

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Peptidyl-prolyl cis-trans isomerase A (Rattus norvegicus) | BDBM50125708 (1-(3,5-Dichloro-phenyl)-3-{3-[3-(3,5-dichloro-phen...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 480 | n/a | n/a | n/a | n/a | n/a | n/a |
Guilford Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description In vitro inhibition of the rotamase activity of cyclophilin A (CyPA) | J Med Chem 46: 1112-5 (2003) Article DOI: 10.1021/jm020409u BindingDB Entry DOI: 10.7270/Q2V1244X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptidyl-prolyl cis-trans isomerase A (Rattus norvegicus) | BDBM50125717 (1-{2,4-Dibromo-5-[3-(4-iodo-phenyl)-ureido]-phenyl...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 590 | n/a | n/a | n/a | n/a | n/a | n/a |
Guilford Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description In vitro inhibition of the rotamase activity of cyclophilin A (CyPA) | J Med Chem 46: 1112-5 (2003) Article DOI: 10.1021/jm020409u BindingDB Entry DOI: 10.7270/Q2V1244X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptidyl-prolyl cis-trans isomerase A (Rattus norvegicus) | BDBM50125729 (3,5-Dichloro-N-{3-[3-(2,4-dibromo-phenyl)-ureido]-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 620 | n/a | n/a | n/a | n/a | n/a | n/a |
Guilford Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description In vitro inhibition of the rotamase activity of cyclophilin A (CyPA) | J Med Chem 46: 1112-5 (2003) Article DOI: 10.1021/jm020409u BindingDB Entry DOI: 10.7270/Q2V1244X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptidyl-prolyl cis-trans isomerase A (Rattus norvegicus) | BDBM50125714 (3,5-Dichloro-N-{3-[3-(3,5-dichloro-phenyl)-ureido]...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 690 | n/a | n/a | n/a | n/a | n/a | n/a |
Guilford Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description In vitro inhibition of the rotamase activity of cyclophilin A (CyPA) | J Med Chem 46: 1112-5 (2003) Article DOI: 10.1021/jm020409u BindingDB Entry DOI: 10.7270/Q2V1244X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptidyl-prolyl cis-trans isomerase A (Rattus norvegicus) | BDBM50125731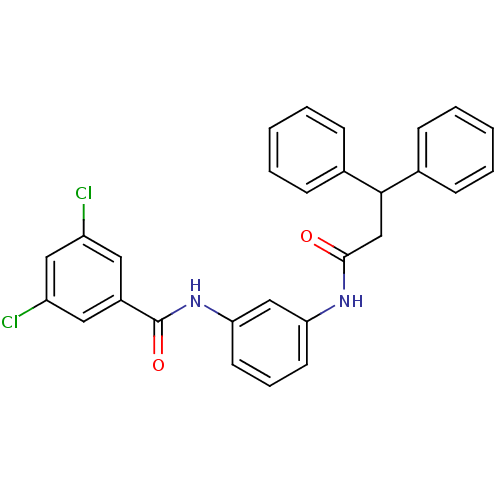 (3,5-Dichloro-N-[3-(3,3-diphenyl-propionylamino)-ph...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 870 | n/a | n/a | n/a | n/a | n/a | n/a |
Guilford Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description In vitro inhibition of the rotamase activity of cyclophilin A (CyPA) | J Med Chem 46: 1112-5 (2003) Article DOI: 10.1021/jm020409u BindingDB Entry DOI: 10.7270/Q2V1244X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptidyl-prolyl cis-trans isomerase A (Rattus norvegicus) | BDBM50125720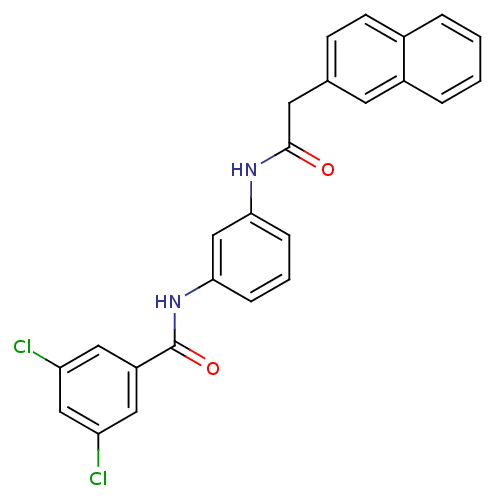 (3,5-Dichloro-N-[3-(2-naphthalen-2-yl-acetylamino)-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 880 | n/a | n/a | n/a | n/a | n/a | n/a |
