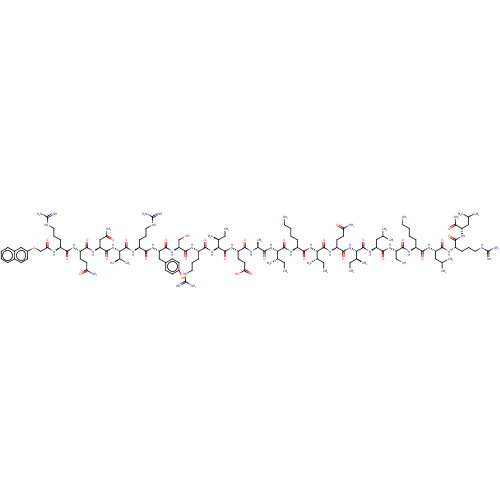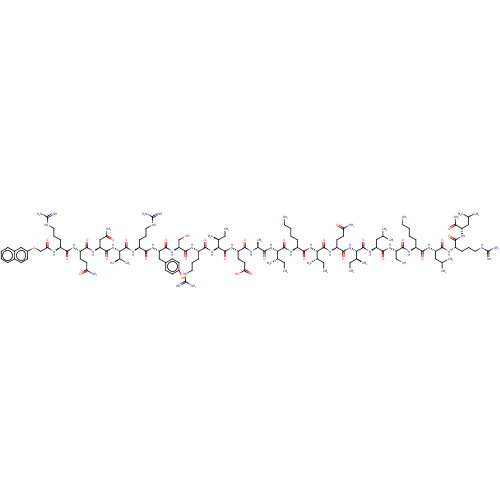Found 47 hits with Last Name = 'taguchi' and Initial = 'a'
Found 47 hits with Last Name = 'taguchi' and Initial = 'a' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Growth/differentiation factor 8
(Homo sapiens (Human)) | BDBM50608178
(CHEMBL5268044)Show SMILES CC[C@@H](C)[C@@H](NC(=O)[C@@H](CCCCN)NC(=O)[C@H](NC(=O)[C@@H](CCCNC(N)=N)NC(=O)[C@H](NC(=O)[C@@H](Cc1ccc2ccccc2c1)NC(=O)[C@@H](CCCNC(N)=N)NC(=O)[C@@H](CCCCN)NC(=O)[C@H](NC(=O)[C@@H](CCCNC(N)=N)NC(=O)[C@@H](CC(C)C)NC(=O)CCc1cn(CCN2CCCc3cc(\C=C\C4=C(Br)C(\C=C\c5cc6CCCN7CCCc(c5)c67)=[O+][B-](F)(O4)C(F)(F)F)ccc23)nn1)C1CCCCC1)[C@H](C)CC)C1CCCCC1)C(=O)N[C@H](CCc1ccccc1)C(=O)N[C@H](CCCNC(N)=N)C(=O)N[C@H]([C@H](C)CC)C(=O)N[C@H](Cc1ccc(O)cc1)C(=O)N[C@H](CCc1ccccc1)C(N)=O |r,c:112,133| | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| UniChem
| | n/a | n/a | 0.890 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Growth/differentiation factor 8
(Homo sapiens (Human)) | BDBM50608179
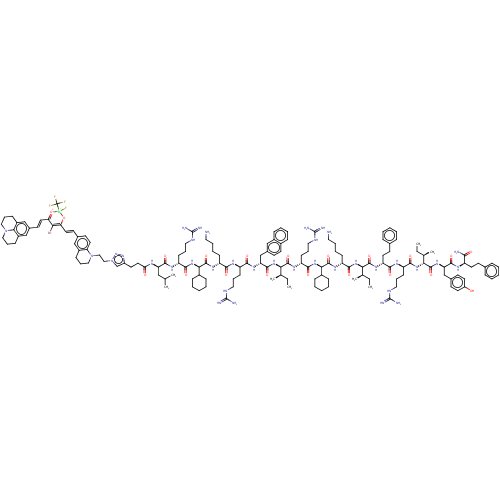
(CHEMBL5285634)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@H](C)NC(=O)[C@H](CCC(O)=O)NC(=O)[C@@H](NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](CO)NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@@H](NC(=O)[C@H](CC(N)=O)NC(=O)[C@H](CCC(N)=O)NC(=O)[C@H](CCCNC(N)=N)NC(=O)CC(c1ccccc1)c1ccccc1)[C@@H](C)O)[C@@H](C)CC)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H]([C@@H](C)CC)C(=O)N[C@@H](Cc1cn(CCN2CCCc3cc(\C=C\C4=C(Br)C(\C=C\c5cc6CCCN7CCCc(c5)c67)=[O+][B-](F)(O4)C(F)(F)F)ccc23)nn1)C(=O)N[C@@H]([C@@H](C)CC)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CO)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CC(C)C)C(O)=O |r,c:158,179| | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| UniChem
| | n/a | n/a | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Growth/differentiation factor 8
(Homo sapiens (Human)) | BDBM50539387

(CHEMBL4648474)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@H](C)NC(=O)[C@H](CCC(O)=O)NC(=O)[C@@H](NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](CO)NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@@H](NC(=O)[C@H](CC(N)=O)NC(=O)[C@H](CCC(N)=O)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@@H](N)Cc1c[nH]c2ccccc12)[C@@H](C)O)[C@@H](C)CC)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H]([C@@H](C)CC)C(=O)N[C@@H](CCC(N)=O)C(=O)N[C@@H]([C@@H](C)CC)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CO)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CCCNC(N)=N)C(N)=O |r| Show InChI InChI=1S/C124H211N41O31/c1-15-63(9)94(116(192)151-77(32-22-24-48-126)107(183)162-95(64(10)16-2)117(193)153-82(42-45-91(129)171)109(185)164-97(66(12)18-4)119(195)158-85(54-62(7)8)111(187)159-88(59-166)114(190)148-76(31-21-23-47-125)104(180)155-84(53-61(5)6)110(186)146-75(99(131)175)33-25-49-140-121(132)133)161-100(176)67(13)145-102(178)83(43-46-93(173)174)154-118(194)96(65(11)17-3)163-108(184)80(36-28-52-143-124(138)139)149-115(191)89(60-167)160-112(188)86(55-69-37-39-71(169)40-38-69)156-105(181)79(35-27-51-142-123(136)137)152-120(196)98(68(14)168)165-113(189)87(57-92(130)172)157-106(182)81(41-44-90(128)170)150-103(179)78(34-26-50-141-122(134)135)147-101(177)73(127)56-70-58-144-74-30-20-19-29-72(70)74/h19-20,29-30,37-40,58,61-68,73,75-89,94-98,144,166-169H,15-18,21-28,31-36,41-57,59-60,125-127H2,1-14H3,(H2,128,170)(H2,129,171)(H2,130,172)(H2,131,175)(H,145,178)(H,146,186)(H,147,177)(H,148,190)(H,149,191)(H,150,179)(H,151,192)(H,152,196)(H,153,193)(H,154,194)(H,155,180)(H,156,181)(H,157,182)(H,158,195)(H,159,187)(H,160,188)(H,161,176)(H,162,183)(H,163,184)(H,164,185)(H,165,189)(H,173,174)(H4,132,133,140)(H4,134,135,141)(H4,136,137,142)(H4,138,139,143)/t63-,64-,65-,66-,67-,68+,73-,75-,76-,77-,78-,79-,80-,81-,82-,83-,84-,85-,86-,87-,88-,89-,94-,95-,96-,97-,98-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 130 | n/a | n/a | n/a | n/a | n/a | n/a |
Tokyo University of Pharmacy and Life Sciences
Curated by ChEMBL
| Assay Description
Inhibition of myostatin (unknown origin) expressed in HEK293 cells incubated for 4 hrs by dual luciferase reporter gene assay |
Bioorg Med Chem Lett 30: (2020)
Article DOI: 10.1016/j.bmcl.2019.126892
BindingDB Entry DOI: 10.7270/Q2G73J8Q |
More data for this
Ligand-Target Pair | |
Growth/differentiation factor 8
(Homo sapiens (Human)) | BDBM50525413

(CHEMBL4448117)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@H](CCC(N)=O)NC(=O)[C@@H](NC(=O)[C@H](CCCCN)NC(=O)[C@@H](NC(=O)[C@@H](Cc1c[nH]c2ccccc12)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@@H](NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@@H](N)Cc1c[nH]c2ccccc12)[C@@H](C)CC)[C@@H](C)CC)C1CCCCC1)C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)N[C@@H](CO)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](C1CCCCC1)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CC(C)C)C(N)=O |r| Show InChI InChI=1S/C114H173N29O19/c1-9-64(6)92(140-105(155)87(55-67-44-46-73(145)47-45-67)135-98(148)77(117)56-70-59-126-78-37-21-18-34-74(70)78)109(159)131-84(43-29-53-125-114(122)123)100(150)136-89(58-72-61-128-80-39-23-20-36-76(72)80)106(156)141-93(65(7)10-2)108(158)130-82(41-25-27-51-116)102(152)143-96(69-32-16-13-17-33-69)112(162)133-85(48-49-91(118)146)103(153)139-94(66(8)11-3)110(160)137-88(57-71-60-127-79-38-22-19-35-75(71)79)104(154)138-90(62-144)107(157)129-81(40-24-26-50-115)101(151)142-95(68-30-14-12-15-31-68)111(161)132-83(42-28-52-124-113(120)121)99(149)134-86(97(119)147)54-63(4)5/h18-23,34-39,44-47,59-61,63-66,68-69,77,81-90,92-96,126-128,144-145H,9-17,24-33,40-43,48-58,62,115-117H2,1-8H3,(H2,118,146)(H2,119,147)(H,129,157)(H,130,158)(H,131,159)(H,132,161)(H,133,162)(H,134,149)(H,135,148)(H,136,150)(H,137,160)(H,138,154)(H,139,153)(H,140,155)(H,141,156)(H,142,151)(H,143,152)(H4,120,121,124)(H4,122,123,125)/t64-,65-,66-,77-,81-,82-,83-,84-,85-,86-,87-,88-,89+,90-,92-,93-,94-,95-,96-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 130 | n/a | n/a | n/a | n/a | n/a | n/a |
Tokyo University of Pharmacy and Life Sciences
Curated by ChEMBL
| Assay Description
Inhibition of myostatin (unknown origin) expressed in HEK293 cells incubated for 4 hrs by dual luciferase reporter gene assay |
ACS Med Chem Lett 10: 985-990 (2019)
Article DOI: 10.1021/acsmedchemlett.9b00174
BindingDB Entry DOI: 10.7270/Q2V1287X |
More data for this
Ligand-Target Pair | |
Growth/differentiation factor 8
(Homo sapiens (Human)) | BDBM50585028
(CHEMBL5078185)Show SMILES CC[C@@H](C)[C@@H](NC(=O)[C@@H](Cc1c[nH]c2ccccc12)NC(=O)[C@@H](CCCNC(N)=N)NC(=O)[C@@H](CCCCN)NC(=O)[C@H](NC(=O)[C@@H](CCCNC(N)=N)NC(=O)[C@H](N)CC(C)C)C1CCCCC1)C(=O)N[C@H](CCCNC(N)=N)C(=O)N[C@H](C1CCCCC1)C(=O)N[C@H](CCCCN)C(=O)N[C@H]([C@H](C)CC)C(=O)N[C@H](Cc1c[nH]c2ccccc12)C(=O)N[C@H](CCCNC(N)=N)C(=O)N[C@H]([C@H](C)CC)C(=O)N[C@H](Cc1ccc(O)cc1)C(=O)N[C@H](Cc1c[nH]c2ccccc12)C(N)=O |r| | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 190 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Growth/differentiation factor 8
(Homo sapiens (Human)) | BDBM50585028

(CHEMBL5078185)Show SMILES CC[C@@H](C)[C@@H](NC(=O)[C@@H](Cc1c[nH]c2ccccc12)NC(=O)[C@@H](CCCNC(N)=N)NC(=O)[C@@H](CCCCN)NC(=O)[C@H](NC(=O)[C@@H](CCCNC(N)=N)NC(=O)[C@H](N)CC(C)C)C1CCCCC1)C(=O)N[C@H](CCCNC(N)=N)C(=O)N[C@H](C1CCCCC1)C(=O)N[C@H](CCCCN)C(=O)N[C@H]([C@H](C)CC)C(=O)N[C@H](Cc1c[nH]c2ccccc12)C(=O)N[C@H](CCCNC(N)=N)C(=O)N[C@H]([C@H](C)CC)C(=O)N[C@H](Cc1ccc(O)cc1)C(=O)N[C@H](Cc1c[nH]c2ccccc12)C(N)=O |r| | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 190 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of myostatin (unknown origin) expressed in HEK293 cells transfected with Smad2/3 responsive reporter plasmid incubated for 4 hrs by dual l... |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.1c00705
BindingDB Entry DOI: 10.7270/Q28S4TT7 |
More data for this
Ligand-Target Pair | |
Growth/differentiation factor 8
(Homo sapiens (Human)) | BDBM50525413

