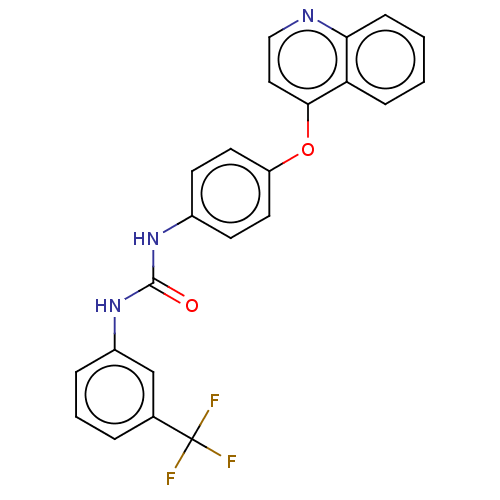
(CHEMBL4870490)Show SMILES FC(F)(F)c1cccc(NC(=O)Nc2ccc(Oc3ccnc4ccccc34)cc2)c1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of GST-6His tagged VEGFR2 (unknown origin) using biotin-aminohexyl-EEEEYFELVAKKKK-NH2 peptide substrate incubated for 90 mins by HTRF assa... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00700
BindingDB Entry DOI: 10.7270/Q23X8BG9 |
More data for this
Ligand-Target Pair | |
Transient receptor potential cation channel subfamily V member 4
(Homo sapiens (Human)) | BDBM50502642
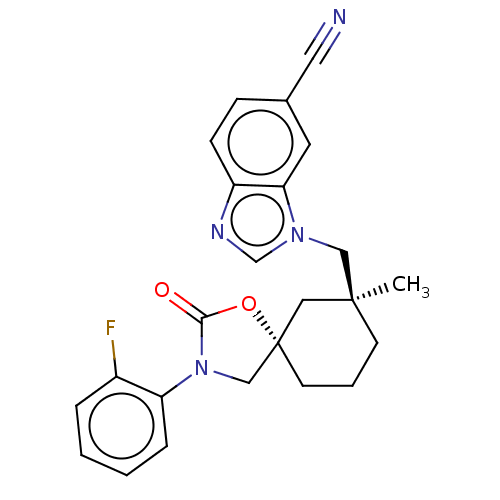
(CHEMBL4560266)Show SMILES C[C@]1(Cn2cnc3ccc(cc23)C#N)CCC[C@@]2(CN(C(=O)O2)c2ccccc2F)C1 |r| Show InChI InChI=1S/C24H23FN4O2/c1-23(14-28-16-27-19-8-7-17(12-26)11-21(19)28)9-4-10-24(13-23)15-29(22(30)31-24)20-6-3-2-5-18(20)25/h2-3,5-8,11,16H,4,9-10,13-15H2,1H3/t23-,24-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 5.20 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human TRPV4 expressed in baculovirus infected HEK/MSR 2 cells pre-incubated for 10 mins followed by GSK634775A addition by Fura-2 dye b... |
ACS Med Chem Lett 10: 1228-1233 (2019)
Article DOI: 10.1021/acsmedchemlett.9b00274
BindingDB Entry DOI: 10.7270/Q2RX9GBF |
More data for this
Ligand-Target Pair | |
Transient receptor potential cation channel subfamily V member 4
(Homo sapiens (Human)) | BDBM50502646
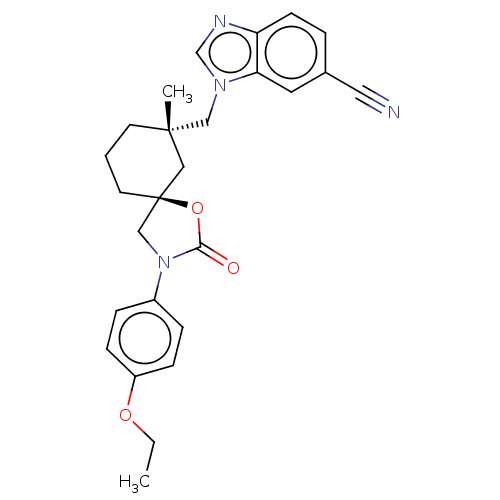
(CHEMBL4579838)Show SMILES CCOc1ccc(cc1)N1C[C@@]2(CCC[C@](C)(Cn3cnc4ccc(cc34)C#N)C2)OC1=O |r| Show InChI InChI=1S/C26H28N4O3/c1-3-32-21-8-6-20(7-9-21)30-17-26(33-24(30)31)12-4-11-25(2,15-26)16-29-18-28-22-10-5-19(14-27)13-23(22)29/h5-10,13,18H,3-4,11-12,15-17H2,1-2H3/t25-,26-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 5.20 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human TRPV4 expressed in baculovirus infected HEK/MSR 2 cells pre-incubated for 10 mins followed by GSK634775A addition by Fura-2 dye b... |