Guilford Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description In vitro inhibition of the rotamase activity of cyclophilin A (CyPA) | J Med Chem 46: 1112-5 (2003) Article DOI: 10.1021/jm020409u BindingDB Entry DOI: 10.7270/Q2V1244X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptidyl-prolyl cis-trans isomerase A (Rattus norvegicus) | BDBM50125716 (3,5-dichloro-N-{3-[(3,5-dichlorobenzoyl)amino]phen...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 930 | n/a | n/a | n/a | n/a | n/a | n/a |
Guilford Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description In vitro inhibition of the rotamase activity of cyclophilin A (CyPA) | J Med Chem 46: 1112-5 (2003) Article DOI: 10.1021/jm020409u BindingDB Entry DOI: 10.7270/Q2V1244X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptidyl-prolyl cis-trans isomerase A (Rattus norvegicus) | BDBM50125706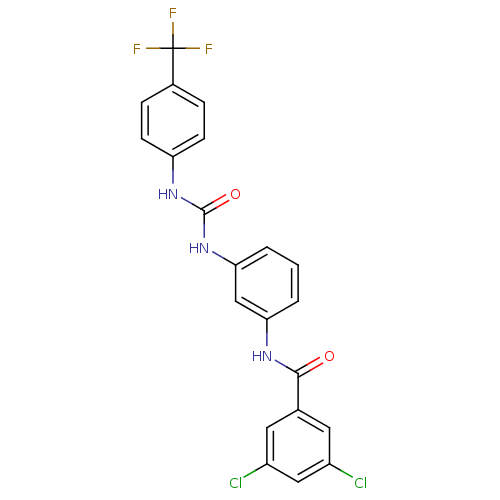 (3,5-Dichloro-N-{3-[3-(4-trifluoromethyl-phenyl)-ur...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 940 | n/a | n/a | n/a | n/a | n/a | n/a |
Guilford Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description In vitro inhibition of the rotamase activity of cyclophilin A (CyPA) | J Med Chem 46: 1112-5 (2003) Article DOI: 10.1021/jm020409u BindingDB Entry DOI: 10.7270/Q2V1244X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptidyl-prolyl cis-trans isomerase A (Rattus norvegicus) | BDBM50125718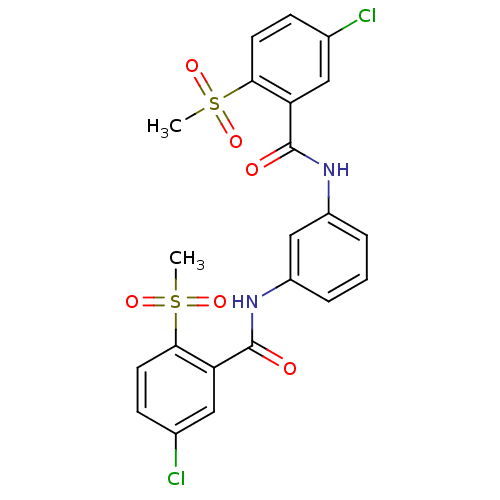 (1,3-di(5-chloro-2-methylsulfonylphenylcarboxamido)...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.02E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Guilford Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description In vitro inhibition of the rotamase activity of cyclophilin A (CyPA) | J Med Chem 46: 1112-5 (2003) Article DOI: 10.1021/jm020409u BindingDB Entry DOI: 10.7270/Q2V1244X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptidyl-prolyl cis-trans isomerase A (Rattus norvegicus) | BDBM50125721 (3,5-Dichloro-N-[3-(2-naphthalen-1-yl-acetylamino)-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.12E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Guilford Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description In vitro inhibition of the rotamase activity of cyclophilin A (CyPA) | J Med Chem 46: 1112-5 (2003) Article DOI: 10.1021/jm020409u BindingDB Entry DOI: 10.7270/Q2V1244X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptidyl-prolyl cis-trans isomerase A (Rattus norvegicus) | BDBM50125715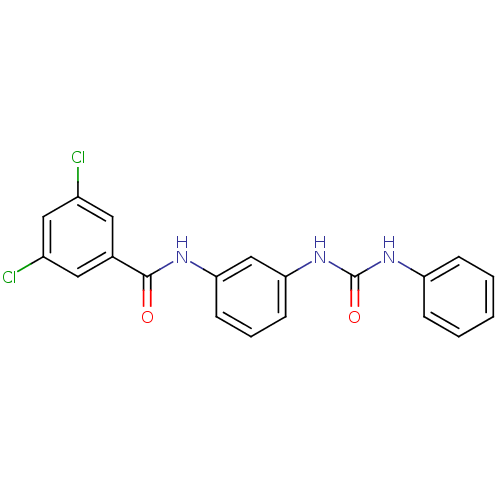 (3,5-Dichloro-N-[3-(3-phenyl-ureido)-phenyl]-benzam...