 Found 360 hits with Last Name = 'svensson' and Initial = 'm'
Found 360 hits with Last Name = 'svensson' and Initial = 'm' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Genome polyprotein
(Hepacivirus C) | BDBM50496205

(CHEMBL3125043)Show SMILES COc1cc(Oc2ccc(cc2C=C)[C@H](NC(=O)[C@@H](NC(=O)OC(C)(C)C)C(C)(C)C)C(=O)Nc2cccc(c2)C(=O)NS(=O)(=O)c2ccc(cc2)C(F)(F)F)nc(n1)-c1ccccc1 |r| Show InChI InChI=1S/C46H47F3N6O9S/c1-9-27-24-29(18-23-34(27)63-36-26-35(62-8)51-39(52-36)28-14-11-10-12-15-28)37(53-42(58)38(44(2,3)4)54-43(59)64-45(5,6)7)41(57)50-32-17-13-16-30(25-32)40(56)55-65(60,61)33-21-19-31(20-22-33)46(47,48)49/h9-26,37-38H,1H2,2-8H3,(H,50,57)(H,53,58)(H,54,59)(H,55,56)/t37-,38+/m0/s1 | PDB
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 53 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Uppsala University
Curated by ChEMBL
| Assay Description
Inhibition of Hepatitis C virus genotype 1a full length NS3/2K-NS4A protease A156T mutant using Ac-DED(Edans)EEAbupsi[COO]ASK(Dabcyl)-NH2 as substrat... |
ACS Med Chem Lett 5: 249-54 (2014)
Article DOI: 10.1021/ml400217r
BindingDB Entry DOI: 10.7270/Q2C250FF |
More data for this
Ligand-Target Pair | |
Fatty-acid amide hydrolase 1 [30-579]
(Rattus norvegicus (rat)) | BDBM50282653

(CHEMBL4163689)Show SMILES CC(C(=O)Nc1ncccc1Cl)c1ccc(Nc2ccnc(c2)C(F)(F)F)cc1 Show InChI InChI=1S/C20H16ClF3N4O/c1-12(19(29)28-18-16(21)3-2-9-26-18)13-4-6-14(7-5-13)27-15-8-10-25-17(11-15)20(22,23)24/h2-12H,1H3,(H,25,27)(H,26,28,29) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Cagliari
Curated by ChEMBL
| Assay Description
Noncompetitive inhibition of rat brain FAAH using [3H]AEA as substrate after 10 mins by Dixon plot analysis |
Eur J Med Chem 136: 523-542 (2017)
Article DOI: 10.1016/j.ejmech.2017.05.033
BindingDB Entry DOI: 10.7270/Q2H134JK |
More data for this
Ligand-Target Pair | |
Genome polyprotein
(Hepacivirus C) | BDBM50496205

(CHEMBL3125043)Show SMILES COc1cc(Oc2ccc(cc2C=C)[C@H](NC(=O)[C@@H](NC(=O)OC(C)(C)C)C(C)(C)C)C(=O)Nc2cccc(c2)C(=O)NS(=O)(=O)c2ccc(cc2)C(F)(F)F)nc(n1)-c1ccccc1 |r| Show InChI InChI=1S/C46H47F3N6O9S/c1-9-27-24-29(18-23-34(27)63-36-26-35(62-8)51-39(52-36)28-14-11-10-12-15-28)37(53-42(58)38(44(2,3)4)54-43(59)64-45(5,6)7)41(57)50-32-17-13-16-30(25-32)40(56)55-65(60,61)33-21-19-31(20-22-33)46(47,48)49/h9-26,37-38H,1H2,2-8H3,(H,50,57)(H,53,58)(H,54,59)(H,55,56)/t37-,38+/m0/s1 | PDB
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Uppsala University
Curated by ChEMBL
| Assay Description
Inhibition of Hepatitis C virus genotype 1a full length NS3/2K-NS4A protease D168V mutant using Ac-DED(Edans)EEAbupsi[COO]ASK(Dabcyl)-NH2 as substrat... |
ACS Med Chem Lett 5: 249-54 (2014)
Article DOI: 10.1021/ml400217r
BindingDB Entry DOI: 10.7270/Q2C250FF |
More data for this
Ligand-Target Pair | |
Genome polyprotein
(Hepacivirus C) | BDBM50496204

(CHEMBL3125169)Show SMILES COc1cc(Oc2ccc(cc2C=C)C(NC(=O)OC(C)(C)C)C(=O)Nc2cccc(c2)C(=O)NS(=O)(=O)c2ccc(cc2)C(F)(F)F)nc(n1)-c1ccccc1 Show InChI InChI=1S/C40H36F3N5O8S/c1-6-24-21-26(15-20-31(24)55-33-23-32(54-5)45-35(46-33)25-11-8-7-9-12-25)34(47-38(51)56-39(2,3)4)37(50)44-29-14-10-13-27(22-29)36(49)48-57(52,53)30-18-16-28(17-19-30)40(41,42)43/h6-23,34H,1H2,2-5H3,(H,44,50)(H,47,51)(H,48,49) | PDB
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Uppsala University
Curated by ChEMBL
| Assay Description
Inhibition of Hepatitis C virus genotype 1a full length NS3/2K-NS4A protease A156T mutant using Ac-DED(Edans)EEAbupsi[COO]ASK(Dabcyl)-NH2 as substrat... |
ACS Med Chem Lett 5: 249-54 (2014)
Article DOI: 10.1021/ml400217r
BindingDB Entry DOI: 10.7270/Q2C250FF |
More data for this
Ligand-Target Pair | |
Genome polyprotein
(Hepacivirus C) | BDBM50496204