(CHEMBL4448117)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@H](CCC(N)=O)NC(=O)[C@@H](NC(=O)[C@H](CCCCN)NC(=O)[C@@H](NC(=O)[C@@H](Cc1c[nH]c2ccccc12)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@@H](NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@@H](N)Cc1c[nH]c2ccccc12)[C@@H](C)CC)[C@@H](C)CC)C1CCCCC1)C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)N[C@@H](CO)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](C1CCCCC1)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CC(C)C)C(N)=O |r| Show InChI InChI=1S/C114H173N29O19/c1-9-64(6)92(140-105(155)87(55-67-44-46-73(145)47-45-67)135-98(148)77(117)56-70-59-126-78-37-21-18-34-74(70)78)109(159)131-84(43-29-53-125-114(122)123)100(150)136-89(58-72-61-128-80-39-23-20-36-76(72)80)106(156)141-93(65(7)10-2)108(158)130-82(41-25-27-51-116)102(152)143-96(69-32-16-13-17-33-69)112(162)133-85(48-49-91(118)146)103(153)139-94(66(8)11-3)110(160)137-88(57-71-60-127-79-38-22-19-35-75(71)79)104(154)138-90(62-144)107(157)129-81(40-24-26-50-115)101(151)142-95(68-30-14-12-15-31-68)111(161)132-83(42-28-52-124-113(120)121)99(149)134-86(97(119)147)54-63(4)5/h18-23,34-39,44-47,59-61,63-66,68-69,77,81-90,92-96,126-128,144-145H,9-17,24-33,40-43,48-58,62,115-117H2,1-8H3,(H2,118,146)(H2,119,147)(H,129,157)(H,130,158)(H,131,159)(H,132,161)(H,133,162)(H,134,149)(H,135,148)(H,136,150)(H,137,160)(H,138,154)(H,139,153)(H,140,155)(H,141,156)(H,142,151)(H,143,152)(H4,120,121,124)(H4,122,123,125)/t64-,65-,66-,77-,81-,82-,83-,84-,85-,86-,87-,88-,89+,90-,92-,93-,94-,95-,96-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 260 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of myostatin (unknown origin) expressed in HEK293 cells transfected with Smad2/3 responsive reporter plasmid incubated for 4 hrs by dual l... |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.1c00705
BindingDB Entry DOI: 10.7270/Q28S4TT7 |
More data for this
Ligand-Target Pair | |
Growth/differentiation factor 8
(Homo sapiens (Human)) | BDBM50537170
(CHEMBL4516740)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@H](CCCCN)NC(=O)[C@@H](NC(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)[C@H](CCC(O)=O)NC(=O)[C@@H](NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@@H]1COC(=O)CCCC(=O)OC[C@H](NC(=O)[C@H](CCC(N)=O)NC(=O)[C@H](CCCNC(N)=N)NC(=O)COc2ccc3ccccc3c2)C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](Cc2ccc(O)cc2)C(=O)N1)[C@@H](C)CC)[C@@H](C)CC)C(=O)N[C@@H](CCC(N)=O)C(=O)N[C@@H]([C@@H](C)CC)C(=O)N[C@@H]([C@@H](C)CC)C(=O)N[C@@H](CO)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CC(C)C)C(N)=O |r| Show InChI InChI=1S/C143H228N40O35/c1-16-76(10)112(135(211)169-97(53-56-107(147)188)127(203)180-116(80(14)20-5)138(214)182-115(79(13)19-4)137(213)175-103(70-184)131(207)164-91(38-25-27-58-144)121(197)172-100(65-75(8)9)128(204)163-93(41-31-61-158-141(151)152)120(196)171-99(118(148)194)64-74(6)7)178-125(201)92(39-26-28-59-145)167-134(210)114(78(12)18-3)181-130(206)102(68-85-69-161-89-37-24-23-36-88(85)89)174-123(199)98(54-57-109(190)191)170-136(212)113(77(11)17-2)179-126(202)95(43-33-63-160-143(155)156)165-132(208)104-71-217-110(192)44-29-45-111(193)218-72-105(133(209)183-117(81(15)185)139(215)168-94(42-32-62-159-142(153)154)122(198)173-101(129(205)177-104)66-82-46-49-86(186)50-47-82)176-124(200)96(52-55-106(146)187)166-119(195)90(40-30-60-157-140(149)150)162-108(189)73-216-87-51-48-83-34-21-22-35-84(83)67-87/h21-24,34-37,46-51,67,69,74-81,90-105,112-117,161,184-186H,16-20,25-33,38-45,52-66,68,70-73,144-145H2,1-15H3,(H2,146,187)(H2,147,188)(H2,148,194)(H,162,189)(H,163,204)(H,164,207)(H,165,208)(H,166,195)(H,167,210)(H,168,215)(H,169,211)(H,170,212)(H,171,196)(H,172,197)(H,173,198)(H,174,199)(H,175,213)(H,176,200)(H,177,205)(H,178,201)(H,179,202)(H,180,203)(H,181,206)(H,182,214)(H,183,209)(H,190,191)(H4,149,150,157)(H4,151,152,158)(H4,153,154,159)(H4,155,156,160)/t76-,77-,78-,79-,80-,81+,90-,91-,92-,93-,94-,95-,96-,97-,98-,99-,100-,101-,102-,103-,104-,105-,112-,113-,114-,115-,116-,117-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 260 | n/a | n/a | n/a | n/a | n/a | n/a |
Tokyo University of Pharmacy and Life Sciences
Curated by ChEMBL
| Assay Description
Inhibition of myostatin (unknown origin) expressed in HEK293 cells after 4 hrs by dual luciferase reporter gene assay |
Bioorg Med Chem 27: 1437-1443 (2019)
Article DOI: 10.1016/j.bmc.2019.02.019
BindingDB Entry DOI: 10.7270/Q2W099FN |
More data for this
Ligand-Target Pair | |
Growth/differentiation factor 8
(Homo sapiens (Human)) | BDBM50254771

(CHEMBL4061416)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@H](CCCCN)NC(=O)[C@@H](NC(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)[C@H](CCC(O)=O)NC(=O)[C@@H](NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](CO)NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@@H](NC(=O)[C@H](CC(N)=O)NC(=O)[C@H](CCC(N)=O)NC(=O)[C@H](CCCNC(N)=N)NC(=O)COc1ccc2ccccc2c1)[C@@H](C)O)[C@@H](C)CC)[C@@H](C)CC)C(=O)N[C@@H](CCC(N)=O)C(=O)N[C@@H]([C@@H](C)CC)C(=O)N[C@@H]([C@@H](C)CC)C(=O)N[C@@H](CO)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CC(C)C)C(N)=O |r| Show InChI InChI=1S/C139H225N41O33/c1-16-73(10)108(131(208)166-94(50-53-104(143)186)123(200)177-112(77(14)20-5)134(211)179-111(76(13)19-4)133(210)174-102(69-182)129(206)161-88(37-25-27-55-140)117(194)169-97(62-72(8)9)124(201)160-90(40-30-58-155-137(148)149)116(193)168-96(114(145)191)61-71(6)7)175-121(198)89(38-26-28-56-141)164-130(207)110(75(12)18-3)178-126(203)99(65-82-67-158-86-36-24-23-35-85(82)86)171-120(197)95(51-54-107(189)190)167-132(209)109(74(11)17-2)176-122(199)92(42-32-60-157-139(152)153)162-128(205)101(68-181)173-125(202)98(63-79-43-46-83(184)47-44-79)170-118(195)91(41-31-59-156-138(150)151)165-135(212)113(78(15)183)180-127(204)100(66-105(144)187)172-119(196)93(49-52-103(142)185)163-115(192)87(39-29-57-154-136(146)147)159-106(188)70-213-84-48-45-80-33-21-22-34-81(80)64-84/h21-24,33-36,43-48,64,67,71-78,87-102,108-113,158,181-184H,16-20,25-32,37-42,49-63,65-66,68-70,140-141H2,1-15H3,(H2,142,185)(H2,143,186)(H2,144,187)(H2,145,191)(H,159,188)(H,160,201)(H,161,206)(H,162,205)(H,163,192)(H,164,207)(H,165,212)(H,166,208)(H,167,209)(H,168,193)(H,169,194)(H,170,195)(H,171,197)(H,172,196)(H,173,202)(H,174,210)(H,175,198)(H,176,199)(H,177,200)(H,178,203)(H,179,211)(H,180,204)(H,189,190)(H4,146,147,154)(H4,148,149,155)(H4,150,151,156)(H4,152,153,157)/t73-,74-,75-,76-,77-,78+,87-,88-,89-,90-,91-,92-,93-,94-,95-,96-,97-,98-,99-,100-,101-,102-,108-,109-,110-,111-,112-,113-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 290 | n/a | n/a | n/a | n/a | n/a | n/a |
Tokyo University of Pharmacy and Life Sciences
Curated by ChEMBL
| Assay Description
Inhibition of myostatin (unknown origin) expressed in HEK293 cells incubated for 4 hrs by dual luciferase reporter gene assay |
ACS Med Chem Lett 10: 985-990 (2019)
Article DOI: 10.1021/acsmedchemlett.9b00174
BindingDB Entry DOI: 10.7270/Q2V1287X |
More data for this
Ligand-Target Pair | |
Growth/differentiation factor 8
(Homo sapiens (Human)) | BDBM50585029
(CHEMBL5092917)Show SMILES CC[C@@H](C)[C@@H](NC(=O)[C@@H](Cc1c[nH]c2ccccc12)NC(=O)[C@@H](CCCNC(N)=N)NC(=O)[C@@H](CCCCN)NC(=O)[C@H](NC(=O)[C@H](N)CCCNC(N)=N)C1CCCCC1)C(=O)N[C@H](CCCNC(N)=N)C(=O)N[C@@H](C1CCCCC1)C(=O)N[C@H](CCCCN)C(=O)N[C@H]([C@H](C)CC)C(=O)N[C@H](Cc1c[nH]c2ccccc12)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@H]([C@H](C)CC)C(=O)N[C@H](Cc1ccc(O)cc1)C(=O)N[C@H](Cc1c[nH]c2ccccc12)C(N)=O |r| | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 300 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of myostatin (unknown origin) expressed in HEK293 cells transfected with Smad2/3 responsive reporter plasmid incubated for 4 hrs by dual l... |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.1c00705
BindingDB Entry DOI: 10.7270/Q28S4TT7 |
More data for this
Ligand-Target Pair | |
Growth/differentiation factor 8
(Homo sapiens (Human)) | BDBM50254771

(CHEMBL4061416)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@H](CCCCN)NC(=O)[C@@H](NC(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)[C@H](CCC(O)=O)NC(=O)[C@@H](NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](CO)NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@@H](NC(=O)[C@H](CC(N)=O)NC(=O)[C@H](CCC(N)=O)NC(=O)[C@H](CCCNC(N)=N)NC(=O)COc1ccc2ccccc2c1)[C@@H](C)O)[C@@H](C)CC)[C@@H](C)CC)C(=O)N[C@@H](CCC(N)=O)C(=O)N[C@@H]([C@@H](C)CC)C(=O)N[C@@H]([C@@H](C)CC)C(=O)N[C@@H](CO)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CC(C)C)C(N)=O |r| Show InChI InChI=1S/C139H225N41O33/c1-16-73(10)108(131(208)166-94(50-53-104(143)186)123(200)177-112(77(14)20-5)134(211)179-111(76(13)19-4)133(210)174-102(69-182)129(206)161-88(37-25-27-55-140)117(194)169-97(62-72(8)9)124(201)160-90(40-30-58-155-137(148)149)116(193)168-96(114(145)191)61-71(6)7)175-121(198)89(38-26-28-56-141)164-130(207)110(75(12)18-3)178-126(203)99(65-82-67-158-86-36-24-23-35-85(82)86)171-120(197)95(51-54-107(189)190)167-132(209)109(74(11)17-2)176-122(199)92(42-32-60-157-139(152)153)162-128(205)101(68-181)173-125(202)98(63-79-43-46-83(184)47-44-79)170-118(195)91(41-31-59-156-138(150)151)165-135(212)113(78(15)183)180-127(204)100(66-105(144)187)172-119(196)93(49-52-103(142)185)163-115(192)87(39-29-57-154-136(146)147)159-106(188)70-213-84-48-45-80-33-21-22-34-81(80)64-84/h21-24,33-36,43-48,64,67,71-78,87-102,108-113,158,181-184H,16-20,25-32,37-42,49-63,65-66,68-70,140-141H2,1-15H3,(H2,142,185)(H2,143,186)(H2,144,187)(H2,145,191)(H,159,188)(H,160,201)(H,161,206)(H,162,205)(H,163,192)(H,164,207)(H,165,212)(H,166,208)(H,167,209)(H,168,193)(H,169,194)(H,170,195)(H,171,197)(H,172,196)(H,173,202)(H,174,210)(H,175,198)(H,176,199)(H,177,200)(H,178,203)(H,179,211)(H,180,204)(H,189,190)(H4,146,147,154)(H4,148,149,155)(H4,150,151,156)(H4,152,153,157)/t73-,74-,75-,76-,77-,78+,87-,88-,89-,90-,91-,92-,93-,94-,95-,96-,97-,98-,99-,100-,101-,102-,108-,109-,110-,111-,112-,113-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 320 | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Medicinal Chemistry and Department of Drug Delivery and Molecular Biopharmaceutics, Tokyo University of Pharmacy and Life Sciences, Hachioji, Tokyo 192-0392, Japan.
Curated by ChEMBL
| Assay Description
Inhibition of recombinant myostatin (unknown origin) expressed in HEK293 cells after 4 hrs by dual-luciferase reporter gene assay |
ACS Med Chem Lett 8: 751-756 (2017)
Article DOI: 10.1021/acsmedchemlett.7b00168
BindingDB Entry DOI: 10.7270/Q2QC05ZJ |
More data for this
Ligand-Target Pair | |
Growth/differentiation factor 8
(Homo sapiens (Human)) | BDBM50254771