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.18E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Guilford Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description In vitro inhibition of the rotamase activity of cyclophilin A (CyPA) | J Med Chem 46: 1112-5 (2003) Article DOI: 10.1021/jm020409u BindingDB Entry DOI: 10.7270/Q2V1244X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptidyl-prolyl cis-trans isomerase A (Rattus norvegicus) | BDBM50125719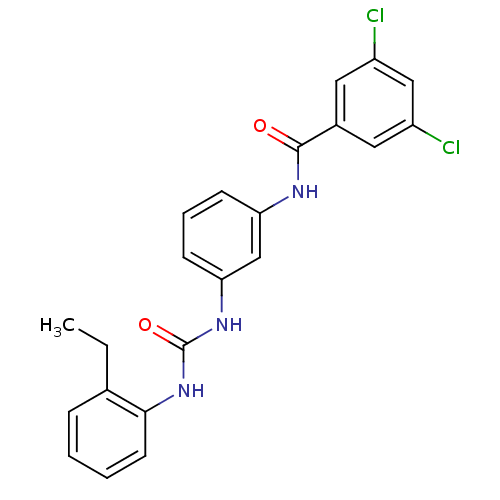 (3,5-Dichloro-N-{3-[3-(2-ethyl-phenyl)-ureido]-phen...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.41E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Guilford Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description In vitro inhibition of the rotamase activity of cyclophilin A (CyPA) | J Med Chem 46: 1112-5 (2003) Article DOI: 10.1021/jm020409u BindingDB Entry DOI: 10.7270/Q2V1244X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptidyl-prolyl cis-trans isomerase A (Rattus norvegicus) | BDBM50125722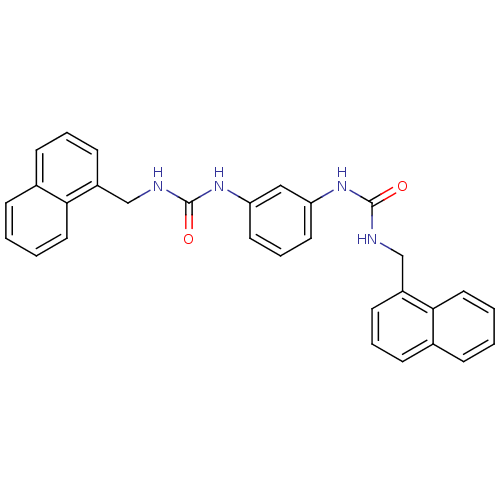 (1-Naphthalen-1-ylmethyl-3-[3-(3-naphthalen-1-ylmet...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 1.92E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Guilford Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description In vitro inhibition of the rotamase activity of cyclophilin A (CyPA) | J Med Chem 46: 1112-5 (2003) Article DOI: 10.1021/jm020409u BindingDB Entry DOI: 10.7270/Q2V1244X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptidyl-prolyl cis-trans isomerase A (Rattus norvegicus) | BDBM50125724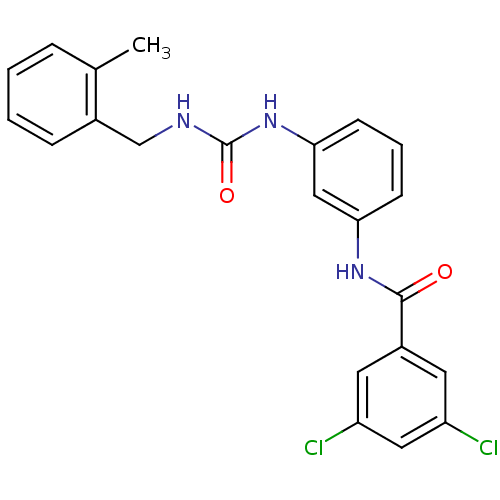 (3,5-Dichloro-N-{3-[3-(2-methyl-benzyl)-ureido]-phe...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.12E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Guilford Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description In vitro inhibition of the rotamase activity of cyclophilin A (CyPA) | J Med Chem 46: 1112-5 (2003) Article DOI: 10.1021/jm020409u BindingDB Entry DOI: 10.7270/Q2V1244X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptidyl-prolyl cis-trans isomerase A (Rattus norvegicus) | BDBM50125709 (1-{2,4-Dibromo-5-[3-(4-dimethylamino-phenyl)-ureid...