(CHEMBL3125169)Show SMILES COc1cc(Oc2ccc(cc2C=C)C(NC(=O)OC(C)(C)C)C(=O)Nc2cccc(c2)C(=O)NS(=O)(=O)c2ccc(cc2)C(F)(F)F)nc(n1)-c1ccccc1 Show InChI InChI=1S/C40H36F3N5O8S/c1-6-24-21-26(15-20-31(24)55-33-23-32(54-5)45-35(46-33)25-11-8-7-9-12-25)34(47-38(51)56-39(2,3)4)37(50)44-29-14-10-13-27(22-29)36(49)48-57(52,53)30-18-16-28(17-19-30)40(41,42)43/h6-23,34H,1H2,2-5H3,(H,44,50)(H,47,51)(H,48,49) | PDB
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Uppsala University
Curated by ChEMBL
| Assay Description
Inhibition of Hepatitis C virus genotype 1a full length NS3/2K-NS4A protease D168V mutant using Ac-DED(Edans)EEAbupsi[COO]ASK(Dabcyl)-NH2 as substrat... |
ACS Med Chem Lett 5: 249-54 (2014)
Article DOI: 10.1021/ml400217r
BindingDB Entry DOI: 10.7270/Q2C250FF |
More data for this
Ligand-Target Pair | |
Genome polyprotein
(Hepacivirus C) | BDBM50496205

(CHEMBL3125043)Show SMILES COc1cc(Oc2ccc(cc2C=C)[C@H](NC(=O)[C@@H](NC(=O)OC(C)(C)C)C(C)(C)C)C(=O)Nc2cccc(c2)C(=O)NS(=O)(=O)c2ccc(cc2)C(F)(F)F)nc(n1)-c1ccccc1 |r| Show InChI InChI=1S/C46H47F3N6O9S/c1-9-27-24-29(18-23-34(27)63-36-26-35(62-8)51-39(52-36)28-14-11-10-12-15-28)37(53-42(58)38(44(2,3)4)54-43(59)64-45(5,6)7)41(57)50-32-17-13-16-30(25-32)40(56)55-65(60,61)33-21-19-31(20-22-33)46(47,48)49/h9-26,37-38H,1H2,2-8H3,(H,50,57)(H,53,58)(H,54,59)(H,55,56)/t37-,38+/m0/s1 | PDB
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 210 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Uppsala University
Curated by ChEMBL
| Assay Description
Inhibition of Hepatitis C virus genotype 1a full length NS3/2K-NS4A protease R155K mutant using Ac-DED(Edans)EEAbupsi[COO]ASK(Dabcyl)-NH2 as substrat... |
ACS Med Chem Lett 5: 249-54 (2014)
Article DOI: 10.1021/ml400217r
BindingDB Entry DOI: 10.7270/Q2C250FF |
More data for this
Ligand-Target Pair | |
Fatty-acid amide hydrolase 1 [30-579]
(Rattus norvegicus (rat)) | BDBM50282650
(CHEMBL4173207)Show SMILES CC(C(=O)Nc1ncccc1C)c1ccc(Nc2ccnc(c2)C(F)(F)F)cc1 Show InChI InChI=1S/C21H19F3N4O/c1-13-4-3-10-26-19(13)28-20(29)14(2)15-5-7-16(8-6-15)27-17-9-11-25-18(12-17)21(22,23)24/h3-12,14H,1-2H3,(H,25,27)(H,26,28,29) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 390 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Cagliari
Curated by ChEMBL
| Assay Description
Competitive inhibition of rat brain FAAH using [3H]AEA as substrate after 10 mins by Dixon plot analysis |
Eur J Med Chem 136: 523-542 (2017)
Article DOI: 10.1016/j.ejmech.2017.05.033
BindingDB Entry DOI: 10.7270/Q2H134JK |
More data for this
Ligand-Target Pair | |
Genome polyprotein
(Hepacivirus C) | BDBM50496204

(CHEMBL3125169)Show SMILES COc1cc(Oc2ccc(cc2C=C)C(NC(=O)OC(C)(C)C)C(=O)Nc2cccc(c2)C(=O)NS(=O)(=O)c2ccc(cc2)C(F)(F)F)nc(n1)-c1ccccc1 Show InChI InChI=1S/C40H36F3N5O8S/c1-6-24-21-26(15-20-31(24)55-33-23-32(54-5)45-35(46-33)25-11-8-7-9-12-25)34(47-38(51)56-39(2,3)4)37(50)44-29-14-10-13-27(22-29)36(49)48-57(52,53)30-18-16-28(17-19-30)40(41,42)43/h6-23,34H,1H2,2-5H3,(H,44,50)(H,47,51)(H,48,49) | PDB
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 580 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Uppsala University
Curated by ChEMBL
| Assay Description
Inhibition of Hepatitis C virus genotype 1a full length NS3/2K-NS4A protease R155K mutant using Ac-DED(Edans)EEAbupsi[COO]ASK(Dabcyl)-NH2 as substrat... |
ACS Med Chem Lett 5: 249-54 (2014)
Article DOI: 10.1021/ml400217r
BindingDB Entry DOI: 10.7270/Q2C250FF |
More data for this
Ligand-Target Pair | |
Fatty-acid amide hydrolase 1
(Mus musculus (mouse)) | BDBM50282653