(CHEMBL4061416)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@H](CCCCN)NC(=O)[C@@H](NC(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)[C@H](CCC(O)=O)NC(=O)[C@@H](NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](CO)NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@@H](NC(=O)[C@H](CC(N)=O)NC(=O)[C@H](CCC(N)=O)NC(=O)[C@H](CCCNC(N)=N)NC(=O)COc1ccc2ccccc2c1)[C@@H](C)O)[C@@H](C)CC)[C@@H](C)CC)C(=O)N[C@@H](CCC(N)=O)C(=O)N[C@@H]([C@@H](C)CC)C(=O)N[C@@H]([C@@H](C)CC)C(=O)N[C@@H](CO)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CC(C)C)C(N)=O |r| Show InChI InChI=1S/C139H225N41O33/c1-16-73(10)108(131(208)166-94(50-53-104(143)186)123(200)177-112(77(14)20-5)134(211)179-111(76(13)19-4)133(210)174-102(69-182)129(206)161-88(37-25-27-55-140)117(194)169-97(62-72(8)9)124(201)160-90(40-30-58-155-137(148)149)116(193)168-96(114(145)191)61-71(6)7)175-121(198)89(38-26-28-56-141)164-130(207)110(75(12)18-3)178-126(203)99(65-82-67-158-86-36-24-23-35-85(82)86)171-120(197)95(51-54-107(189)190)167-132(209)109(74(11)17-2)176-122(199)92(42-32-60-157-139(152)153)162-128(205)101(68-181)173-125(202)98(63-79-43-46-83(184)47-44-79)170-118(195)91(41-31-59-156-138(150)151)165-135(212)113(78(15)183)180-127(204)100(66-105(144)187)172-119(196)93(49-52-103(142)185)163-115(192)87(39-29-57-154-136(146)147)159-106(188)70-213-84-48-45-80-33-21-22-34-81(80)64-84/h21-24,33-36,43-48,64,67,71-78,87-102,108-113,158,181-184H,16-20,25-32,37-42,49-63,65-66,68-70,140-141H2,1-15H3,(H2,142,185)(H2,143,186)(H2,144,187)(H2,145,191)(H,159,188)(H,160,201)(H,161,206)(H,162,205)(H,163,192)(H,164,207)(H,165,212)(H,166,208)(H,167,209)(H,168,193)(H,169,194)(H,170,195)(H,171,197)(H,172,196)(H,173,202)(H,174,210)(H,175,198)(H,176,199)(H,177,200)(H,178,203)(H,179,211)(H,180,204)(H,189,190)(H4,146,147,154)(H4,148,149,155)(H4,150,151,156)(H4,152,153,157)/t73-,74-,75-,76-,77-,78+,87-,88-,89-,90-,91-,92-,93-,94-,95-,96-,97-,98-,99-,100-,101-,102-,108-,109-,110-,111-,112-,113-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 360 | n/a | n/a | n/a | n/a | n/a | n/a |
Tokyo University of Pharmacy and Life Sciences
Curated by ChEMBL
| Assay Description
Inhibition of myostatin (unknown origin) expressed in HEK293 cells after 4 hrs by dual luciferase reporter gene assay |
Bioorg Med Chem 27: 1437-1443 (2019)
Article DOI: 10.1016/j.bmc.2019.02.019
BindingDB Entry DOI: 10.7270/Q2W099FN |
More data for this
Ligand-Target Pair | |
Growth/differentiation factor 8
(Homo sapiens (Human)) | BDBM50206029
(CHEMBL3892004)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@H](C)NC(=O)[C@H](CCC(O)=O)NC(=O)[C@@H](NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](CO)NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@@H](NC(=O)[C@H](CC(N)=O)NC(=O)[C@H](CCC(N)=O)NC(=O)[C@H](CCCNC(N)=N)NC(=O)COc1ccc2ccccc2c1)[C@@H](C)O)[C@@H](C)CC)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H]([C@@H](C)CC)C(=O)N[C@@H](CCC(N)=O)C(=O)N[C@@H]([C@@H](C)CC)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CO)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CC(C)C)C(N)=O |r| Show InChI InChI=1S/C131H220N40O33/c1-17-69(11)101(123(199)156-82(34-24-26-52-133)114(190)168-102(70(12)18-2)124(200)158-87(46-49-97(135)177)116(192)170-104(72(14)20-4)126(202)164-91(59-68(9)10)118(194)165-94(63-172)121(197)153-81(33-23-25-51-132)111(187)161-90(58-67(7)8)117(193)152-83(36-28-54-147-129(140)141)110(186)160-89(106(137)182)57-66(5)6)167-107(183)73(15)150-108(184)88(47-50-100(180)181)159-125(201)103(71(13)19-3)169-115(191)85(38-30-56-149-131(144)145)154-122(198)95(64-173)166-119(195)92(60-75-39-42-78(175)43-40-75)162-112(188)84(37-29-55-148-130(142)143)157-127(203)105(74(16)174)171-120(196)93(62-98(136)178)163-113(189)86(45-48-96(134)176)155-109(185)80(35-27-53-146-128(138)139)151-99(179)65-204-79-44-41-76-31-21-22-32-77(76)61-79/h21-22,31-32,39-44,61,66-74,80-95,101-105,172-175H,17-20,23-30,33-38,45-60,62-65,132-133H2,1-16H3,(H2,134,176)(H2,135,177)(H2,136,178)(H2,137,182)(H,150,184)(H,151,179)(H,152,193)(H,153,197)(H,154,198)(H,155,185)(H,156,199)(H,157,203)(H,158,200)(H,159,201)(H,160,186)(H,161,187)(H,162,188)(H,163,189)(H,164,202)(H,165,194)(H,166,195)(H,167,183)(H,168,190)(H,169,191)(H,170,192)(H,171,196)(H,180,181)(H4,138,139,146)(H4,140,141,147)(H4,142,143,148)(H4,144,145,149)/t69-,70-,71-,72-,73-,74+,80-,81-,82-,83-,84-,85-,86-,87-,88-,89-,90-,91-,92-,93-,94-,95-,101-,102-,103-,104-,105-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.19E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Medicinal Chemistry and Department of Drug Delivery and Molecular Biopharmaceutics, Tokyo University of Pharmacy and Life Sciences, Hachioji, Tokyo 192-0392, Japan.
Curated by ChEMBL
| Assay Description
Inhibition of recombinant myostatin (unknown origin) expressed in HEK293 cells after 4 hrs by dual-luciferase reporter gene assay |
ACS Med Chem Lett 8: 751-756 (2017)
Article DOI: 10.1021/acsmedchemlett.7b00168
BindingDB Entry DOI: 10.7270/Q2QC05ZJ |
More data for this
Ligand-Target Pair | |
Tubulin beta chain
(Sus scrofa) | BDBM50030766
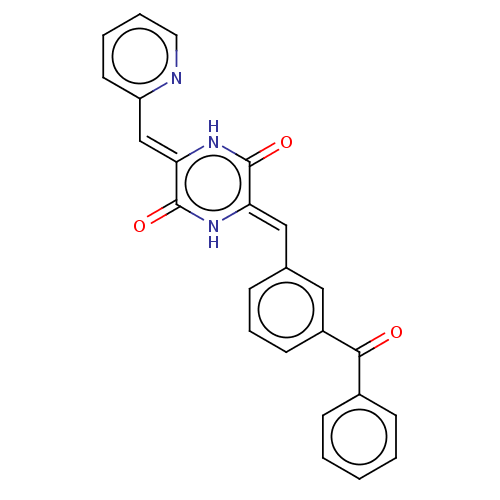
(CHEMBL3342339)Show SMILES O=C(c1ccccc1)c1cccc(\C=c2/[nH]c(=O)\c(=C\c3ccccn3)[nH]c2=O)c1 Show InChI InChI=1S/C24H17N3O3/c28-22(17-8-2-1-3-9-17)18-10-6-7-16(13-18)14-20-23(29)27-21(24(30)26-20)15-19-11-4-5-12-25-19/h1-15H,(H,26,30)(H,27,29)/b20-14-,21-15- | PDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Tokyo University of Pharmacy and Life Sciences
Curated by ChEMBL
| Assay Description
Inhibition of porcine tubulin polymerization by spectrophotometry |
ACS Med Chem Lett 5: 1094-8 (2014)
Article DOI: 10.1021/ml5001883
BindingDB Entry DOI: 10.7270/Q2R49SCP |
More data for this
Ligand-Target Pair | |
Growth/differentiation factor 8
(Homo sapiens (Human)) | BDBM50206029
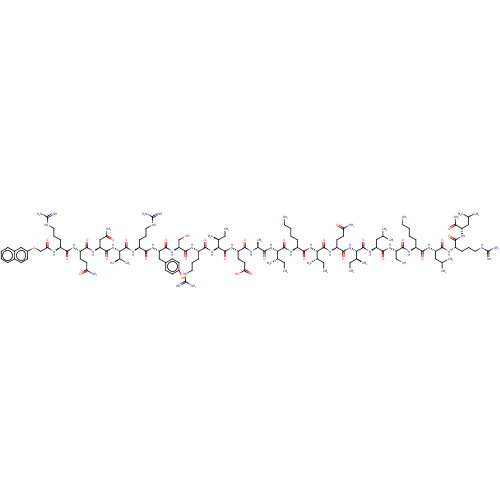
(CHEMBL3892004)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@H](C)NC(=O)[C@H](CCC(O)=O)NC(=O)[C@@H](NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](CO)NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@@H](NC(=O)[C@H](CC(N)=O)NC(=O)[C@H](CCC(N)=O)NC(=O)[C@H](CCCNC(N)=N)NC(=O)COc1ccc2ccccc2c1)[C@@H](C)O)[C@@H](C)CC)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H]([C@@H](C)CC)C(=O)N[C@@H](CCC(N)=O)C(=O)N[C@@H]([C@@H](C)CC)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CO)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CC(C)C)C(N)=O |r| Show InChI InChI=1S/C131H220N40O33/c1-17-69(11)101(123(199)156-82(34-24-26-52-133)114(190)168-102(70(12)18-2)124(200)158-87(46-49-97(135)177)116(192)170-104(72(14)20-4)126(202)164-91(59-68(9)10)118(194)165-94(63-172)121(197)153-81(33-23-25-51-132)111(187)161-90(58-67(7)8)117(193)152-83(36-28-54-147-129(140)141)110(186)160-89(106(137)182)57-66(5)6)167-107(183)73(15)150-108(184)88(47-50-100(180)181)159-125(201)103(71(13)19-3)169-115(191)85(38-30-56-149-131(144)145)154-122(198)95(64-173)166-119(195)92(60-75-39-42-78(175)43-40-75)162-112(188)84(37-29-55-148-130(142)143)157-127(203)105(74(16)174)171-120(196)93(62-98(136)178)163-113(189)86(45-48-96(134)176)155-109(185)80(35-27-53-146-128(138)139)151-99(179)65-204-79-44-41-76-31-21-22-32-77(76)61-79/h21-22,31-32,39-44,61,66-74,80-95,101-105,172-175H,17-20,23-30,33-38,45-60,62-65,132-133H2,1-16H3,(H2,134,176)(H2,135,177)(H2,136,178)(H2,137,182)(H,150,184)(H,151,179)(H,152,193)(H,153,197)(H,154,198)(H,155,185)(H,156,199)(H,157,203)(H,158,200)(H,159,201)(H,160,186)(H,161,187)(H,162,188)(H,163,189)(H,164,202)(H,165,194)(H,166,195)(H,167,183)(H,168,190)(H,169,191)(H,170,192)(H,171,196)(H,180,181)(H4,138,139,146)(H4,140,141,147)(H4,142,143,148)(H4,144,145,149)/t69-,70-,71-,72-,73-,74+,80-,81-,82-,83-,84-,85-,86-,87-,88-,89-,90-,91-,92-,93-,94-,95-,101-,102-,103-,104-,105-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Tokyo University of Pharmacy and Life Sciences
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant myostatin expressed in HEK293 cells after 4 hrs by SBE4 based luciferase reporter gene assay |
ACS Med Chem Lett 8: 113-117 (2017)
Article DOI: 10.1021/acsmedchemlett.6b00420
BindingDB Entry DOI: 10.7270/Q2MG7RHH |
More data for this
Ligand-Target Pair | |
Transforming growth factor beta-1 proprotein
(Homo sapiens (Human)) | BDBM50585028

