

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Angiotensin-converting enzyme (Homo sapiens (Human)) | BDBM50017125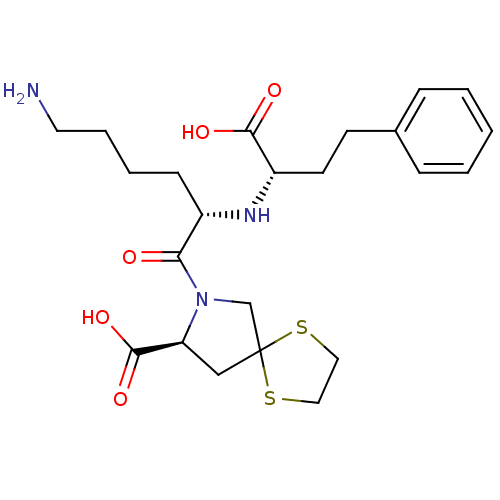 (1-[6-Amino-2-(1-carboxy-3-phenyl-propylamino)-hexa...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Corporation Curated by ChEMBL | Assay Description Compound tested in vitro for inhibition of Angiotensin I converting enzyme | J Med Chem 32: 1600-6 (1989) BindingDB Entry DOI: 10.7270/Q2M61KVW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Homo sapiens (Human)) | BDBM50017122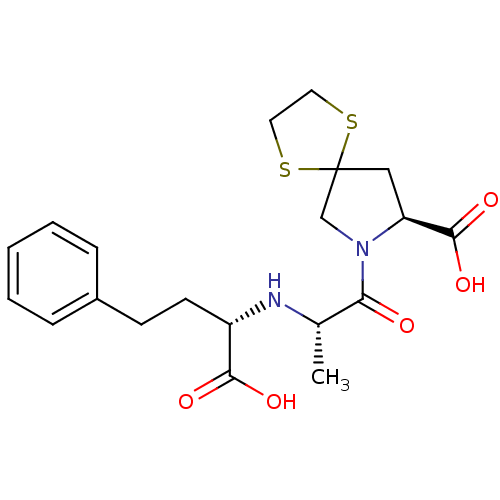 (7-[2-(1-Carboxy-3-phenyl-propylamino)-propionyl]-1...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Corporation Curated by ChEMBL | Assay Description Compound tested in vitro for inhibition of Angiotensin I converting enzyme | J Med Chem 32: 1600-6 (1989) BindingDB Entry DOI: 10.7270/Q2M61KVW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Rattus norvegicus) | BDBM50017122 (7-[2-(1-Carboxy-3-phenyl-propylamino)-propionyl]-1...) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Corporation Curated by ChEMBL | Assay Description Compound tested in vivo for inhibition of Angiotensin I converting enzyme in rat | J Med Chem 32: 1600-6 (1989) BindingDB Entry DOI: 10.7270/Q2M61KVW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Homo sapiens (Human)) | BDBM50367254 (ENALAPRILAT) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE KEGG MMDB PC cid PC sid PDB UniChem Similars | PDB PubMed | n/a | n/a | 2.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Corporation Curated by ChEMBL | Assay Description Compound tested in vitro for inhibition of Angiotensin I converting enzyme | J Med Chem 32: 1600-6 (1989) BindingDB Entry DOI: 10.7270/Q2M61KVW | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Angiotensin-converting enzyme (Homo sapiens (Human)) | BDBM21642 ((2S)-1-[(2S)-2-methyl-3-sulfanylpropanoyl]pyrrolid...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank PDB PubMed | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Corporation Curated by ChEMBL | Assay Description Compound tested in vitro for inhibition of Angiotensin I converting enzyme | J Med Chem 32: 1600-6 (1989) BindingDB Entry DOI: 10.7270/Q2M61KVW | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Angiotensin-converting enzyme (Oryctolagus cuniculus) | BDBM21642 ((2S)-1-[(2S)-2-methyl-3-sulfanylpropanoyl]pyrrolid...