(CHEMBL4163689)Show SMILES CC(C(=O)Nc1ncccc1Cl)c1ccc(Nc2ccnc(c2)C(F)(F)F)cc1 Show InChI InChI=1S/C20H16ClF3N4O/c1-12(19(29)28-18-16(21)3-2-9-26-18)13-4-6-14(7-5-13)27-15-8-10-25-17(11-15)20(22,23)24/h2-12H,1H3,(H,25,27)(H,26,28,29) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Cagliari
Curated by ChEMBL
| Assay Description
Mixed-type inhibition of B6CBAF1/J mouse brain FAAH using [3H]AEA as substrate after 10 mins by Dixon plot analysis |
Eur J Med Chem 136: 523-542 (2017)
Article DOI: 10.1016/j.ejmech.2017.05.033
BindingDB Entry DOI: 10.7270/Q2H134JK |
More data for this
Ligand-Target Pair | |
Fatty-acid amide hydrolase 1
(Mus musculus (mouse)) | BDBM50282650

(CHEMBL4173207)Show SMILES CC(C(=O)Nc1ncccc1C)c1ccc(Nc2ccnc(c2)C(F)(F)F)cc1 Show InChI InChI=1S/C21H19F3N4O/c1-13-4-3-10-26-19(13)28-20(29)14(2)15-5-7-16(8-6-15)27-17-9-11-25-18(12-17)21(22,23)24/h3-12,14H,1-2H3,(H,25,27)(H,26,28,29) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 6.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Cagliari
Curated by ChEMBL
| Assay Description
Mixed-type inhibition of B6CBAF1/J mouse brain FAAH using [3H]AEA as substrate after 10 mins by Dixon plot analysis |
Eur J Med Chem 136: 523-542 (2017)
Article DOI: 10.1016/j.ejmech.2017.05.033
BindingDB Entry DOI: 10.7270/Q2H134JK |
More data for this
Ligand-Target Pair | |
Transient receptor potential cation channel subfamily V member 1
(Homo sapiens (Human)) | BDBM50391055
(CHEMBL2088399)Show InChI InChI=1S/C19H20N2O2S/c1-19(2,3)13-6-4-12(5-7-13)18(23)20-14-8-9-16-15(10-14)21-17(11-22)24-16/h4-10,22H,11H2,1-3H3,(H,20,23) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.70 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Antagonist activity at human recombinant TRPV1 receptor assessed as inhibition of capsaicin-induced calcium uptake by FLIPR assay |
Bioorg Med Chem Lett 22: 6205-11 (2012)
Article DOI: 10.1016/j.bmcl.2012.08.018
BindingDB Entry DOI: 10.7270/Q29C6ZGB |
More data for this
Ligand-Target Pair | |
Lysine-specific demethylase 5A
(Homo sapiens (Human)) | BDBM615666

((5-isopropyl-1H-pyrazol-3-yl)[(1R,5S,6r)-6-(1H-tet...)Show SMILES CC(C)c1cc(n[nH]1)C(=O)N1C[C@H]2[C@@H](C1)[C@@H]2c1nnn[nH]1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Celgene Quanticel Research, Inc.
US Patent
| |
US Patent US9828343 (2017)
BindingDB Entry DOI: 10.7270/Q2639TWH |
More data for this
Ligand-Target Pair | |
Lysine-specific demethylase 5A
(Homo sapiens (Human)) | BDBM608104

(US11691968, Example 134)Show SMILES CC(C)c1cc(n[nH]1)C(=O)N1CC2C(C1)C2c1nnn[nH]1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2QJ7NC3 |
More data for this
Ligand-Target Pair | |
D-amino-acid oxidase
(Homo sapiens (Human)) | BDBM50605601
(CHEMBL5184138)Show SMILES Fc1cccc(COCc2ccc3S[C@@H](COc3c2)c2c[nH]c(=O)c(=O)[nH]2)c1 |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00118
BindingDB Entry DOI: 10.7270/Q2MG7TK7 |
More data for this
Ligand-Target Pair | |
D-amino-acid oxidase
(Homo sapiens (Human)) | BDBM50605597
(CHEMBL5204161)Show SMILES C[C@]1(COc2cc(ccc2S1)C(F)(F)F)c1c[nH]c(=O)c(=O)[nH]1 |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00118
BindingDB Entry DOI: 10.7270/Q2MG7TK7 |
More data for this
Ligand-Target Pair | |
D-amino-acid oxidase
(Homo sapiens (Human)) | BDBM50605598