(CHEMBL5078185)Show SMILES CC[C@@H](C)[C@@H](NC(=O)[C@@H](Cc1c[nH]c2ccccc12)NC(=O)[C@@H](CCCNC(N)=N)NC(=O)[C@@H](CCCCN)NC(=O)[C@H](NC(=O)[C@@H](CCCNC(N)=N)NC(=O)[C@H](N)CC(C)C)C1CCCCC1)C(=O)N[C@H](CCCNC(N)=N)C(=O)N[C@H](C1CCCCC1)C(=O)N[C@H](CCCCN)C(=O)N[C@H]([C@H](C)CC)C(=O)N[C@H](Cc1c[nH]c2ccccc12)C(=O)N[C@H](CCCNC(N)=N)C(=O)N[C@H]([C@H](C)CC)C(=O)N[C@H](Cc1ccc(O)cc1)C(=O)N[C@H](Cc1c[nH]c2ccccc12)C(N)=O |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of TGF beta 1 (unknown origin) expressed in HEK293 cells transfected with Smad2/3 responsive reporter plasmid incubated for 4 hrs by dual ... |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.1c00705
BindingDB Entry DOI: 10.7270/Q28S4TT7 |
More data for this
Ligand-Target Pair | |
Tubulin beta chain
(Sus scrofa) | BDBM50030765
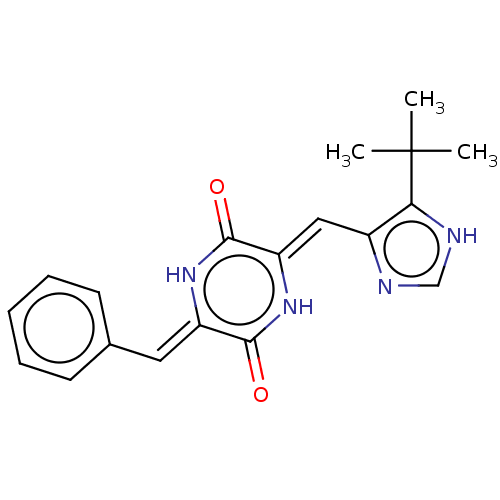
(NPI-2358 | Plinabulin)Show SMILES CC(C)(C)c1[nH]cnc1\C=c1/[nH]c(=O)\c(=C\c2ccccc2)[nH]c1=O Show InChI InChI=1S/C19H20N4O2/c1-19(2,3)16-13(20-11-21-16)10-15-18(25)22-14(17(24)23-15)9-12-7-5-4-6-8-12/h4-11H,1-3H3,(H,20,21)(H,22,25)(H,23,24)/b14-9-,15-10- | PDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 1.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Tokyo University of Pharmacy and Life Sciences
Curated by ChEMBL
| Assay Description
Inhibition of porcine tubulin polymerization by spectrophotometry |
ACS Med Chem Lett 5: 1094-8 (2014)
Article DOI: 10.1021/ml5001883
BindingDB Entry DOI: 10.7270/Q2R49SCP |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Growth/differentiation factor 8
(Homo sapiens (Human)) | BDBM50071380
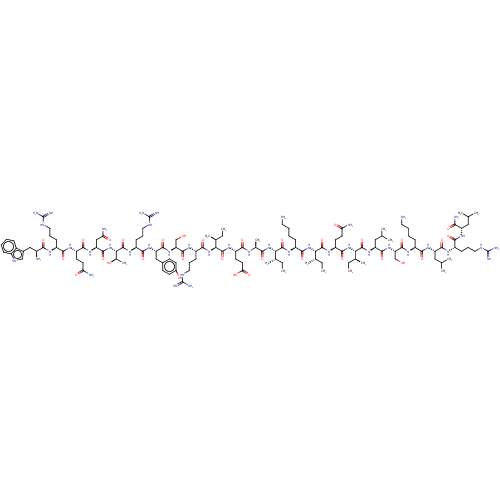
(CHEMBL3410232)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@H](C)NC(=O)[C@H](CCC(O)=O)NC(=O)[C@@H](NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](CO)NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@@H](NC(=O)[C@H](CC(N)=O)NC(=O)[C@H](CCC(N)=O)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@@H](N)Cc1c[nH]c2ccccc12)[C@@H](C)O)[C@@H](C)CC)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H]([C@@H](C)CC)C(=O)N[C@@H](CCC(N)=O)C(=O)N[C@@H]([C@@H](C)CC)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CO)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CC(C)C)C(N)=O |r| | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.56E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Medicinal Chemistry and Department of Drug Delivery and Molecular Biopharmaceutics, Tokyo University of Pharmacy and Life Sciences, Hachioji, Tokyo 192-0392, Japan.
Curated by ChEMBL
| Assay Description
Inhibition of recombinant myostatin (unknown origin) expressed in HEK293 cells after 4 hrs by dual-luciferase reporter gene assay |
ACS Med Chem Lett 8: 751-756 (2017)
Article DOI: 10.1021/acsmedchemlett.7b00168
BindingDB Entry DOI: 10.7270/Q2QC05ZJ |
More data for this
Ligand-Target Pair | |
Growth/differentiation factor 8
(Homo sapiens (Human)) | BDBM50525413

(CHEMBL4448117)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@H](CCC(N)=O)NC(=O)[C@@H](NC(=O)[C@H](CCCCN)NC(=O)[C@@H](NC(=O)[C@@H](Cc1c[nH]c2ccccc12)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@@H](NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@@H](N)Cc1c[nH]c2ccccc12)[C@@H](C)CC)[C@@H](C)CC)C1CCCCC1)C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)N[C@@H](CO)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](C1CCCCC1)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CC(C)C)C(N)=O |r| Show InChI InChI=1S/C114H173N29O19/c1-9-64(6)92(140-105(155)87(55-67-44-46-73(145)47-45-67)135-98(148)77(117)56-70-59-126-78-37-21-18-34-74(70)78)109(159)131-84(43-29-53-125-114(122)123)100(150)136-89(58-72-61-128-80-39-23-20-36-76(72)80)106(156)141-93(65(7)10-2)108(158)130-82(41-25-27-51-116)102(152)143-96(69-32-16-13-17-33-69)112(162)133-85(48-49-91(118)146)103(153)139-94(66(8)11-3)110(160)137-88(57-71-60-127-79-38-22-19-35-75(71)79)104(154)138-90(62-144)107(157)129-81(40-24-26-50-115)101(151)142-95(68-30-14-12-15-31-68)111(161)132-83(42-28-52-124-113(120)121)99(149)134-86(97(119)147)54-63(4)5/h18-23,34-39,44-47,59-61,63-66,68-69,77,81-90,92-96,126-128,144-145H,9-17,24-33,40-43,48-58,62,115-117H2,1-8H3,(H2,118,146)(H2,119,147)(H,129,157)(H,130,158)(H,131,159)(H,132,161)(H,133,162)(H,134,149)(H,135,148)(H,136,150)(H,137,160)(H,138,154)(H,139,153)(H,140,155)(H,141,156)(H,142,151)(H,143,152)(H4,120,121,124)(H4,122,123,125)/t64-,65-,66-,77-,81-,82-,83-,84-,85-,86-,87-,88-,89+,90-,92-,93-,94-,95-,96-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.56E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Tokyo University of Pharmacy and Life Sciences
Curated by ChEMBL
| Assay Description
Inhibition of myostatin (unknown origin) expressed in HEK293 cells incubated for 4 hrs by dual luciferase reporter gene assay |
Bioorg Med Chem Lett 30: (2020)
Article DOI: 10.1016/j.bmcl.2019.126892
BindingDB Entry DOI: 10.7270/Q2G73J8Q |
More data for this
Ligand-Target Pair | |
Growth/differentiation factor 8
(Homo sapiens (Human)) | BDBM50539386
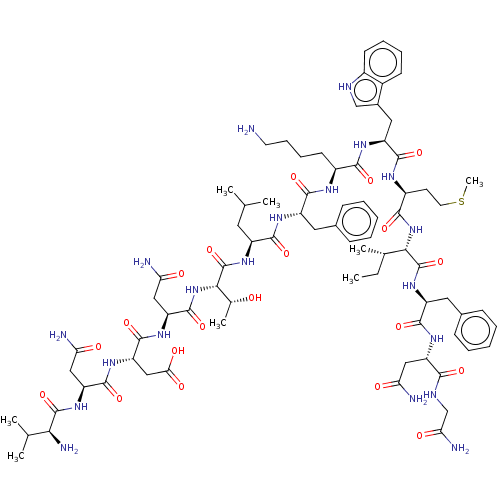
(CHEMBL4646947)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@H](CCSC)NC(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)[C@H](CCCCN)NC(=O)[C@H](Cc1ccccc1)NC(=O)[C@H](CC(C)C)NC(=O)[C@@H](NC(=O)[C@H](CC(N)=O)NC(=O)[C@H](CC(O)=O)NC(=O)[C@H](CC(N)=O)NC(=O)[C@@H](N)C(C)C)[C@@H](C)O)C(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@@H](CC(N)=O)C(=O)NCC(N)=O |r| Show InChI InChI=1S/C79H116N20O20S/c1-9-42(6)65(78(118)97-53(32-45-22-14-11-15-23-45)72(112)92-55(34-59(81)101)67(107)87-39-62(84)104)98-69(109)50(27-29-120-8)89-73(113)54(33-46-38-86-48-25-17-16-24-47(46)48)91-68(108)49(26-18-19-28-80)88-71(111)52(31-44-20-12-10-13-21-44)90-70(110)51(30-40(2)3)96-79(119)66(43(7)100)99-76(116)57(36-61(83)103)93-75(115)58(37-63(105)106)94-74(114)56(35-60(82)102)95-77(117)64(85)41(4)5/h10-17,20-25,38,40-43,49-58,64-66,86,100H,9,18-19,26-37,39,80,85H2,1-8H3,(H2,81,101)(H2,82,102)(H2,83,103)(H2,84,104)(H,87,107)(H,88,111)(H,89,113)(H,90,110)(H,91,108)(H,92,112)(H,93,115)(H,94,114)(H,95,117)(H,96,119)(H,97,118)(H,98,109)(H,99,116)(H,105,106)/t42-,43+,49-,50-,51-,52-,53-,54-,55-,56-,57-,58-,64-,65-,66-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 6.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Tokyo University of Pharmacy and Life Sciences
Curated by ChEMBL
| Assay Description
Inhibition of myostatin (unknown origin) expressed in HEK293 cells incubated for 4 hrs by dual luciferase reporter gene assay |
Bioorg Med Chem Lett 30: (2020)
Article DOI: 10.1016/j.bmcl.2019.126892
BindingDB Entry DOI: 10.7270/Q2G73J8Q |
More data for this
Ligand-Target Pair | |
Transforming growth factor beta-1 proprotein
(Homo sapiens (Human)) | BDBM50525413

(CHEMBL4448117)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@H](CCC(N)=O)NC(=O)[C@@H](NC(=O)[C@H](CCCCN)NC(=O)[C@@H](NC(=O)[C@@H](Cc1c[nH]c2ccccc12)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@@H](NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@@H](N)Cc1c[nH]c2ccccc12)[C@@H](C)CC)[C@@H](C)CC)C1CCCCC1)C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)N[C@@H](CO)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](C1CCCCC1)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CC(C)C)C(N)=O |r| Show InChI InChI=1S/C114H173N29O19/c1-9-64(6)92(140-105(155)87(55-67-44-46-73(145)47-45-67)135-98(148)77(117)56-70-59-126-78-37-21-18-34-74(70)78)109(159)131-84(43-29-53-125-114(122)123)100(150)136-89(58-72-61-128-80-39-23-20-36-76(72)80)106(156)141-93(65(7)10-2)108(158)130-82(41-25-27-51-116)102(152)143-96(69-32-16-13-17-33-69)112(162)133-85(48-49-91(118)146)103(153)139-94(66(8)11-3)110(160)137-88(57-71-60-127-79-38-22-19-35-75(71)79)104(154)138-90(62-144)107(157)129-81(40-24-26-50-115)101(151)142-95(68-30-14-12-15-31-68)111(161)132-83(42-28-52-124-113(120)121)99(149)134-86(97(119)147)54-63(4)5/h18-23,34-39,44-47,59-61,63-66,68-69,77,81-90,92-96,126-128,144-145H,9-17,24-33,40-43,48-58,62,115-117H2,1-8H3,(H2,118,146)(H2,119,147)(H,129,157)(H,130,158)(H,131,159)(H,132,161)(H,133,162)(H,134,149)(H,135,148)(H,136,150)(H,137,160)(H,138,154)(H,139,153)(H,140,155)(H,141,156)(H,142,151)(H,143,152)(H4,120,121,124)(H4,122,123,125)/t64-,65-,66-,77-,81-,82-,83-,84-,85-,86-,87-,88-,89+,90-,92-,93-,94-,95-,96-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 6.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of TGF beta 1 (unknown origin) expressed in HEK293 cells transfected with Smad2/3 responsive reporter plasmid incubated for 4 hrs by dual ... |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.1c00705
BindingDB Entry DOI: 10.7270/Q28S4TT7 |
More data for this
Ligand-Target Pair | |
Neuromedin-U receptor 1
(Homo sapiens (Human)) | BDBM50560260