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Guilford Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description In vitro inhibition of the rotamase activity of cyclophilin A (CyPA) | J Med Chem 46: 1112-5 (2003) Article DOI: 10.1021/jm020409u BindingDB Entry DOI: 10.7270/Q2V1244X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptidyl-prolyl cis-trans isomerase A (Rattus norvegicus) | BDBM50125732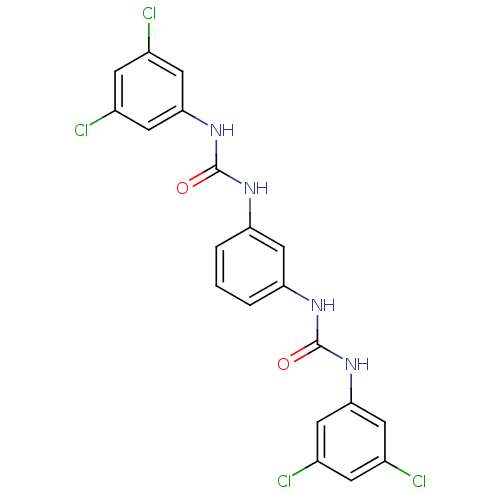 (1-(3,5-Dichloro-phenyl)-3-{3-[3-(3,5-dichloro-phen...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2.95E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Guilford Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description In vitro inhibition of the rotamase activity of cyclophilin A (CyPA) | J Med Chem 46: 1112-5 (2003) Article DOI: 10.1021/jm020409u BindingDB Entry DOI: 10.7270/Q2V1244X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptidyl-prolyl cis-trans isomerase A (Rattus norvegicus) | BDBM50125713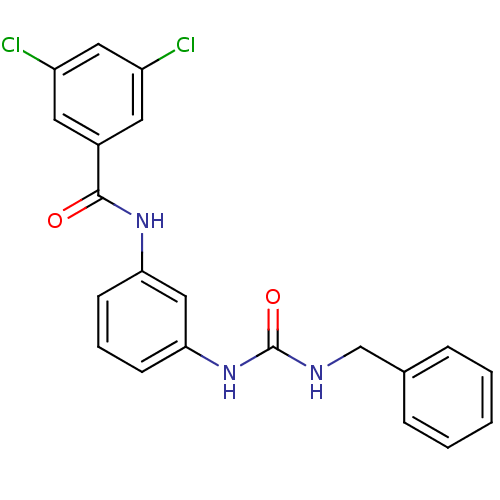 (CHEMBL276713 | N-[3-(3-Benzyl-ureido)-phenyl]-3,5-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.35E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Guilford Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description In vitro inhibition of the rotamase activity of cyclophilin A (CyPA) | J Med Chem 46: 1112-5 (2003) Article DOI: 10.1021/jm020409u BindingDB Entry DOI: 10.7270/Q2V1244X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptidyl-prolyl cis-trans isomerase A (Rattus norvegicus) | BDBM50125728 (CHEMBL13550 | N-(3,5-dichlorophenyl)-N'-{3-[({[(3,...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 3.95E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Guilford Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description In vitro inhibition of the rotamase activity of cyclophilin A (CyPA) | J Med Chem 46: 1112-5 (2003) Article DOI: 10.1021/jm020409u BindingDB Entry DOI: 10.7270/Q2V1244X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptidyl-prolyl cis-trans isomerase A (Rattus norvegicus) | BDBM50125707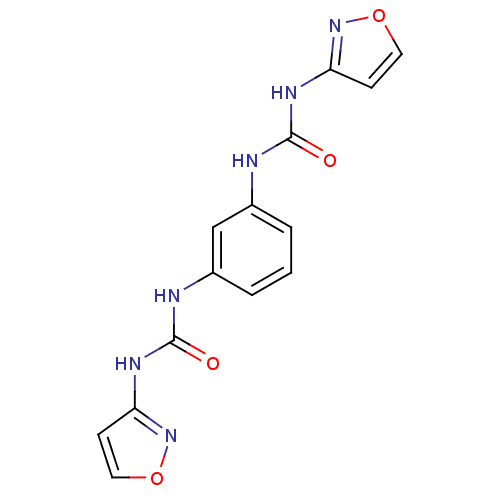 (1-Isoxazol-3-yl-3-[3-(3-isoxazol-3-yl-ureido)-phen...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 4.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Guilford Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description In vitro inhibition of the rotamase activity of cyclophilin A (CyPA) | J Med Chem 46: 1112-5 (2003) Article DOI: 10.1021/jm020409u BindingDB Entry DOI: 10.7270/Q2V1244X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptidyl-prolyl cis-trans isomerase A (Rattus norvegicus) | BDBM50125727 (1-(3,5-Dichloro-phenyl)-3-{3-[3-(3,5-dichloro-phen...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 4.