(CHEMBL5188193)Show SMILES C[C@]1(COc2cc(Cl)ccc2S1)c1c[nH]c(=O)c(=O)[nH]1 |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00118
BindingDB Entry DOI: 10.7270/Q2MG7TK7 |
More data for this
Ligand-Target Pair | |
Transient receptor potential cation channel subfamily V member 1
(Homo sapiens (Human)) | BDBM50391031
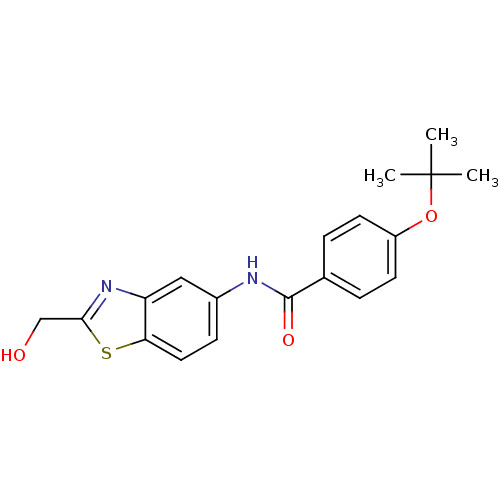
(CHEMBL2088393)Show InChI InChI=1S/C19H20N2O3S/c1-19(2,3)24-14-7-4-12(5-8-14)18(23)20-13-6-9-16-15(10-13)21-17(11-22)25-16/h4-10,22H,11H2,1-3H3,(H,20,23) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 9.80 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Antagonist activity at human recombinant TRPV1 receptor assessed as inhibition of capsaicin-induced calcium uptake by FLIPR assay |
Bioorg Med Chem Lett 22: 6205-11 (2012)
Article DOI: 10.1016/j.bmcl.2012.08.018
BindingDB Entry DOI: 10.7270/Q29C6ZGB |
More data for this
Ligand-Target Pair | |
Lysine-specific demethylase 5A
(Homo sapiens (Human)) | BDBM615664
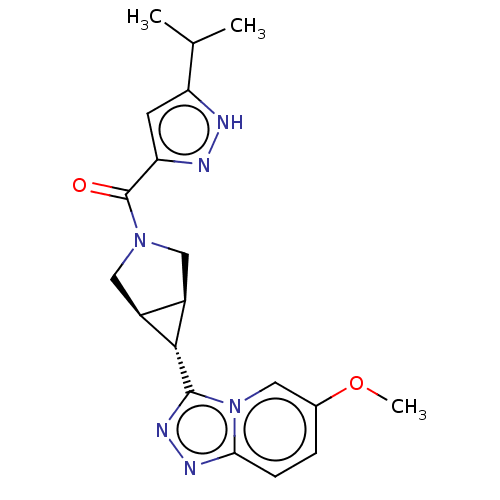
((5-isopropyl-1H-pyrazol-3-yl)[(1R,5S,6r)-6-(6-meth...)Show SMILES COc1ccc2nnc([C@@H]3[C@H]4CN(C[C@@H]34)C(=O)c3cc([nH]n3)C(C)C)n2c1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Celgene Quanticel Research, Inc.
US Patent
| |
US Patent US9828343 (2017)
BindingDB Entry DOI: 10.7270/Q2639TWH |
More data for this
Ligand-Target Pair | |
Lysine-specific demethylase 5A
(Homo sapiens (Human)) | BDBM615641
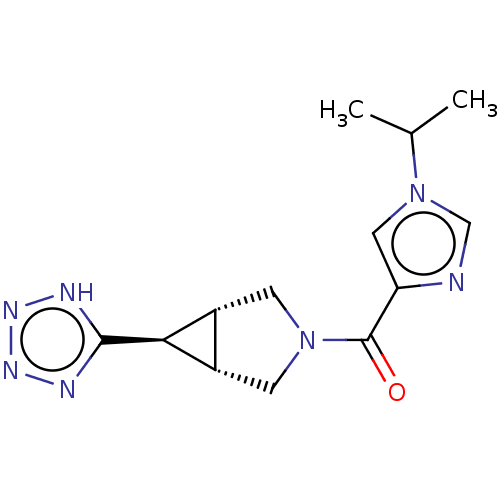
((1-isopropyl-1H-imidazol-4-yl)[(1R,5S,6r)-6-(1H-te...)Show SMILES CC(C)n1cnc(c1)C(=O)N1C[C@H]2[C@@H](C1)[C@@H]2c1nnn[nH]1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Celgene Quanticel Research, Inc.
US Patent
| |
US Patent US9828343 (2017)
BindingDB Entry DOI: 10.7270/Q2639TWH |
More data for this
Ligand-Target Pair | |
Lysine-specific demethylase 5A
(Homo sapiens (Human)) | BDBM608078

(US11691968, Example 62)Show SMILES CC(C)n1cnc(c1)C(=O)N1CC2C(C1)C2c1nnn2ccccc12 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2QJ7NC3 |
More data for this
Ligand-Target Pair | |
Lysine-specific demethylase 5A
(Homo sapiens (Human)) | BDBM615640
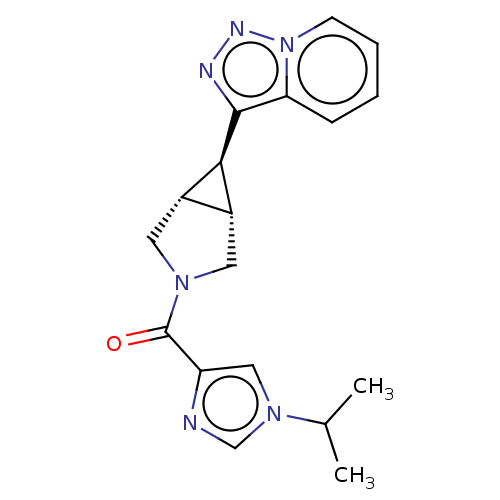
((1-isopropyl-1H-imidazol-4-yl)[(1R,5S,6r)-6-([1,2,...)Show SMILES CC(C)n1cnc(c1)C(=O)N1C[C@H]2[C@@H](C1)[C@@H]2c1nnn2ccccc12 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Celgene Quanticel Research, Inc.
US Patent
| |
US Patent US9828343 (2017)
BindingDB Entry DOI: 10.7270/Q2639TWH |
More data for this
Ligand-Target Pair | |
Lysine-specific demethylase 5A
(Homo sapiens (Human)) | BDBM608102

(US11691968, Example 131)Show SMILES COc1ccc2nnc(C3C4CN(CC34)C(=O)c3cc([nH]n3)C(C)C)n2c1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2QJ7NC3 |
More data for this
Ligand-Target Pair | |
Lysine-specific demethylase 5A
(Homo sapiens (Human)) | BDBM608079