(CHEMBL4798983)Show SMILES NC(=O)C[C@H](NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@@H]1CCCN1C(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](Cc1ccc(cc1)-c1ccccc1)NC(=O)[C@H](Cc1ccc2ccccc2c1)NC(=O)CCc1cccnc1)C(N)=O |r| | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 3.20 | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Agonist activity at human NMUR1 expressed in CHO cells by calcium mobilization assay |
Citation and Details
Article DOI: 10.1016/j.bmcl.2020.127436
BindingDB Entry DOI: 10.7270/Q2QC077Z |
More data for this
Ligand-Target Pair | |
Neuromedin-U receptor 1
(Homo sapiens (Human)) | BDBM50560261
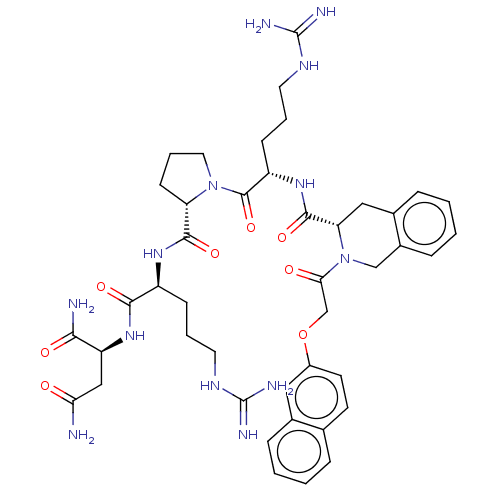
(CHEMBL4757331)Show SMILES NC(=O)C[C@H](NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@@H]1CCCN1C(=O)[C@H](CCCNC(N)=N)NC(=O)[C@@H]1Cc2ccccc2CN1C(=O)COc1ccc2ccccc2c1)C(N)=O |r| | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 30 | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Agonist activity at human NMUR1 expressed in CHO cells by calcium mobilization assay |
Citation and Details
Article DOI: 10.1016/j.bmcl.2020.127436
BindingDB Entry DOI: 10.7270/Q2QC077Z |
More data for this
Ligand-Target Pair | |
Neuromedin-U receptor 1
(Homo sapiens (Human)) | BDBM50049424
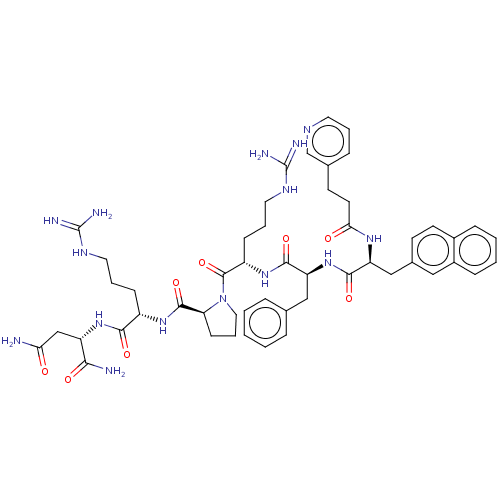
(CHEMBL3315348)Show SMILES NC(=O)C[C@H](NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@@H]1CCCN1C(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](Cc1ccccc1)NC(=O)[C@H](Cc1ccc2ccccc2c1)NC(=O)CCc1cccnc1)C(N)=O |r| Show InChI InChI=1S/C51H67N15O8/c52-42(67)29-38(44(53)69)64-45(70)36(15-7-23-59-50(54)55)62-48(73)41-17-9-25-66(41)49(74)37(16-8-24-60-51(56)57)63-47(72)40(27-31-10-2-1-3-11-31)65-46(71)39(61-43(68)21-19-32-12-6-22-58-30-32)28-33-18-20-34-13-4-5-14-35(34)26-33/h1-6,10-14,18,20,22,26,30,36-41H,7-9,15-17,19,21,23-25,27-29H2,(H2,52,67)(H2,53,69)(H,61,68)(H,62,73)(H,63,72)(H,64,70)(H,65,71)(H4,54,55,59)(H4,56,57,60)/t36-,37-,38-,39-,40-,41-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 1.70 | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Partial agonist activity at human NMUR1 expressed in CHO cells by calcium mobilization assay |
Citation and Details
Article DOI: 10.1016/j.bmcl.2020.127436
BindingDB Entry DOI: 10.7270/Q2QC077Z |
More data for this
Ligand-Target Pair | |
Neuromedin-U receptor 2
(Homo sapiens (Human)) | BDBM50560260

(CHEMBL4798983)Show SMILES NC(=O)C[C@H](NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@@H]1CCCN1C(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](Cc1ccc(cc1)-c1ccccc1)NC(=O)[C@H](Cc1ccc2ccccc2c1)NC(=O)CCc1cccnc1)C(N)=O |r| | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Agonist activity at human NMUR2 expressed in CHO cells by calcium mobilization assay |
Citation and Details
Article DOI: 10.1016/j.bmcl.2020.127436
BindingDB Entry DOI: 10.7270/Q2QC077Z |
More data for this
Ligand-Target Pair | |
Neuromedin-U receptor 2
(Homo sapiens (Human)) | BDBM50049422

(CHEMBL3315335)Show SMILES CC(C)C[C@H](NC(=O)CCC1CCCCC1)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CN)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CC(N)=O)C(N)=O |r| Show InChI InChI=1S/C39H70N12O8/c1-22(2)18-27(46-32(53)15-14-24-10-6-5-7-11-24)35(56)49-28(19-23(3)4)36(57)50-29(21-40)38(59)51-17-9-13-30(51)37(58)47-25(12-8-16-45-39(43)44)34(55)48-26(33(42)54)20-31(41)52/h22-30H,5-21,40H2,1-4H3,(H2,41,52)(H2,42,54)(H,46,53)(H,47,58)(H,48,55)(H,49,56)(H,50,57)(H4,43,44,45)/t25-,26-,27-,28-,29-,30-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 6.40 | n/a | n/a | n/a | n/a |
Tokyo University of Pharmacy and Life Sciences
Curated by ChEMBL
| Assay Description
Agonist activity at human NMUR2 expressed in CHO cells by Fluo-4-AM dye based calcium mobilization assay |
Bioorg Med Chem 28: (2020)
Article DOI: 10.1016/j.bmc.2020.115454
BindingDB Entry DOI: 10.7270/Q2JW8JDK |
More data for this
Ligand-Target Pair | |
Neuromedin-U receptor 2
(Homo sapiens (Human)) | BDBM50542392

(CHEMBL4647192)Show SMILES CC(C)C[C@H](NC(=O)CCC1CCCCC1)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CCN)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CC(N)=O)C(N)=O |r| Show InChI InChI=1S/C40H72N12O8/c1-23(2)20-29(47-33(54)15-14-25-10-6-5-7-11-25)36(57)51-30(21-24(3)4)37(58)49-27(16-17-41)39(60)52-19-9-13-31(52)38(59)48-26(12-8-18-46-40(44)45)35(56)50-28(34(43)55)22-32(42)53/h23-31H,5-22,41H2,1-4H3,(H2,42,53)(H2,43,55)(H,47,54)(H,48,59)(H,49,58)(H,50,56)(H,51,57)(H4,44,45,46)/t26-,27-,28-,29-,30-,31-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 2.20 | n/a | n/a | n/a | n/a |
Tokyo University of Pharmacy and Life Sciences
Curated by ChEMBL
| Assay Description
Agonist activity at human NMUR2 expressed in CHO cells by Fluo-4-AM dye based calcium mobilization assay |
Bioorg Med Chem 28: (2020)
Article DOI: 10.1016/j.bmc.2020.115454
BindingDB Entry DOI: 10.7270/Q2JW8JDK |
More data for this
Ligand-Target Pair | |
Neuromedin-U receptor 1
(Homo sapiens (Human)) | BDBM50239756
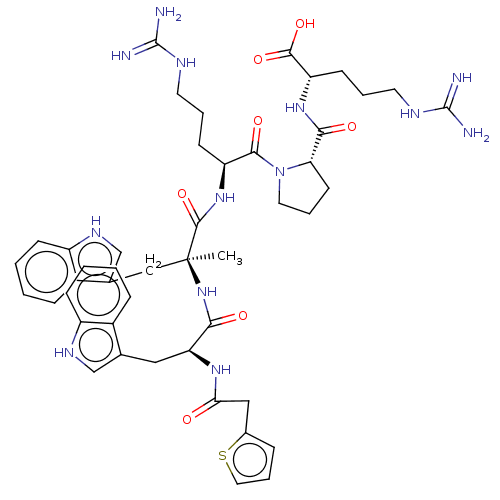
(CHEMBL4105671)Show SMILES C[C@@](Cc1c[nH]c2ccccc12)(NC(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)Cc1cccs1)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CCCNC(N)=N)C(O)=O |r| Show InChI InChI=1S/C46H59N13O7S/c1-46(24-28-26-54-33-14-5-3-12-31(28)33,58-39(61)36(55-38(60)23-29-10-9-21-67-29)22-27-25-53-32-13-4-2-11-30(27)32)43(66)57-34(15-6-18-51-44(47)48)41(63)59-20-8-17-37(59)40(62)56-35(42(64)65)16-7-19-52-45(49)50/h2-5,9-14,21,25-26,34-37,53-54H,6-8,15-20,22-24H2,1H3,(H,55,60)(H,56,62)(H,57,66)(H,58,61)(H,64,65)(H4,47,48,51)(H4,49,50,52)/t34-,35-,36-,37-,46-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 0.25 | n/a | n/a | n/a | n/a |
Tokyo University of Pharmacy and Life Sciences
Curated by ChEMBL
| Assay Description
Agonist activity at human NMUR1 expressed in CHO cells assessed as induction of Ca2+ flux after 18 hrs by Fluo-4 AM dye based FLIPR assay |
J Med Chem 60: 5228-5234 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00694
BindingDB Entry DOI: 10.7270/Q2S46V4J |
More data for this
Ligand-Target Pair | |
Neuromedin-U receptor 1
(Mus musculus) | BDBM50239756