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Guilford Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description In vitro inhibition of the rotamase activity of cyclophilin A (CyPA) | J Med Chem 46: 1112-5 (2003) Article DOI: 10.1021/jm020409u BindingDB Entry DOI: 10.7270/Q2V1244X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptidyl-prolyl cis-trans isomerase A (Rattus norvegicus) | BDBM50125710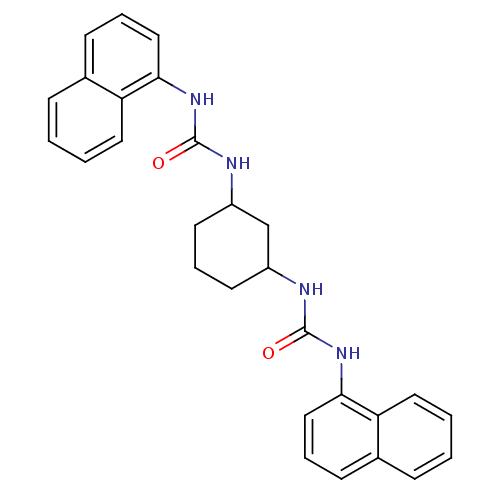 (1-Naphthalen-1-yl-3-[3-(3-naphthalen-1-yl-ureido)-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 4.55E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Guilford Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description In vitro inhibition of the rotamase activity of cyclophilin A (CyPA) | J Med Chem 46: 1112-5 (2003) Article DOI: 10.1021/jm020409u BindingDB Entry DOI: 10.7270/Q2V1244X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptidyl-prolyl cis-trans isomerase A (Rattus norvegicus) | BDBM50125712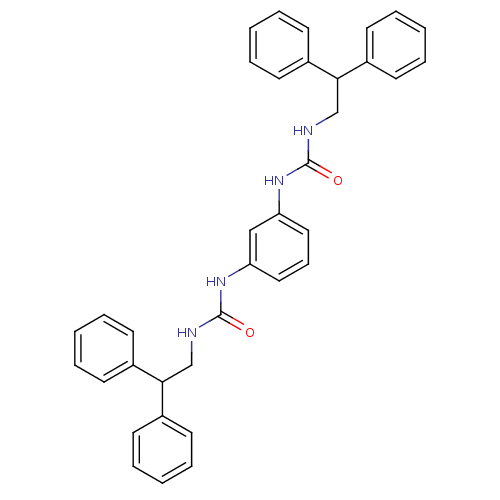 (1-(2,2-Diphenyl-ethyl)-3-{3-[3-(2,2-diphenyl-ethyl...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 4.85E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Guilford Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description In vitro inhibition of the rotamase activity of cyclophilin A (CyPA) | J Med Chem 46: 1112-5 (2003) Article DOI: 10.1021/jm020409u BindingDB Entry DOI: 10.7270/Q2V1244X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptidyl-prolyl cis-trans isomerase A (Rattus norvegicus) | BDBM50125723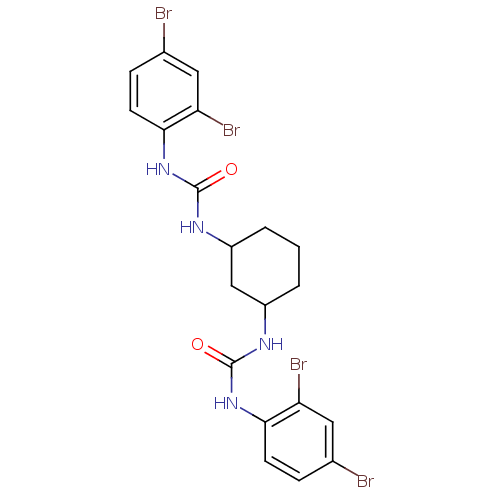 (1-(2,4-Dibromo-phenyl)-3-{3-[3-(2,4-dibromo-phenyl...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 5.09E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Guilford Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description In vitro inhibition of the rotamase activity of cyclophilin A (CyPA) | J Med Chem 46: 1112-5 (2003) Article DOI: 10.1021/jm020409u BindingDB Entry DOI: 10.7270/Q2V1244X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptidyl-prolyl cis-trans isomerase A (Rattus norvegicus) | BDBM50125725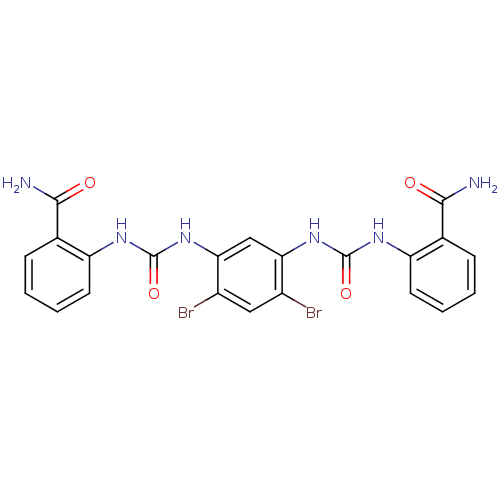 (1,1'-(4,6-dibromo-1,3-phenylene)bis(3-(2-carbamoyl...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 5.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Guilford Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description In vitro inhibition of the rotamase activity of cyclophilin A (CyPA) | J Med Chem 46: 1112-5 (2003) Article DOI: 10.1021/jm020409u BindingDB Entry DOI: 10.7270/Q2V1244X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptidyl-prolyl cis-trans isomerase A (Rattus norvegicus) | BDBM50125730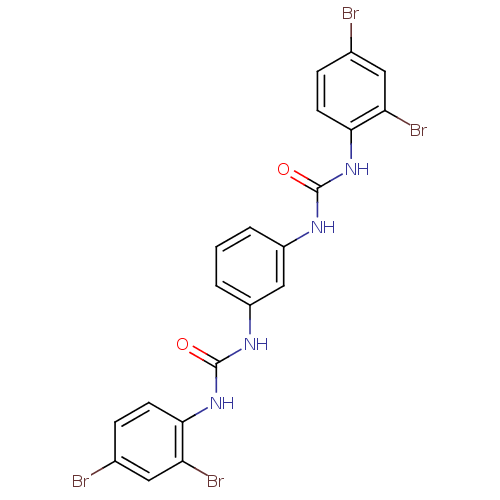 (1-(2,4-Dibromo-phenyl)-3-{3-[3-(2,4-dibromo-phenyl...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 6.