ACS Med Chem Lett 10: 1228-1233 (2019)
Article DOI: 10.1021/acsmedchemlett.9b00274
BindingDB Entry DOI: 10.7270/Q2RX9GBF |
More data for this
Ligand-Target Pair | |
Transient receptor potential cation channel subfamily V member 4
(Rattus norvegicus) | BDBM50502650
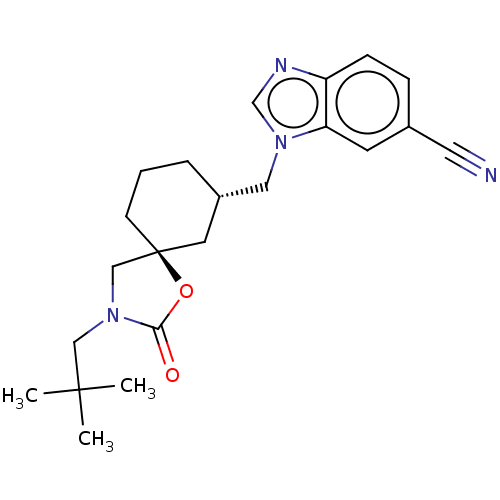
(CHEMBL4549613)Show SMILES CC(C)(C)CN1C[C@@]2(CCC[C@H](Cn3cnc4ccc(cc34)C#N)C2)OC1=O |r| Show InChI InChI=1S/C22H28N4O2/c1-21(2,3)13-26-14-22(28-20(26)27)8-4-5-17(10-22)12-25-15-24-18-7-6-16(11-23)9-19(18)25/h6-7,9,15,17H,4-5,8,10,12-14H2,1-3H3/t17-,22-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 5.5 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of rat TRPV4 expressed in baculovirus infected HEK/MSR 2 cells pre-incubated for 10 mins followed by GSK634775A addition by Fura-2 dye bas... |
ACS Med Chem Lett 10: 1228-1233 (2019)
Article DOI: 10.1021/acsmedchemlett.9b00274
BindingDB Entry DOI: 10.7270/Q2RX9GBF |
More data for this
Ligand-Target Pair | |
Progesterone receptor
(Homo sapiens (Human)) | BDBM50304972

((S)-2-chloro-4-((2-chlorobenzyl)(1-(methylsulfonyl...)Show SMILES CS(=O)(=O)N1CC[C@@H](C1)N(Cc1ccccc1Cl)c1ccc(C#N)c(Cl)c1 |r| Show InChI InChI=1S/C19H19Cl2N3O2S/c1-27(25,26)23-9-8-17(13-23)24(12-15-4-2-3-5-18(15)20)16-7-6-14(11-22)19(21)10-16/h2-7,10,17H,8-9,12-13H2,1H3/t17-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of PR |
Bioorg Med Chem Lett 20: 371-4 (2010)
Article DOI: 10.1016/j.bmcl.2009.10.092
BindingDB Entry DOI: 10.7270/Q2057G17 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase B-raf
(Homo sapiens (Human)) | BDBM50118379
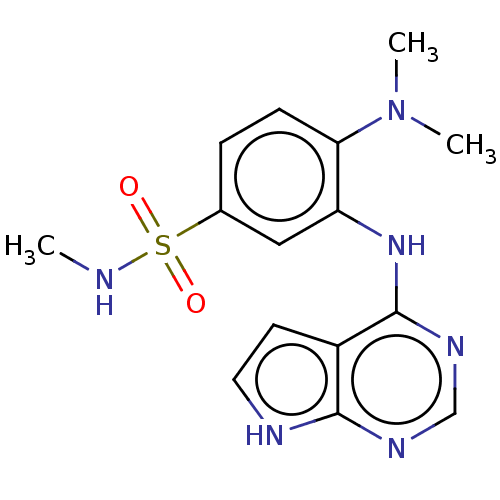
(CHEMBL3613195)Show SMILES CNS(=O)(=O)c1ccc(N(C)C)c(Nc2ncnc3[nH]ccc23)c1 Show InChI InChI=1S/C15H18N6O2S/c1-16-24(22,23)10-4-5-13(21(2)3)12(8-10)20-15-11-6-7-17-14(11)18-9-19-15/h4-9,16H,1-3H3,(H2,17,18,19,20) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of B-Raf (unknown origin) |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00700