(CHEMBL4105671)Show SMILES C[C@@](Cc1c[nH]c2ccccc12)(NC(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)Cc1cccs1)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CCCNC(N)=N)C(O)=O |r| Show InChI InChI=1S/C46H59N13O7S/c1-46(24-28-26-54-33-14-5-3-12-31(28)33,58-39(61)36(55-38(60)23-29-10-9-21-67-29)22-27-25-53-32-13-4-2-11-30(27)32)43(66)57-34(15-6-18-51-44(47)48)41(63)59-20-8-17-37(59)40(62)56-35(42(64)65)16-7-19-52-45(49)50/h2-5,9-14,21,25-26,34-37,53-54H,6-8,15-20,22-24H2,1H3,(H,55,60)(H,56,62)(H,57,66)(H,58,61)(H,64,65)(H4,47,48,51)(H4,49,50,52)/t34-,35-,36-,37-,46-/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 8.90 | n/a | n/a | n/a | n/a |
Tokyo University of Pharmacy and Life Sciences
Curated by ChEMBL
| Assay Description
Antisecretory activity evaluated by the inhibition of 14C -AP uptake in isolated rabbit parietal cells stimulated by dibutyryl cyclic adenosine 3', 5... |
J Med Chem 60: 5228-5234 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00694
BindingDB Entry DOI: 10.7270/Q2S46V4J |
More data for this
Ligand-Target Pair | |
Neuromedin-U receptor 1
(Homo sapiens (Human)) | BDBM50239755
(CHEMBL4069151)Show SMILES CC(C)C[C@H](NC(=O)[C@H](Cc1ccccc1)NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)CNC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](CO)NC(=O)[C@H](CCC(N)=O)NC(=O)[C@H](CO)NC(=O)[C@H](C)NC(=O)[C@H](Cc1ccccc1)NC(=O)[C@@H]1CCCN1C(=O)[C@@H](CO)NC(=O)[C@@H](CCC(N)=O)NC(=O)[C@@H](Cc1ccccc1)NC(=O)[C@@H](CCC(O)=O)NC(=O)[C@@H](CCC(O)=O)NC(=O)[C@@H](CC(O)=O)NC(=O)[C@H](NC(=O)[C@@H](CCCNC(N)=N)NC(=O)[C@H](N)Cc1ccccc1)C(C)C)C(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CC(N)=O)C(N)=O |r| Show InChI InChI=1S/C141H203N41O38/c1-74(2)61-94(125(208)173-98(65-80-33-17-9-18-34-80)128(211)169-92(40-24-58-158-141(153)154)136(219)181-59-25-41-104(181)133(216)168-86(38-22-56-156-139(149)150)118(201)170-93(114(146)197)68-108(145)189)171-129(212)99(66-81-35-19-10-20-36-81)174-126(209)95(67-82-43-45-83(186)46-44-82)161-109(190)70-159-117(200)85(37-21-55-155-138(147)148)163-132(215)102(72-184)178-121(204)88(47-51-106(143)187)167-131(214)101(71-183)177-115(198)76(5)160-124(207)96(63-78-29-13-7-14-30-78)175-134(217)105-42-26-60-182(105)137(220)103(73-185)179-122(205)89(48-52-107(144)188)165-127(210)97(64-79-31-15-8-16-32-79)172-120(203)91(50-54-111(193)194)164-119(202)90(49-53-110(191)192)166-130(213)100(69-112(195)196)176-135(218)113(75(3)4)180-123(206)87(39-23-57-157-140(151)152)162-116(199)84(142)62-77-27-11-6-12-28-77/h6-20,27-36,43-46,74-76,84-105,113,183-186H,21-26,37-42,47-73,142H2,1-5H3,(H2,143,187)(H2,144,188)(H2,145,189)(H2,146,197)(H,159,200)(H,160,207)(H,161,190)(H,162,199)(H,163,215)(H,164,202)(H,165,210)(H,166,213)(H,167,214)(H,168,216)(H,169,211)(H,170,201)(H,171,212)(H,172,203)(H,173,208)(H,174,209)(H,175,217)(H,176,218)(H,177,198)(H,178,204)(H,179,205)(H,180,206)(H,191,192)(H,193,194)(H,195,196)(H4,147,148,155)(H4,149,150,156)(H4,151,152,157)(H4,153,154,158)/t76-,84+,85-,86-,87+,88-,89+,90+,91+,92-,93-,94-,95-,96-,97+,98-,99-,100+,101-,102-,103+,104-,105-,113+/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 0.170 | n/a | n/a | n/a | n/a |
Tokyo University of Pharmacy and Life Sciences
Curated by ChEMBL
| Assay Description
Antisecretory activity evaluated by the inhibition of 14C -AP uptake in isolated rabbit parietal cells stimulated by exogenous histamine |
J Med Chem 60: 5228-5234 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00694
BindingDB Entry DOI: 10.7270/Q2S46V4J |
More data for this
Ligand-Target Pair | |
Neuromedin-U receptor 1
(Mus musculus) | BDBM50239753
(CHEMBL4097923)Show SMILES CC(C)C[C@H](NC(=O)[C@H](Cc1ccccc1)NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)CNC(=O)[C@H](CCCCN)NC(=O)[C@H](CO)NC(=O)[C@H](CCC(N)=O)NC(=O)CNC(=O)[C@@H](NC(=O)[C@H](CO)NC(=O)[C@@H]1CCCN1C(=O)[C@H](CO)NC(=O)[C@H](CCC(N)=O)NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@H](CCC(O)=O)NC(=O)[C@H](C)NC(=O)[C@H](CCCCN)NC(=O)[C@@H](N)Cc1ccccc1)C(C)C)C(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CC(N)=O)C(N)=O |r| Show InChI InChI=1S/C125H185N35O33/c1-67(2)55-86(112(182)153-88(57-71-25-11-7-12-26-71)115(185)149-84(32-20-52-138-125(135)136)122(192)159-53-21-33-94(159)119(189)148-80(31-19-51-137-124(133)134)108(178)150-85(103(132)173)61-98(131)168)151-116(186)89(58-72-27-13-8-14-28-72)154-113(183)87(59-73-35-39-75(164)40-36-73)143-100(170)62-139-106(176)78(29-15-17-49-126)146-117(187)91(64-161)155-109(179)81(43-46-96(129)166)142-99(169)63-140-121(191)102(68(3)4)158-118(188)92(65-162)156-120(190)95-34-22-54-160(95)123(193)93(66-163)157-111(181)82(44-47-97(130)167)147-114(184)90(60-74-37-41-76(165)42-38-74)152-110(180)83(45-48-101(171)172)144-104(174)69(5)141-107(177)79(30-16-18-50-127)145-105(175)77(128)56-70-23-9-6-10-24-70/h6-14,23-28,35-42,67-69,77-95,102,161-165H,15-22,29-34,43-66,126-128H2,1-5H3,(H2,129,166)(H2,130,167)(H2,131,168)(H2,132,173)(H,139,176)(H,140,191)(H,141,177)(H,142,169)(H,143,170)(H,144,174)(H,145,175)(H,146,187)(H,147,184)(H,148,189)(H,149,185)(H,150,178)(H,151,186)(H,152,180)(H,153,182)(H,154,183)(H,155,179)(H,156,190)(H,157,181)(H,158,188)(H,171,172)(H4,133,134,137)(H4,135,136,138)/t69-,77-,78-,79-,80-,81-,82-,83-,84-,85-,86-,87-,88-,89-,90-,91-,92-,93-,94-,95-,102-/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 0.420 | n/a | n/a | n/a | n/a |
Tokyo University of Pharmacy and Life Sciences
Curated by ChEMBL
| Assay Description
Antisecretory activity evaluated by the inhibition of 14C -AP uptake in isolated rabbit parietal cells stimulated by exogenous histamine |
J Med Chem 60: 5228-5234 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00694
BindingDB Entry DOI: 10.7270/Q2S46V4J |
More data for this
Ligand-Target Pair | |
Neuromedin-U receptor 1
(Homo sapiens (Human)) | BDBM50085928

(CHEMBL3425593)Show SMILES NC(=O)C[C@H](NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@@H]1CCCN1C(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](Cc1ccc(F)cc1)NC(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)Cc1cccs1)C(N)=O |r| Show InChI InChI=1S/C47H62FN15O8S/c48-28-15-13-26(14-16-28)21-35(62-43(69)36(58-39(65)23-29-7-6-20-72-29)22-27-25-57-31-9-2-1-8-30(27)31)42(68)60-33(11-4-18-56-47(53)54)45(71)63-19-5-12-37(63)44(70)59-32(10-3-17-55-46(51)52)41(67)61-34(40(50)66)24-38(49)64/h1-2,6-9,13-16,20,25,32-37,57H,3-5,10-12,17-19,21-24H2,(H2,49,64)(H2,50,66)(H,58,65)(H,59,70)(H,60,68)(H,61,67)(H,62,69)(H4,51,52,55)(H4,53,54,56)/t32-,33-,34-,35-,36-,37-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 0.0830 | n/a | n/a | n/a | n/a |
Tokyo University of Pharmacy and Life Sciences
Curated by ChEMBL
| Assay Description
Agonist activity at human NMUR1 expressed in CHO cells assessed as intracellular calcium flux at by Fluo-4 AM dye based fluorometric imaging method |
ACS Med Chem Lett 6: 302-7 (2015)
Article DOI: 10.1021/ml500494j
BindingDB Entry DOI: 10.7270/Q2TM7CVP |
More data for this
Ligand-Target Pair | |
Neuromedin-U receptor 1
(Homo sapiens (Human)) | BDBM50049421
(CHEMBL3315349)Show SMILES CC(C)C[C@H](NC(=O)[C@H](Cc1ccccc1)NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)CNC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](CO)NC(=O)[C@H](CCC(N)=O)NC(=O)[C@H](CO)NC(=O)[C@H](C)NC(=O)[C@H](Cc1ccccc1)NC(=O)[C@@H]1CCCN1C(=O)[C@H](CO)NC(=O)[C@H](CCC(N)=O)NC(=O)[C@H](Cc1ccccc1)NC(=O)[C@H](CCC(O)=O)NC(=O)[C@H](CCC(O)=O)NC(=O)[C@H](CC(O)=O)NC(=O)[C@@H](NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@@H](N)Cc1ccccc1)C(C)C)C(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CC(N)=O)C(N)=O |r| Show InChI InChI=1S/C141H203N41O38/c1-74(2)61-94(125(208)173-98(65-80-33-17-9-18-34-80)128(211)169-92(40-24-58-158-141(153)154)136(219)181-59-25-41-104(181)133(216)168-86(38-22-56-156-139(149)150)118(201)170-93(114(146)197)68-108(145)189)171-129(212)99(66-81-35-19-10-20-36-81)174-126(209)95(67-82-43-45-83(186)46-44-82)161-109(190)70-159-117(200)85(37-21-55-155-138(147)148)163-132(215)102(72-184)178-121(204)88(47-51-106(143)187)167-131(214)101(71-183)177-115(198)76(5)160-124(207)96(63-78-29-13-7-14-30-78)175-134(217)105-42-26-60-182(105)137(220)103(73-185)179-122(205)89(48-52-107(144)188)165-127(210)97(64-79-31-15-8-16-32-79)172-120(203)91(50-54-111(193)194)164-119(202)90(49-53-110(191)192)166-130(213)100(69-112(195)196)176-135(218)113(75(3)4)180-123(206)87(39-23-57-157-140(151)152)162-116(199)84(142)62-77-27-11-6-12-28-77/h6-20,27-36,43-46,74-76,84-105,113,183-186H,21-26,37-42,47-73,142H2,1-5H3,(H2,143,187)(H2,144,188)(H2,145,189)(H2,146,197)(H,159,200)(H,160,207)(H,161,190)(H,162,199)(H,163,215)(H,164,202)(H,165,210)(H,166,213)(H,167,214)(H,168,216)(H,169,211)(H,170,201)(H,171,212)(H,172,203)(H,173,208)(H,174,209)(H,175,217)(H,176,218)(H,177,198)(H,178,204)(H,179,205)(H,180,206)(H,191,192)(H,193,194)(H,195,196)(H4,147,148,155)(H4,149,150,156)(H4,151,152,157)(H4,153,154,158)/t76-,84-,85-,86-,87-,88-,89-,90-,91-,92-,93-,94-,95-,96-,97-,98-,99-,100-,101-,102-,103-,104-,105-,113-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 0.140 | n/a | n/a | n/a | n/a |
Tokyo University of Pharmacy and Life Sciences
Curated by ChEMBL
| Assay Description
Agonist activity at human NMUR1 expressed in CHO cells assessed as intracellular calcium flux at by Fluo-4 AM dye based fluorometric imaging method |
ACS Med Chem Lett 6: 302-7 (2015)
Article DOI: 10.1021/ml500494j
BindingDB Entry DOI: 10.7270/Q2TM7CVP |
More data for this
Ligand-Target Pair | |
Neuromedin-U receptor 2
(Homo sapiens (Human)) | BDBM50085928

(CHEMBL3425593)Show SMILES NC(=O)C[C@H](NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@@H]1CCCN1C(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](Cc1ccc(F)cc1)NC(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)Cc1cccs1)C(N)=O |r| Show InChI InChI=1S/C47H62FN15O8S/c48-28-15-13-26(14-16-28)21-35(62-43(69)36(58-39(65)23-29-7-6-20-72-29)22-27-25-57-31-9-2-1-8-30(27)31)42(68)60-33(11-4-18-56-47(53)54)45(71)63-19-5-12-37(63)44(70)59-32(10-3-17-55-46(51)52)41(67)61-34(40(50)66)24-38(49)64/h1-2,6-9,13-16,20,25,32-37,57H,3-5,10-12,17-19,21-24H2,(H2,49,64)(H2,50,66)(H,58,65)(H,59,70)(H,60,68)(H,61,67)(H,62,69)(H4,51,52,55)(H4,53,54,56)/t32-,33-,34-,35-,36-,37-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 2.60 | n/a | n/a | n/a | n/a |
Tokyo University of Pharmacy and Life Sciences
Curated by ChEMBL
| Assay Description
Agonist activity at human NMUR2 expressed in CHO cells assessed as intracellular calcium flux at by Fluo-4 AM dye based fluorometric imaging method |
ACS Med Chem Lett 6: 302-7 (2015)
Article DOI: 10.1021/ml500494j
BindingDB Entry DOI: 10.7270/Q2TM7CVP |
More data for this
Ligand-Target Pair | |
Neuromedin-U receptor 2
(Homo sapiens (Human)) | BDBM50049421