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Guilford Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description In vitro inhibition of the rotamase activity of cyclophilin A (CyPA) | J Med Chem 46: 1112-5 (2003) Article DOI: 10.1021/jm020409u BindingDB Entry DOI: 10.7270/Q2V1244X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptidyl-prolyl cis-trans isomerase A (Rattus norvegicus) | BDBM50125711 (1-(2,4-Dimethyl-phenyl)-3-{3-[3-(2,4-dimethyl-phen...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 8.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Guilford Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description In vitro inhibition of the rotamase activity of cyclophilin A (CyPA) | J Med Chem 46: 1112-5 (2003) Article DOI: 10.1021/jm020409u BindingDB Entry DOI: 10.7270/Q2V1244X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptidyl-prolyl cis-trans isomerase A (Rattus norvegicus) | BDBM50125726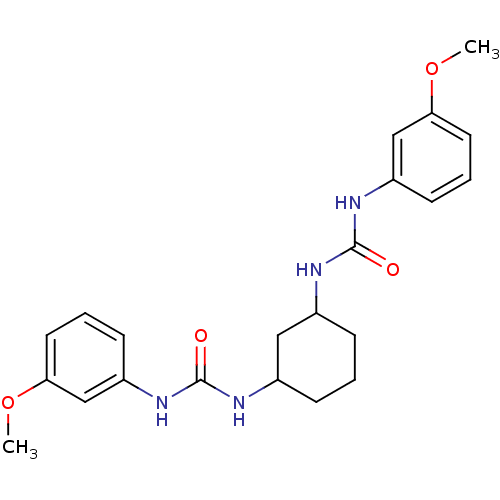 (1-(3-Methoxy-phenyl)-3-{3-[3-(3-methoxy-phenyl)-ur...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 9.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Guilford Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description In vitro inhibition of the rotamase activity of cyclophilin A (CyPA) | J Med Chem 46: 1112-5 (2003) Article DOI: 10.1021/jm020409u BindingDB Entry DOI: 10.7270/Q2V1244X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| E-selectin (Homo sapiens (Human)) | BDBM50450369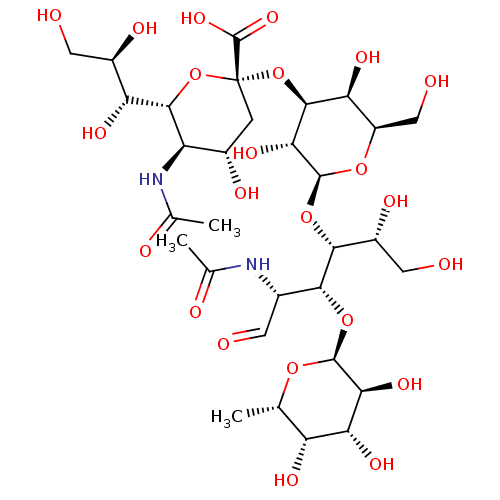 (SIALYL LEWIS X | Sialyl LeX | Sialyl lewis-x | sLe...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE KEGG PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 3.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research Curated by ChEMBL | Assay Description In vitro inhibitory activity against binding of Selectin E to human recombinant AGP (alpha-1 acid glycoprotein) containing sLex derivative | Bioorg Med Chem Lett 11: 151-5 (2001) BindingDB Entry DOI: 10.7270/Q2G73F71 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| E-selectin (Homo sapiens (Human)) | BDBM50096284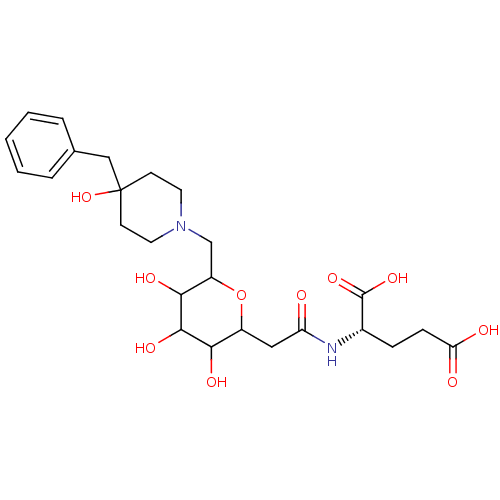 (2-{2-[6-(4-Benzyl-4-hydroxy-piperidin-1-ylmethyl)-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.60E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research Curated by ChEMBL | Assay Description In vitro inhibitory activity against Selectin E binding to human recombinant AGP (alpha-1 acid glycoprotein) | Bioorg Med Chem Lett 11: 151-5 (2001) BindingDB Entry DOI: 10.7270/Q2G73F71 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P-selectin glycoprotein ligand 1 (Homo sapiens (Human)) | BDBM50111451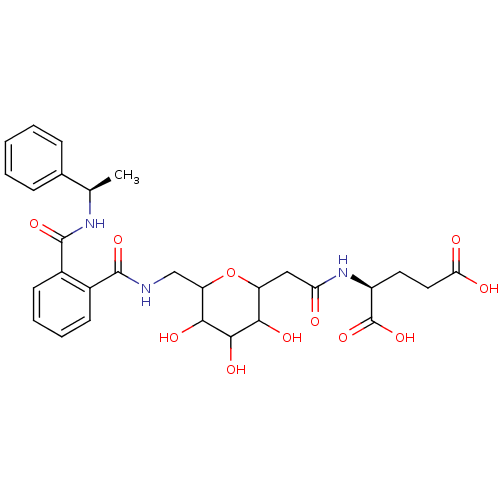 (2-[2-(3,4,5-Trihydroxy-6-{[2-(1-phenyl-ethylcarbam...) | NCI pathway Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem | PubMed | n/a | n/a | 2.50E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research Curated by ChEMBL | Assay Description Ability to inhibit the binding of P-selectin glycoprotein ligand 1 (PSGL-1) fusion protein to immobilized soluble P-selectin in a P-selectin assay. | J Med Chem 45: 1563-6 (2002) BindingDB Entry DOI: 10.7270/Q27W6CZ4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| E-selectin (Homo sapiens (Human)) | BDBM50096291 (2-[2-(3,4,5-Trihydroxy-6-hydroxymethyl-tetrahydro-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 3.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research Curated by ChEMBL | Assay Description In vitro inhibitory activity against Selectin E binding to human recombinant AGP (alpha-1 acid glycoprotein) | Bioorg Med Chem Lett 11: 151-5 (2001) BindingDB Entry DOI: 10.7270/Q2G73F71 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1-acid glycoprotein 1 (Homo sapiens (Human)) | BDBM50111451 (2-[2-(3,4,5-Trihydroxy-6-{[2-(1-phenyl-ethylcarbam...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem | PubMed | n/a | n/a | 5.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research Curated by ChEMBL | Assay Description Ability to inhibit the binding of E-selectin to human recombinant AGP (alpha-1 acid glycoprotein) in a E-selectin assay | J Med Chem 45: 1563-6 (2002) BindingDB Entry DOI: 10.7270/Q27W6CZ4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| E-selectin (Homo sapiens (Human)) | BDBM50096293 (2-[2-(3,4,5-Trihydroxy-6-hydroxymethyl-tetrahydro-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 6.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research Curated by ChEMBL | Assay Description In vitro inhibitory activity against Selectin E binding to human recombinant AGP (alpha-1 acid glycoprotein) | Bioorg Med Chem Lett 11: 151-5 (2001) BindingDB Entry DOI: 10.7270/Q2G73F71 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| E-selectin (Homo sapiens (Human)) | BDBM50096287 (5-({6-[(1,3-Dicarboxy-propylcarbamoyl)-methyl]-3,4...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem | PubMed | n/a | n/a | 6.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research Curated by ChEMBL | Assay Description In vitro inhibitory activity against Selectin E binding to human recombinant AGP (alpha-1 acid glycoprotein) | Bioorg Med Chem Lett 11: 151-5 (2001) BindingDB Entry DOI: 10.7270/Q2G73F71 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| E-selectin (Homo sapiens (Human)) | BDBM50096290 (2-(2-{6-[(1-Carbamoyl-2-phenyl-ethylamino)-methyl]...