(US11691968, Example 63) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2QJ7NC3 |
More data for this
Ligand-Target Pair | |
D-amino-acid oxidase
(Homo sapiens (Human)) | BDBM50605555
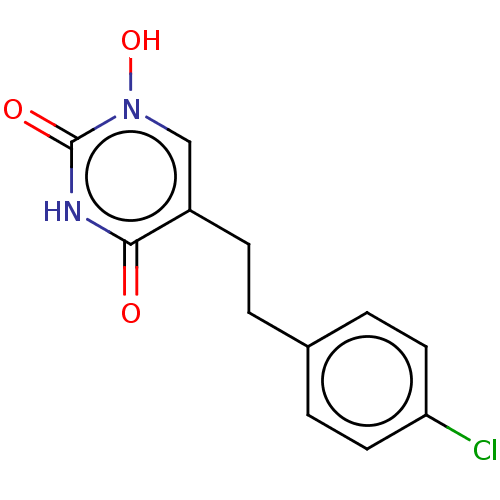
(CHEMBL5173250) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00118
BindingDB Entry DOI: 10.7270/Q2MG7TK7 |
More data for this
Ligand-Target Pair | |
Transient receptor potential cation channel subfamily V member 1
(Homo sapiens (Human)) | BDBM50391029
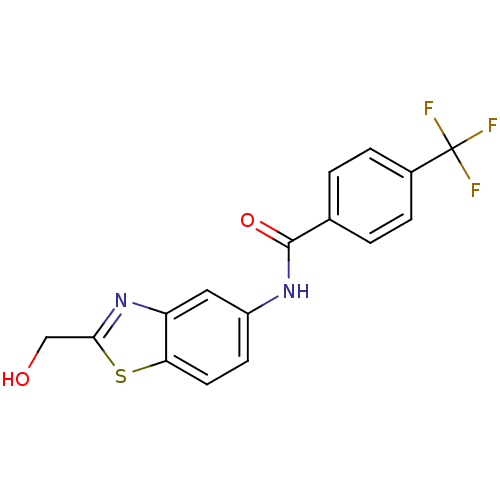
(CHEMBL2088391)Show InChI InChI=1S/C16H11F3N2O2S/c17-16(18,19)10-3-1-9(2-4-10)15(23)20-11-5-6-13-12(7-11)21-14(8-22)24-13/h1-7,22H,8H2,(H,20,23) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Antagonist activity at human recombinant TRPV1 receptor assessed as inhibition of capsaicin-induced calcium uptake by FLIPR assay |
Bioorg Med Chem Lett 22: 6205-11 (2012)
Article DOI: 10.1016/j.bmcl.2012.08.018
BindingDB Entry DOI: 10.7270/Q29C6ZGB |
More data for this
Ligand-Target Pair | |
D-amino-acid oxidase
(Homo sapiens (Human)) | BDBM50605593
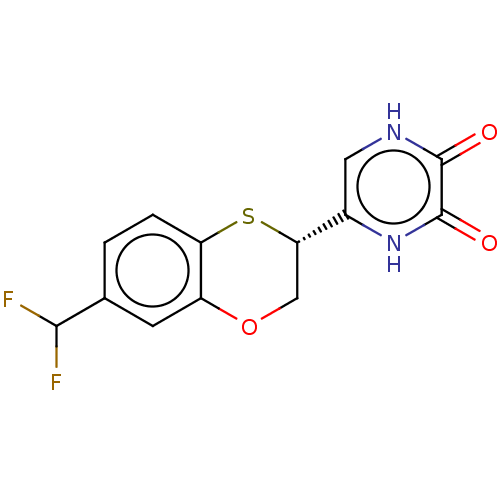
(CHEMBL5179642)Show SMILES FC(F)c1ccc2S[C@@H](COc2c1)c1c[nH]c(=O)c(=O)[nH]1 |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00118
BindingDB Entry DOI: 10.7270/Q2MG7TK7 |
More data for this
Ligand-Target Pair | |
D-amino-acid oxidase
(Homo sapiens (Human)) | BDBM210802
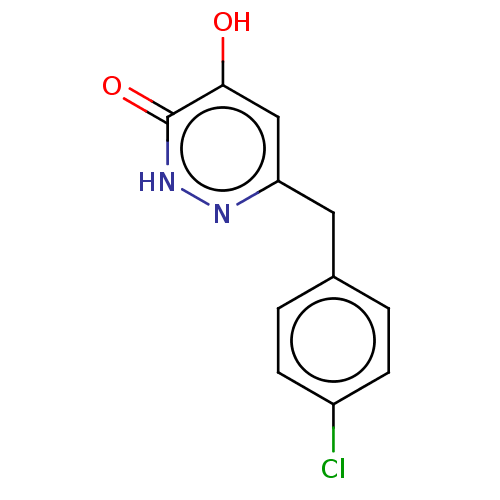
(US10463663, Example 40 | US11129828, Example 40 | ...)Show InChI InChI=1S/C11H9ClN2O2/c12-8-3-1-7(2-4-8)5-9-6-10(15)11(16)14-13-9/h1-4,6H,5H2,(H,13,15)(H,14,16) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00118
BindingDB Entry DOI: 10.7270/Q2MG7TK7 |
More data for this
Ligand-Target Pair | |
Fatty-acid amide hydrolase 1 [30-579]
(Rattus norvegicus (rat)) | BDBM26739