(CHEMBL3315349)Show SMILES CC(C)C[C@H](NC(=O)[C@H](Cc1ccccc1)NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)CNC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](CO)NC(=O)[C@H](CCC(N)=O)NC(=O)[C@H](CO)NC(=O)[C@H](C)NC(=O)[C@H](Cc1ccccc1)NC(=O)[C@@H]1CCCN1C(=O)[C@H](CO)NC(=O)[C@H](CCC(N)=O)NC(=O)[C@H](Cc1ccccc1)NC(=O)[C@H](CCC(O)=O)NC(=O)[C@H](CCC(O)=O)NC(=O)[C@H](CC(O)=O)NC(=O)[C@@H](NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@@H](N)Cc1ccccc1)C(C)C)C(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CC(N)=O)C(N)=O |r| Show InChI InChI=1S/C141H203N41O38/c1-74(2)61-94(125(208)173-98(65-80-33-17-9-18-34-80)128(211)169-92(40-24-58-158-141(153)154)136(219)181-59-25-41-104(181)133(216)168-86(38-22-56-156-139(149)150)118(201)170-93(114(146)197)68-108(145)189)171-129(212)99(66-81-35-19-10-20-36-81)174-126(209)95(67-82-43-45-83(186)46-44-82)161-109(190)70-159-117(200)85(37-21-55-155-138(147)148)163-132(215)102(72-184)178-121(204)88(47-51-106(143)187)167-131(214)101(71-183)177-115(198)76(5)160-124(207)96(63-78-29-13-7-14-30-78)175-134(217)105-42-26-60-182(105)137(220)103(73-185)179-122(205)89(48-52-107(144)188)165-127(210)97(64-79-31-15-8-16-32-79)172-120(203)91(50-54-111(193)194)164-119(202)90(49-53-110(191)192)166-130(213)100(69-112(195)196)176-135(218)113(75(3)4)180-123(206)87(39-23-57-157-140(151)152)162-116(199)84(142)62-77-27-11-6-12-28-77/h6-20,27-36,43-46,74-76,84-105,113,183-186H,21-26,37-42,47-73,142H2,1-5H3,(H2,143,187)(H2,144,188)(H2,145,189)(H2,146,197)(H,159,200)(H,160,207)(H,161,190)(H,162,199)(H,163,215)(H,164,202)(H,165,210)(H,166,213)(H,167,214)(H,168,216)(H,169,211)(H,170,201)(H,171,212)(H,172,203)(H,173,208)(H,174,209)(H,175,217)(H,176,218)(H,177,198)(H,178,204)(H,179,205)(H,180,206)(H,191,192)(H,193,194)(H,195,196)(H4,147,148,155)(H4,149,150,156)(H4,151,152,157)(H4,153,154,158)/t76-,84-,85-,86-,87-,88-,89-,90-,91-,92-,93-,94-,95-,96-,97-,98-,99-,100-,101-,102-,103-,104-,105-,113-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 0.920 | n/a | n/a | n/a | n/a |
Tokyo University of Pharmacy and Life Sciences
Curated by ChEMBL
| Assay Description
Agonist activity at human NMUR2 expressed in CHO cells assessed as intracellular calcium flux at by Fluo-4 AM dye based fluorometric imaging method |
ACS Med Chem Lett 6: 302-7 (2015)
Article DOI: 10.1021/ml500494j
BindingDB Entry DOI: 10.7270/Q2TM7CVP |
More data for this
Ligand-Target Pair | |
Neuromedin-U receptor 2
(Mus musculus) | BDBM50049426
(CHEMBL3356082)Show SMILES CC(C)C[C@H](NC(=O)[C@H](Cc1ccccc1)NC(=O)[C@@H](N)Cc1ccc(O)cc1)C(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@@H](C)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CC(N)=O)C(N)=O |r| Show InChI InChI=1S/C51H71N13O10/c1-29(2)24-38(62-48(72)40(27-32-14-8-5-9-15-32)61-44(68)35(52)25-33-18-20-34(65)21-19-33)47(71)63-39(26-31-12-6-4-7-13-31)46(70)58-30(3)50(74)64-23-11-17-41(64)49(73)59-36(16-10-22-57-51(55)56)45(69)60-37(43(54)67)28-42(53)66/h4-9,12-15,18-21,29-30,35-41,65H,10-11,16-17,22-28,52H2,1-3H3,(H2,53,66)(H2,54,67)(H,58,70)(H,59,73)(H,60,69)(H,61,68)(H,62,72)(H,63,71)(H4,55,56,57)/t30-,35-,36-,37-,38-,39-,40-,41-/m0/s1 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 156 | n/a | n/a | n/a | n/a |
Tokyo University of Pharmacy and Life Sciences
Curated by ChEMBL
| Assay Description
Agonist activity at mouse NMUR2 expressed in HEK293 cells by calcium mobilization assay |
J Med Chem 57: 6583-93 (2014)
Article DOI: 10.1021/jm500599s
BindingDB Entry DOI: 10.7270/Q2FQ9Z71 |
More data for this
Ligand-Target Pair | |
Neuromedin-U receptor 1
(Mus musculus) | BDBM50049426

(CHEMBL3356082)Show SMILES CC(C)C[C@H](NC(=O)[C@H](Cc1ccccc1)NC(=O)[C@@H](N)Cc1ccc(O)cc1)C(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@@H](C)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CC(N)=O)C(N)=O |r| Show InChI InChI=1S/C51H71N13O10/c1-29(2)24-38(62-48(72)40(27-32-14-8-5-9-15-32)61-44(68)35(52)25-33-18-20-34(65)21-19-33)47(71)63-39(26-31-12-6-4-7-13-31)46(70)58-30(3)50(74)64-23-11-17-41(64)49(73)59-36(16-10-22-57-51(55)56)45(69)60-37(43(54)67)28-42(53)66/h4-9,12-15,18-21,29-30,35-41,65H,10-11,16-17,22-28,52H2,1-3H3,(H2,53,66)(H2,54,67)(H,58,70)(H,59,73)(H,60,69)(H,61,68)(H,62,72)(H,63,71)(H4,55,56,57)/t30-,35-,36-,37-,38-,39-,40-,41-/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 2.41E+3 | n/a | n/a | n/a | n/a |
Tokyo University of Pharmacy and Life Sciences
Curated by ChEMBL
| Assay Description
Agonist activity at mouse NMUR1 expressed in HEK293 cells by calcium mobilization assay |
J Med Chem 57: 6583-93 (2014)
Article DOI: 10.1021/jm500599s
BindingDB Entry DOI: 10.7270/Q2FQ9Z71 |
More data for this
Ligand-Target Pair | |
Neuromedin-U receptor 1
(Mus musculus) | BDBM50049425
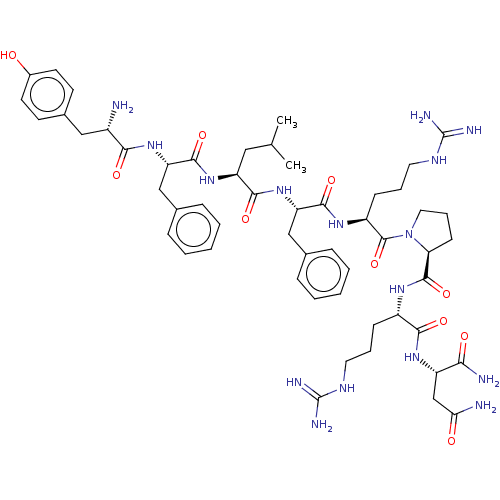
(CHEMBL3356083)Show SMILES CC(C)C[C@H](NC(=O)[C@H](Cc1ccccc1)NC(=O)[C@@H](N)Cc1ccc(O)cc1)C(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CC(N)=O)C(N)=O |r| Show InChI InChI=1S/C54H78N16O10/c1-31(2)26-40(68-50(78)41(28-32-12-5-3-6-13-32)67-46(74)36(55)27-34-19-21-35(71)22-20-34)48(76)69-42(29-33-14-7-4-8-15-33)49(77)65-38(17-10-24-63-54(60)61)52(80)70-25-11-18-43(70)51(79)64-37(16-9-23-62-53(58)59)47(75)66-39(45(57)73)30-44(56)72/h3-8,12-15,19-22,31,36-43,71H,9-11,16-18,23-30,55H2,1-2H3,(H2,56,72)(H2,57,73)(H,64,79)(H,65,77)(H,66,75)(H,67,74)(H,68,78)(H,69,76)(H4,58,59,62)(H4,60,61,63)/t36-,37-,38-,39-,40-,41-,42-,43-/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 9.5 | n/a | n/a | n/a | n/a |
Tokyo University of Pharmacy and Life Sciences
Curated by ChEMBL
| Assay Description
Agonist activity at mouse NMUR1 expressed in HEK293 cells by calcium mobilization assay |
J Med Chem 57: 6583-93 (2014)
Article DOI: 10.1021/jm500599s
BindingDB Entry DOI: 10.7270/Q2FQ9Z71 |
More data for this
Ligand-Target Pair | |
Neuromedin-U receptor 2
(Mus musculus) | BDBM50049425

(CHEMBL3356083)Show SMILES CC(C)C[C@H](NC(=O)[C@H](Cc1ccccc1)NC(=O)[C@@H](N)Cc1ccc(O)cc1)C(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CC(N)=O)C(N)=O |r| Show InChI InChI=1S/C54H78N16O10/c1-31(2)26-40(68-50(78)41(28-32-12-5-3-6-13-32)67-46(74)36(55)27-34-19-21-35(71)22-20-34)48(76)69-42(29-33-14-7-4-8-15-33)49(77)65-38(17-10-24-63-54(60)61)52(80)70-25-11-18-43(70)51(79)64-37(16-9-23-62-53(58)59)47(75)66-39(45(57)73)30-44(56)72/h3-8,12-15,19-22,31,36-43,71H,9-11,16-18,23-30,55H2,1-2H3,(H2,56,72)(H2,57,73)(H,64,79)(H,65,77)(H,66,75)(H,67,74)(H,68,78)(H,69,76)(H4,58,59,62)(H4,60,61,63)/t36-,37-,38-,39-,40-,41-,42-,43-/m0/s1 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 3 | n/a | n/a | n/a | n/a |
Tokyo University of Pharmacy and Life Sciences
Curated by ChEMBL
| Assay Description
Agonist activity at mouse NMUR2 expressed in HEK293 cells by calcium mobilization assay |
J Med Chem 57: 6583-93 (2014)
Article DOI: 10.1021/jm500599s
BindingDB Entry DOI: 10.7270/Q2FQ9Z71 |
More data for this
Ligand-Target Pair | |
Neuromedin-U receptor 1
(Homo sapiens (Human)) | BDBM50049423
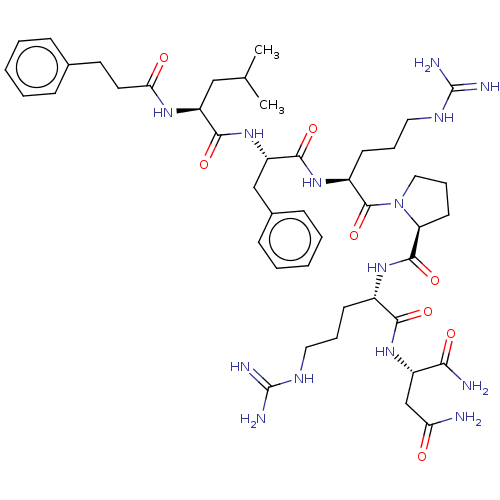
(CHEMBL3315278)Show SMILES CC(C)C[C@H](NC(=O)CCc1ccccc1)C(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CC(N)=O)C(N)=O |r| Show InChI InChI=1S/C45H68N14O8/c1-27(2)24-33(54-37(61)20-19-28-12-5-3-6-13-28)40(64)58-34(25-29-14-7-4-8-15-29)41(65)56-31(17-10-22-53-45(50)51)43(67)59-23-11-18-35(59)42(66)55-30(16-9-21-52-44(48)49)39(63)57-32(38(47)62)26-36(46)60/h3-8,12-15,27,30-35H,9-11,16-26H2,1-2H3,(H2,46,60)(H2,47,62)(H,54,61)(H,55,66)(H,56,65)(H,57,63)(H,58,64)(H4,48,49,52)(H4,50,51,53)/t30-,31-,32-,33-,34-,35-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 9 | n/a | n/a | n/a | n/a |
Tokyo University of Pharmacy and Life Sciences
Curated by ChEMBL
| Assay Description
Partial agonist activity at human NMUR1 expressed in CHO cells by calcium mobilization assay |
J Med Chem 57: 6583-93 (2014)
Article DOI: 10.1021/jm500599s
BindingDB Entry DOI: 10.7270/Q2FQ9Z71 |
More data for this
Ligand-Target Pair | |
Neuromedin-U receptor 1
(Homo sapiens (Human)) | BDBM50049424