BindingDB Entry DOI: 10.7270/Q23X8BG9 |
More data for this
Ligand-Target Pair | |
Vascular endothelial growth factor receptor 2
(Homo sapiens (Human)) | BDBM50578218

(CHEMBL4863203)Show SMILES CNc1cc(Oc2ccc(NC(=O)Nc3cccc(c3)C(F)(F)F)c(F)c2)ncn1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 6.30 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of GST-6His tagged VEGFR2 (unknown origin) using biotin-aminohexyl-EEEEYFELVAKKKK-NH2 peptide substrate incubated for 90 mins by HTRF assa... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00700
BindingDB Entry DOI: 10.7270/Q23X8BG9 |
More data for this
Ligand-Target Pair | |
Vascular endothelial growth factor receptor 2
(Homo sapiens (Human)) | BDBM50578194
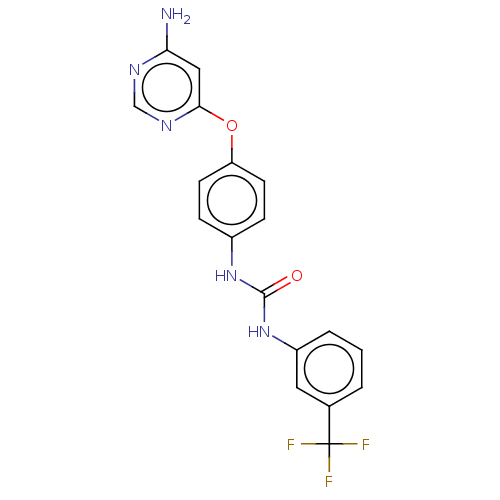
(CHEMBL4849349)Show SMILES Nc1cc(Oc2ccc(NC(=O)Nc3cccc(c3)C(F)(F)F)cc2)ncn1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 7.90 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of GST-6His tagged VEGFR2 (unknown origin) using biotin-aminohexyl-EEEEYFELVAKKKK-NH2 peptide substrate incubated for 90 mins by HTRF assa... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00700
BindingDB Entry DOI: 10.7270/Q23X8BG9 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase TNNI3K
(Homo sapiens (Human)) | BDBM50578223

(CHEMBL4868757)Show SMILES CNc1cc(Oc2c(C)cc(NC(=O)Nc3cccc(c3)C(F)(F)F)cc2C)ncn1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 7.90 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human myc-TNNI3K expressed in HEK-MSR2 cells assessed as reduction in TNNI3K autophosphorylation preincubated for 30 mins followed by p... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00700
BindingDB Entry DOI: 10.7270/Q23X8BG9 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data