(3-(3-carbamoylphenyl)phenyl N-cyclohexylcarbamate ...)Show InChI InChI=1S/C20H22N2O3/c21-19(23)16-8-4-6-14(12-16)15-7-5-11-18(13-15)25-20(24)22-17-9-2-1-3-10-17/h4-8,11-13,17H,1-3,9-10H2,(H2,21,23)(H,22,24) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Cagliari
Curated by ChEMBL
| Assay Description
Inhibition of rat brain FAAH using [3H]AEA as substrate preincubated for 10 mins at pH 8 |
Eur J Med Chem 136: 523-542 (2017)
Article DOI: 10.1016/j.ejmech.2017.05.033
BindingDB Entry DOI: 10.7270/Q2H134JK |
More data for this
Ligand-Target Pair | |
Lysine-specific demethylase 5A
(Homo sapiens (Human)) | BDBM608107

(US11691968, Example 144)Show SMILES CC1(C2CN(CC12)C(=O)c1cc([nH]n1)C1CC1)C1=NOC2(CC2)C1 |t:21| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2QJ7NC3 |
More data for this
Ligand-Target Pair | |
Lysine-specific demethylase 5A
(Homo sapiens (Human)) | BDBM608089
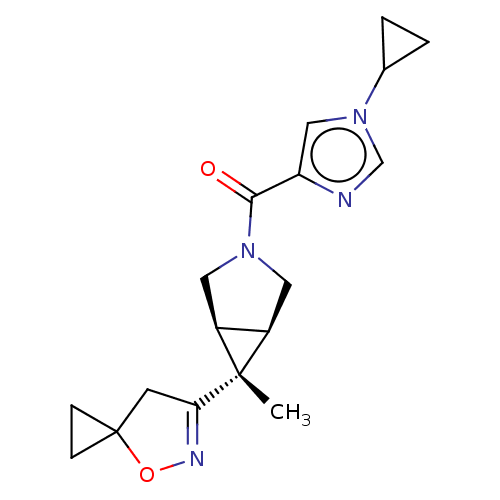
((1-cyclopropyl-1H-imidazol-4-yl)[(1R,5S,6r)-6-meth...)Show SMILES C[C@]1([C@H]2CN(C[C@@H]12)C(=O)c1cn(cn1)C1CC1)C1=NOC2(CC2)C1 |r,t:21| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2QJ7NC3 |
More data for this
Ligand-Target Pair | |
Lysine-specific demethylase 5A
(Homo sapiens (Human)) | BDBM608089

((1-cyclopropyl-1H-imidazol-4-yl)[(1R,5S,6r)-6-meth...)Show SMILES C[C@]1([C@H]2CN(C[C@@H]12)C(=O)c1cn(cn1)C1CC1)C1=NOC2(CC2)C1 |r,t:21| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Celgene Quanticel Research, Inc.
US Patent
| |
US Patent US9828343 (2017)
BindingDB Entry DOI: 10.7270/Q2639TWH |
More data for this
Ligand-Target Pair | |
Lysine-specific demethylase 5A
(Homo sapiens (Human)) | BDBM608080

(US11691968, Example 65)Show SMILES CC(C)n1cnc(c1)C(=O)N1CC2C(C1)C2C(=O)NC(C)(C)C | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2QJ7NC3 |
More data for this
Ligand-Target Pair | |
Lysine-specific demethylase 5A
(Homo sapiens (Human)) | BDBM608076
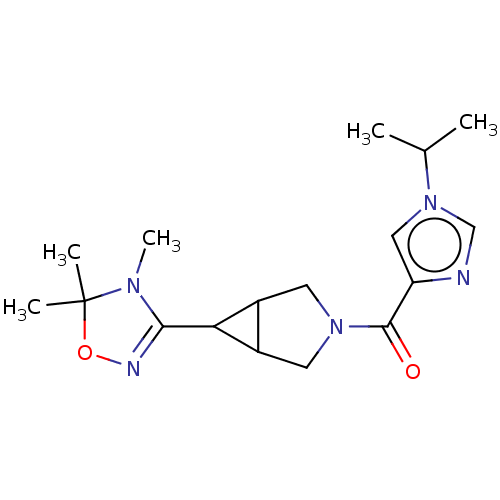
(US11691968, Example 59)Show SMILES CC(C)n1cnc(c1)C(=O)N1CC2C(C1)C2C1=NOC(C)(C)N1C |t:19| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2QJ7NC3 |
More data for this
Ligand-Target Pair | |
Lysine-specific demethylase 5A
(Homo sapiens (Human)) | BDBM608074
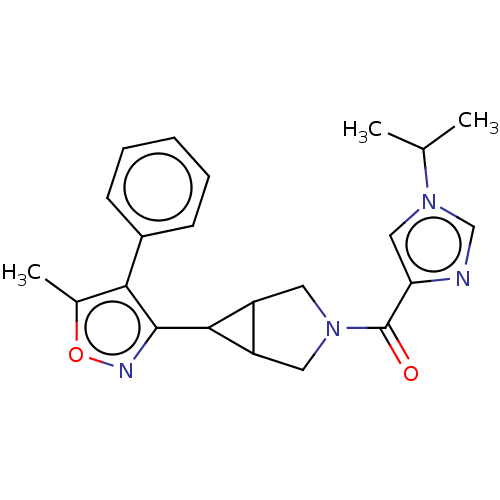
(US11691968, Example 55)Show SMILES CC(C)n1cnc(c1)C(=O)N1CC2C(C1)C2c1noc(C)c1-c1ccccc1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2QJ7NC3 |
More data for this
Ligand-Target Pair | |
Lysine-specific demethylase 5A
(Homo sapiens (Human)) | BDBM608073