(CHEMBL3315348)Show SMILES NC(=O)C[C@H](NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@@H]1CCCN1C(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](Cc1ccccc1)NC(=O)[C@H](Cc1ccc2ccccc2c1)NC(=O)CCc1cccnc1)C(N)=O |r| Show InChI InChI=1S/C51H67N15O8/c52-42(67)29-38(44(53)69)64-45(70)36(15-7-23-59-50(54)55)62-48(73)41-17-9-25-66(41)49(74)37(16-8-24-60-51(56)57)63-47(72)40(27-31-10-2-1-3-11-31)65-46(71)39(61-43(68)21-19-32-12-6-22-58-30-32)28-33-18-20-34-13-4-5-14-35(34)26-33/h1-6,10-14,18,20,22,26,30,36-41H,7-9,15-17,19,21,23-25,27-29H2,(H2,52,67)(H2,53,69)(H,61,68)(H,62,73)(H,63,72)(H,64,70)(H,65,71)(H4,54,55,59)(H4,56,57,60)/t36-,37-,38-,39-,40-,41-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 2.20 | n/a | n/a | n/a | n/a |
Tokyo University of Pharmacy and Life Sciences
Curated by ChEMBL
| Assay Description
Partial agonist activity at human NMUR1 expressed in CHO cells by calcium mobilization assay |
J Med Chem 57: 6583-93 (2014)
Article DOI: 10.1021/jm500599s
BindingDB Entry DOI: 10.7270/Q2FQ9Z71 |
More data for this
Ligand-Target Pair | |
Neuromedin-U receptor 2
(Homo sapiens (Human)) | BDBM50049423

(CHEMBL3315278)Show SMILES CC(C)C[C@H](NC(=O)CCc1ccccc1)C(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CC(N)=O)C(N)=O |r| Show InChI InChI=1S/C45H68N14O8/c1-27(2)24-33(54-37(61)20-19-28-12-5-3-6-13-28)40(64)58-34(25-29-14-7-4-8-15-29)41(65)56-31(17-10-22-53-45(50)51)43(67)59-23-11-18-35(59)42(66)55-30(16-9-21-52-44(48)49)39(63)57-32(38(47)62)26-36(46)60/h3-8,12-15,27,30-35H,9-11,16-26H2,1-2H3,(H2,46,60)(H2,47,62)(H,54,61)(H,55,66)(H,56,65)(H,57,63)(H,58,64)(H4,48,49,52)(H4,50,51,53)/t30-,31-,32-,33-,34-,35-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 8.40 | n/a | n/a | n/a | n/a |
Tokyo University of Pharmacy and Life Sciences
Curated by ChEMBL
| Assay Description
Partial agonist activity at human NMUR2 expressed in CHO cells by calcium mobilization assay |
J Med Chem 57: 6583-93 (2014)
Article DOI: 10.1021/jm500599s
BindingDB Entry DOI: 10.7270/Q2FQ9Z71 |
More data for this
Ligand-Target Pair | |
Neuromedin-U receptor 1
(Homo sapiens (Human)) | BDBM50049421

(CHEMBL3315349)Show SMILES CC(C)C[C@H](NC(=O)[C@H](Cc1ccccc1)NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)CNC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](CO)NC(=O)[C@H](CCC(N)=O)NC(=O)[C@H](CO)NC(=O)[C@H](C)NC(=O)[C@H](Cc1ccccc1)NC(=O)[C@@H]1CCCN1C(=O)[C@H](CO)NC(=O)[C@H](CCC(N)=O)NC(=O)[C@H](Cc1ccccc1)NC(=O)[C@H](CCC(O)=O)NC(=O)[C@H](CCC(O)=O)NC(=O)[C@H](CC(O)=O)NC(=O)[C@@H](NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@@H](N)Cc1ccccc1)C(C)C)C(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CC(N)=O)C(N)=O |r| Show InChI InChI=1S/C141H203N41O38/c1-74(2)61-94(125(208)173-98(65-80-33-17-9-18-34-80)128(211)169-92(40-24-58-158-141(153)154)136(219)181-59-25-41-104(181)133(216)168-86(38-22-56-156-139(149)150)118(201)170-93(114(146)197)68-108(145)189)171-129(212)99(66-81-35-19-10-20-36-81)174-126(209)95(67-82-43-45-83(186)46-44-82)161-109(190)70-159-117(200)85(37-21-55-155-138(147)148)163-132(215)102(72-184)178-121(204)88(47-51-106(143)187)167-131(214)101(71-183)177-115(198)76(5)160-124(207)96(63-78-29-13-7-14-30-78)175-134(217)105-42-26-60-182(105)137(220)103(73-185)179-122(205)89(48-52-107(144)188)165-127(210)97(64-79-31-15-8-16-32-79)172-120(203)91(50-54-111(193)194)164-119(202)90(49-53-110(191)192)166-130(213)100(69-112(195)196)176-135(218)113(75(3)4)180-123(206)87(39-23-57-157-140(151)152)162-116(199)84(142)62-77-27-11-6-12-28-77/h6-20,27-36,43-46,74-76,84-105,113,183-186H,21-26,37-42,47-73,142H2,1-5H3,(H2,143,187)(H2,144,188)(H2,145,189)(H2,146,197)(H,159,200)(H,160,207)(H,161,190)(H,162,199)(H,163,215)(H,164,202)(H,165,210)(H,166,213)(H,167,214)(H,168,216)(H,169,211)(H,170,201)(H,171,212)(H,172,203)(H,173,208)(H,174,209)(H,175,217)(H,176,218)(H,177,198)(H,178,204)(H,179,205)(H,180,206)(H,191,192)(H,193,194)(H,195,196)(H4,147,148,155)(H4,149,150,156)(H4,151,152,157)(H4,153,154,158)/t76-,84-,85-,86-,87-,88-,89-,90-,91-,92-,93-,94-,95-,96-,97-,98-,99-,100-,101-,102-,103-,104-,105-,113-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 0.430 | n/a | n/a | n/a | n/a |
Tokyo University of Pharmacy and Life Sciences
Curated by ChEMBL
| Assay Description
Agonist activity at human NMUR1 expressed in CHO cells by calcium mobilization assay |
J Med Chem 57: 6583-93 (2014)
Article DOI: 10.1021/jm500599s
BindingDB Entry DOI: 10.7270/Q2FQ9Z71 |
More data for this
Ligand-Target Pair | |
Neuromedin-U receptor 2
(Homo sapiens (Human)) | BDBM50049422

(CHEMBL3315335)Show SMILES CC(C)C[C@H](NC(=O)CCC1CCCCC1)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CN)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CC(N)=O)C(N)=O |r| Show InChI InChI=1S/C39H70N12O8/c1-22(2)18-27(46-32(53)15-14-24-10-6-5-7-11-24)35(56)49-28(19-23(3)4)36(57)50-29(21-40)38(59)51-17-9-13-30(51)37(58)47-25(12-8-16-45-39(43)44)34(55)48-26(33(42)54)20-31(41)52/h22-30H,5-21,40H2,1-4H3,(H2,41,52)(H2,42,54)(H,46,53)(H,47,58)(H,48,55)(H,49,56)(H,50,57)(H4,43,44,45)/t25-,26-,27-,28-,29-,30-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 6.40 | n/a | n/a | n/a | n/a |
Tokyo University of Pharmacy and Life Sciences
Curated by ChEMBL
| Assay Description
Agonist activity at human NMUR2 expressed in CHO cells by calcium mobilization assay |
J Med Chem 57: 6583-93 (2014)
Article DOI: 10.1021/jm500599s
BindingDB Entry DOI: 10.7270/Q2FQ9Z71 |
More data for this
Ligand-Target Pair | |
Neuromedin-U receptor 2
(Homo sapiens (Human)) | BDBM50049421

(CHEMBL3315349)Show SMILES CC(C)C[C@H](NC(=O)[C@H](Cc1ccccc1)NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)CNC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](CO)NC(=O)[C@H](CCC(N)=O)NC(=O)[C@H](CO)NC(=O)[C@H](C)NC(=O)[C@H](Cc1ccccc1)NC(=O)[C@@H]1CCCN1C(=O)[C@H](CO)NC(=O)[C@H](CCC(N)=O)NC(=O)[C@H](Cc1ccccc1)NC(=O)[C@H](CCC(O)=O)NC(=O)[C@H](CCC(O)=O)NC(=O)[C@H](CC(O)=O)NC(=O)[C@@H](NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@@H](N)Cc1ccccc1)C(C)C)C(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CC(N)=O)C(N)=O |r| Show InChI InChI=1S/C141H203N41O38/c1-74(2)61-94(125(208)173-98(65-80-33-17-9-18-34-80)128(211)169-92(40-24-58-158-141(153)154)136(219)181-59-25-41-104(181)133(216)168-86(38-22-56-156-139(149)150)118(201)170-93(114(146)197)68-108(145)189)171-129(212)99(66-81-35-19-10-20-36-81)174-126(209)95(67-82-43-45-83(186)46-44-82)161-109(190)70-159-117(200)85(37-21-55-155-138(147)148)163-132(215)102(72-184)178-121(204)88(47-51-106(143)187)167-131(214)101(71-183)177-115(198)76(5)160-124(207)96(63-78-29-13-7-14-30-78)175-134(217)105-42-26-60-182(105)137(220)103(73-185)179-122(205)89(48-52-107(144)188)165-127(210)97(64-79-31-15-8-16-32-79)172-120(203)91(50-54-111(193)194)164-119(202)90(49-53-110(191)192)166-130(213)100(69-112(195)196)176-135(218)113(75(3)4)180-123(206)87(39-23-57-157-140(151)152)162-116(199)84(142)62-77-27-11-6-12-28-77/h6-20,27-36,43-46,74-76,84-105,113,183-186H,21-26,37-42,47-73,142H2,1-5H3,(H2,143,187)(H2,144,188)(H2,145,189)(H2,146,197)(H,159,200)(H,160,207)(H,161,190)(H,162,199)(H,163,215)(H,164,202)(H,165,210)(H,166,213)(H,167,214)(H,168,216)(H,169,211)(H,170,201)(H,171,212)(H,172,203)(H,173,208)(H,174,209)(H,175,217)(H,176,218)(H,177,198)(H,178,204)(H,179,205)(H,180,206)(H,191,192)(H,193,194)(H,195,196)(H4,147,148,155)(H4,149,150,156)(H4,151,152,157)(H4,153,154,158)/t76-,84-,85-,86-,87-,88-,89-,90-,91-,92-,93-,94-,95-,96-,97-,98-,99-,100-,101-,102-,103-,104-,105-,113-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 2.5 | n/a | n/a | n/a | n/a |
Tokyo University of Pharmacy and Life Sciences
Curated by ChEMBL
| Assay Description
Agonist activity at human NMUR2 expressed in CHO cells by calcium mobilization assay |
J Med Chem 57: 6583-93 (2014)
Article DOI: 10.1021/jm500599s
BindingDB Entry DOI: 10.7270/Q2FQ9Z71 |
More data for this
Ligand-Target Pair | |
Tubulin alpha-1A/beta chain
(Sus scrofa (Pig)) | BDBM50030765

(NPI-2358 | Plinabulin)Show SMILES CC(C)(C)c1[nH]cnc1\C=c1/[nH]c(=O)\c(=C\c2ccccc2)[nH]c1=O Show InChI InChI=1S/C19H20N4O2/c1-19(2,3)16-13(20-11-21-16)10-15-18(25)22-14(17(24)23-15)9-12-7-5-4-6-8-12/h4-11H,1-3H3,(H,20,21)(H,22,25)(H,23,24)/b14-9-,15-10- | PDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a |
Tokyo University of Pharmacy and Life Sciences
Curated by ChEMBL
| Assay Description
Binding affinity to porcine tubulin incubated for 1 hr by spectrofluorometry |
ACS Med Chem Lett 5: 1094-8 (2014)
Article DOI: 10.1021/ml5001883
BindingDB Entry DOI: 10.7270/Q2R49SCP |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Tubulin beta chain
(Sus scrofa) | BDBM50030766

(CHEMBL3342339)Show SMILES O=C(c1ccccc1)c1cccc(\C=c2/[nH]c(=O)\c(=C\c3ccccn3)[nH]c2=O)c1 Show InChI InChI=1S/C24H17N3O3/c28-22(17-8-2-1-3-9-17)18-10-6-7-16(13-18)14-20-23(29)27-21(24(30)26-20)15-19-11-4-5-12-25-19/h1-15H,(H,26,30)(H,27,29)/b20-14-,21-15- | PDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | 1.30E+3 | n/a | n/a | n/a | n/a | n/a |
Tokyo University of Pharmacy and Life Sciences
Curated by ChEMBL
| Assay Description
Binding affinity to porcine tubulin incubated for 1 hr by spectrofluorometry |
ACS Med Chem Lett 5: 1094-8 (2014)
Article DOI: 10.1021/ml5001883
BindingDB Entry DOI: 10.7270/Q2R49SCP |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data