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PubMed | n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Corporation Curated by ChEMBL | Assay Description In vitro 50% inhibition of Angiotensin I converting enzyme | J Med Chem 31: 875-85 (1988) BindingDB Entry DOI: 10.7270/Q2GT5NRV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Rattus norvegicus) | BDBM50017124 (7-[2-(1-Ethoxycarbonyl-3-phenyl-propylamino)-propi...) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 67 | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Corporation Curated by ChEMBL | Assay Description Compound tested in vitro for inhibition of Angiotensin I converting enzyme | J Med Chem 32: 1600-6 (1989) BindingDB Entry DOI: 10.7270/Q2M61KVW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Oryctolagus cuniculus) | BDBM50018792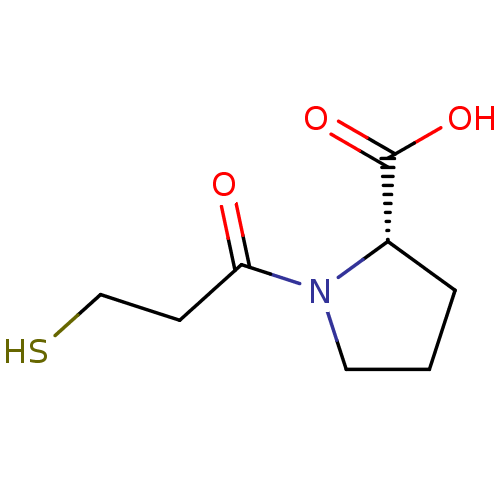 ((L)-1-(3-Mercapto-propionyl)-pyrrolidine-2-carboxy...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Corporation Curated by ChEMBL | Assay Description In vitro inhibition of Angiotensin I converting enzyme with relative to captopril(=1) | J Med Chem 31: 875-85 (1988) BindingDB Entry DOI: 10.7270/Q2GT5NRV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Homo sapiens (Human)) | BDBM50017126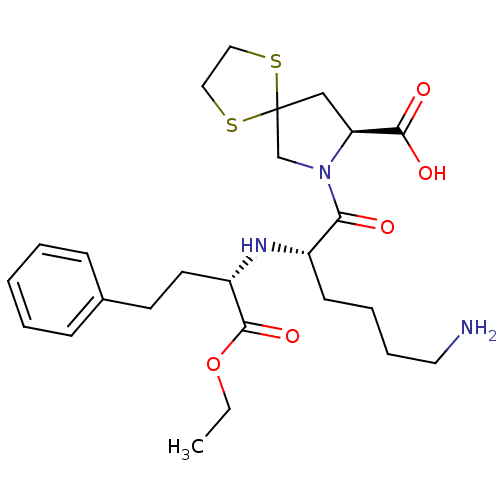 (7-[6-Amino-2-(1-ethoxycarbonyl-3-phenyl-propylamin...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Corporation Curated by ChEMBL | Assay Description Compound tested in vitro for inhibition of Angiotensin I converting enzyme | J Med Chem 32: 1600-6 (1989) BindingDB Entry DOI: 10.7270/Q2M61KVW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Homo sapiens (Human)) | BDBM50017129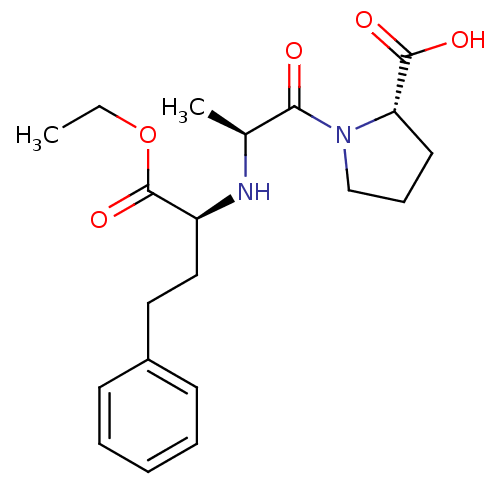 ((S)-1-((S)-2-((R)-1-ethoxy-1-oxo-4-phenylbutan-2-y...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid UniChem Patents Similars | DrugBank PubMed | n/a | n/a | 1.27E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Corporation Curated by ChEMBL | Assay Description Compound tested in vitro for inhibition of Angiotensin I converting enzyme | J Med Chem 32: 1600-6 (1989) BindingDB Entry DOI: 10.7270/Q2M61KVW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||