((1-cyclopropyl-1H-imidazol-4-yl)[(1R,5S,6r)-6-(4-o...)Show SMILES O=C(N1C[C@H]2[C@@H](C1)[C@@H]2C1=NOC2(CC2)C1)c1cn(cn1)C1CC1 |r,t:10| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2QJ7NC3 |
More data for this
Ligand-Target Pair | |
Lysine-specific demethylase 5A
(Homo sapiens (Human)) | BDBM608065

(US11691968, Example 1 | US20230271952, Example 1 |...)Show SMILES CC(C)n1cnc(c1)C(=O)N1C[C@H]2[C@@H](C1)[C@@H]2C1=NOC(C)(C)C1 |r,t:19| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2QJ7NC3 |
More data for this
Ligand-Target Pair | |
Lysine-specific demethylase 5A
(Homo sapiens (Human)) | BDBM615669

((5-cyclopropyl-1H-pyrazol-3-yl)[(1R,5S,6r)-6-methy...)Show SMILES C[C@]1([C@H]2CN(C[C@@H]12)C(=O)c1cc([nH]n1)C1CC1)C1=NOC2(CC2)C1 |t:21| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Celgene Quanticel Research, Inc.
US Patent
| |
US Patent US9828343 (2017)
BindingDB Entry DOI: 10.7270/Q2639TWH |
More data for this
Ligand-Target Pair | |
D-amino-acid oxidase
(Homo sapiens (Human)) | BDBM50605599

(CHEMBL5178413)Show SMILES C[C@]1(COc2cc(ccc2S1)C#N)c1c[nH]c(=O)c(=O)[nH]1 |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00118
BindingDB Entry DOI: 10.7270/Q2MG7TK7 |
More data for this
Ligand-Target Pair | |
D-amino-acid oxidase
(Homo sapiens (Human)) | BDBM50605594

(CHEMBL5186374)Show SMILES O=c1[nH]cc([nH]c1=O)[C@@H]1COc2cc(ccc2S1)C#N |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00118
BindingDB Entry DOI: 10.7270/Q2MG7TK7 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Lysine-specific demethylase 5A
(Homo sapiens (Human)) | BDBM608085
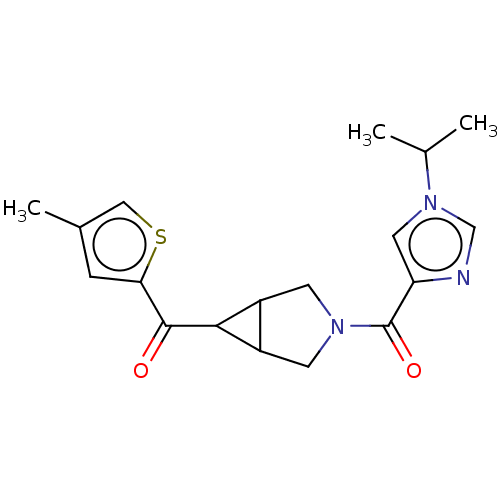
(US11691968, Example 77)Show SMILES CC(C)n1cnc(c1)C(=O)N1CC2C(C1)C2C(=O)c1cc(C)cs1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2QJ7NC3 |
More data for this
Ligand-Target Pair | |
Lysine-specific demethylase 5A
(Homo sapiens (Human)) | BDBM615647

((1-isopropyl-1H-imidazol-4-yl){(1R,5S,6r)-6-[(4-me...)Show SMILES CC(C)n1cnc(c1)C(=O)N1C[C@H]2[C@@H](C1)[C@@H]2C(=O)c1cc(C)cs1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Celgene Quanticel Research, Inc.
US Patent
| |
US Patent US9828343 (2017)
BindingDB Entry DOI: 10.7270/Q2639TWH |
More data for this
Ligand-Target Pair | |
Lysine-specific demethylase 5A
(Homo sapiens (Human)) | BDBM615642
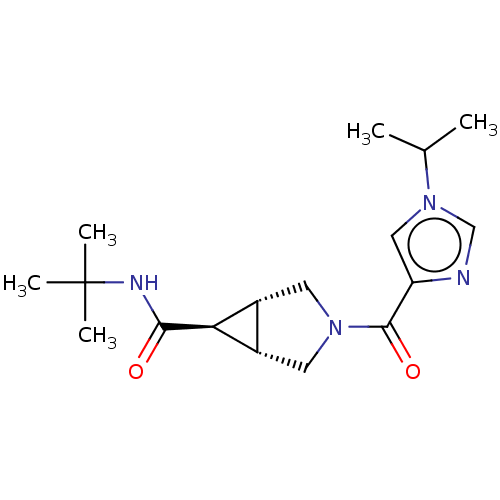
((1R,5S,6r)-N-tert-butyl-3-[1-(propan-2-yl)-1H-imid...)Show SMILES CC(C)n1cnc(c1)C(=O)N1C[C@H]2[C@@H](C1)[C@@H]2C(=O)NC(C)(C)C | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Celgene Quanticel Research, Inc.
US Patent
| |
US Patent US9828343 (2017)
BindingDB Entry DOI: 10.7270/Q2639TWH |
More data for this
Ligand-Target Pair | |
Lysine-specific demethylase 5A
(Homo sapiens (Human)) | BDBM615638

((1-isopropyl-1H-imidazol-4-yl)[(1R,5S,6r)-6-(4,5,5...)Show SMILES CC(C)n1cnc(c1)C(=O)N1C[C@H]2[C@@H](C1)[C@@H]2C1=NOC(C)(C)N1C |t:19| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Celgene Quanticel Research, Inc.
US Patent
| |
US Patent US9828343 (2017)
BindingDB Entry DOI: 10.7270/Q2639TWH |
More data for this
Ligand-Target Pair | |
Lysine-specific demethylase 5A
(Homo sapiens (Human)) | BDBM615636

((1-isopropyl-1H-imidazol-4-yl)[(1R,5S,6r)-6-(5-met...)Show SMILES CC(C)n1cnc(c1)C(=O)N1C[C@H]2[C@@H](C1)[C@@H]2c1noc(C)c1-c1ccccc1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Celgene Quanticel Research, Inc.
US Patent
| |
US Patent US9828343 (2017)
BindingDB Entry DOI: 10.7270/Q2639TWH |
More data for this
Ligand-Target Pair | |
Lysine-specific demethylase 5A
(Homo sapiens (Human)) | BDBM608073

((1-cyclopropyl-1H-imidazol-4-yl)[(1R,5S,6r)-6-(4-o...)Show SMILES O=C(N1C[C@H]2[C@@H](C1)[C@@H]2C1=NOC2(CC2)C1)c1cn(cn1)C1CC1 |r,t:10| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Celgene Quanticel Research, Inc.
US Patent
| |
US Patent US9828343 (2017)
BindingDB Entry DOI: 10.7270/Q2639TWH |
More data for this
Ligand-Target Pair | |
Lysine-specific demethylase 5A
(Homo sapiens (Human)) | BDBM608065

(US11691968, Example 1 | US20230271952, Example 1 |...)Show SMILES CC(C)n1cnc(c1)C(=O)N1C[C@H]2[C@@H](C1)[C@@H]2C1=NOC(C)(C)C1 |r,t:19| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Celgene Quanticel Research, Inc.
US Patent
| |
US Patent US9828343 (2017)
BindingDB Entry DOI: 10.7270/Q2639TWH |
More data for this
Ligand-Target Pair | |
D-amino-acid oxidase
(Homo sapiens (Human)) | BDBM50605592

(CHEMBL5179643)Show SMILES Clc1ccc2S[C@@H](COc2c1)c1c[nH]c(=O)c(=O)[nH]1 |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 21 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00118
BindingDB Entry DOI: 10.7270/Q2MG7TK7 |
More data for this
Ligand-Target Pair | |
D-amino-acid oxidase
(Homo sapiens (Human)) | BDBM50605595

(CHEMBL5174518)Show SMILES CS(=O)(=O)c1ccc2S[C@@H](COc2c1)c1c[nH]c(=O)c(=O)[nH]1 |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00118
BindingDB Entry DOI: 10.7270/Q2MG7TK7 |
More data for this
Ligand-Target Pair | |
D-amino-acid oxidase
(Homo sapiens (Human)) | BDBM50605589
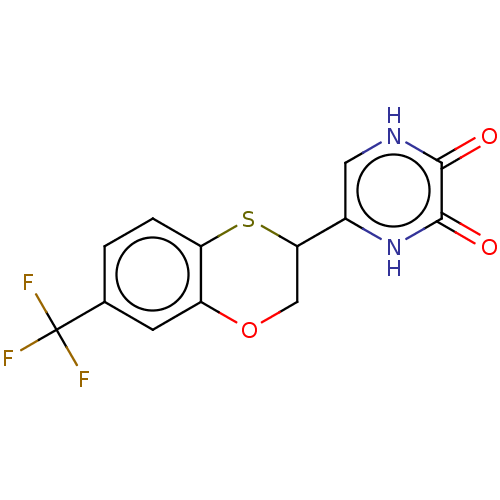
(CHEMBL5196542)Show SMILES FC(F)(F)c1ccc2SC(COc2c1)c1c[nH]c(=O)c(=O)[nH]1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00118
BindingDB Entry DOI: 10.7270/Q2MG7TK7 |
More data for this
Ligand-Target Pair | |
Transient receptor potential cation channel subfamily V member 1
(Homo sapiens (Human)) | BDBM50391025
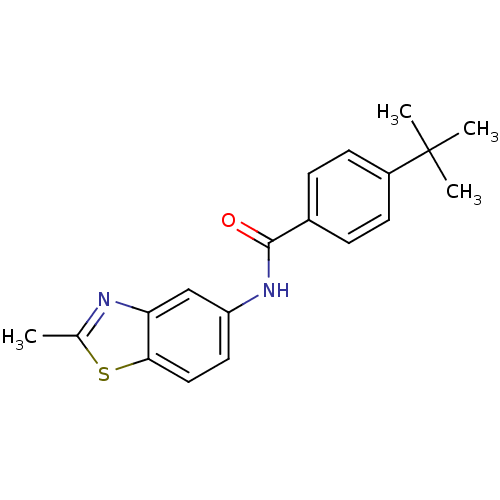
(CHEMBL2088398)Show InChI InChI=1S/C19H20N2OS/c1-12-20-16-11-15(9-10-17(16)23-12)21-18(22)13-5-7-14(8-6-13)19(2,3)4/h5-11H,1-4H3,(H,21,22) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 27 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Antagonist activity at human recombinant TRPV1 receptor assessed as inhibition of capsaicin-induced calcium uptake by FLIPR assay |
Bioorg Med Chem Lett 22: 6205-11 (2012)
Article DOI: 10.1016/j.bmcl.2012.08.018
BindingDB Entry DOI: 10.7270/Q29C6ZGB |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data