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem | PubMed | n/a | n/a | 6.70E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research Curated by ChEMBL | Assay Description In vitro inhibitory activity against Selectin E binding to human recombinant AGP (alpha-1 acid glycoprotein) | Bioorg Med Chem Lett 11: 151-5 (2001) BindingDB Entry DOI: 10.7270/Q2G73F71 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| E-selectin (Homo sapiens (Human)) | BDBM50096289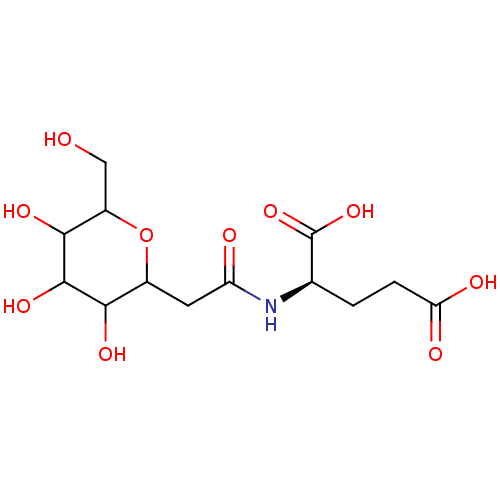 (2-[2-(3,4,5-Trihydroxy-6-hydroxymethyl-tetrahydro-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 7.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research Curated by ChEMBL | Assay Description In vitro inhibitory activity against Selectin E binding to human recombinant AGP (alpha-1 acid glycoprotein) | Bioorg Med Chem Lett 11: 151-5 (2001) BindingDB Entry DOI: 10.7270/Q2G73F71 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| E-selectin (Homo sapiens (Human)) | BDBM50096292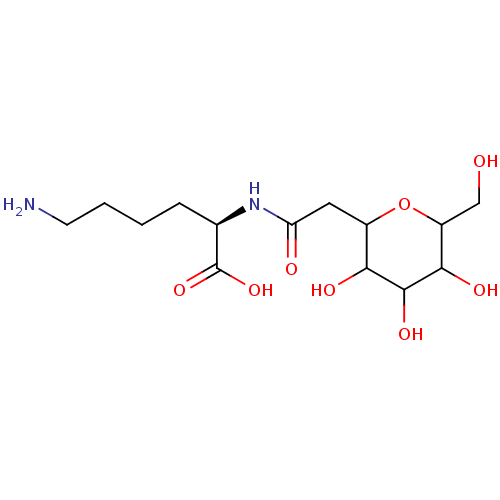 (6-Amino-2-[2-(3,4,5-trihydroxy-6-hydroxymethyl-tet...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 7.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research Curated by ChEMBL | Assay Description In vitro inhibitory activity against Selectin E binding to human recombinant AGP (alpha-1 acid glycoprotein) | Bioorg Med Chem Lett 11: 151-5 (2001) BindingDB Entry DOI: 10.7270/Q2G73F71 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| E-selectin (Homo sapiens (Human)) | BDBM50096286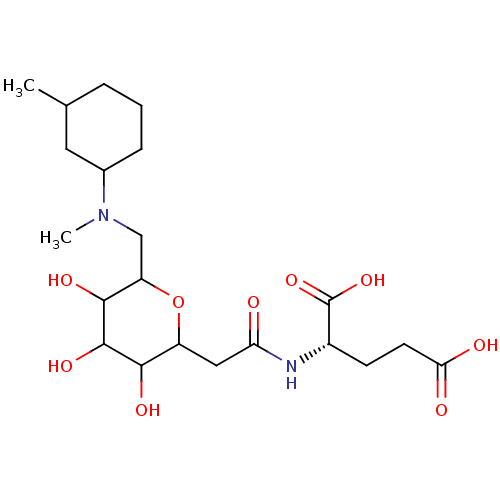 (2-[2-(3,4,5-Trihydroxy-6-{[methyl-(3-methyl-cycloh...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 8.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research Curated by ChEMBL | Assay Description In vitro inhibitory activity against Selectin E binding to human recombinant AGP (alpha-1 acid glycoprotein) | Bioorg Med Chem Lett 11: 151-5 (2001) BindingDB Entry DOI: 10.7270/Q2G73F71 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1-acid glycoprotein 1 (Homo sapiens (Human)) | BDBM50111453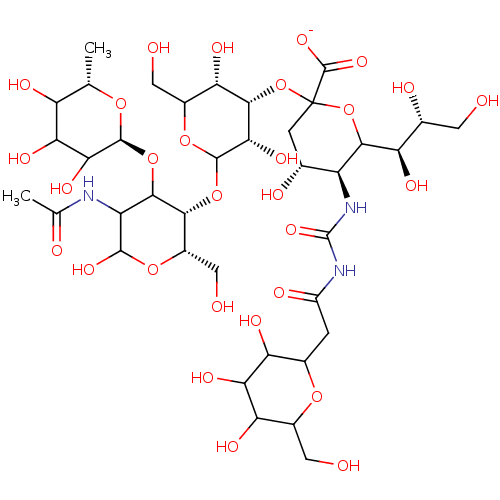 ((4R,5S)-2-{[(3S,4S,5S)-2-{[(2S,3S)-5-acetamido-6-h...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 8.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research Curated by ChEMBL | Assay Description Ability to inhibit the binding of E-selectin to human recombinant AGP (alpha-1 acid glycoprotein) in a E-selectin assay | J Med Chem 45: 1563-6 (2002) BindingDB Entry DOI: 10.7270/Q27W6CZ4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| E-selectin (Homo sapiens (Human)) | BDBM50096294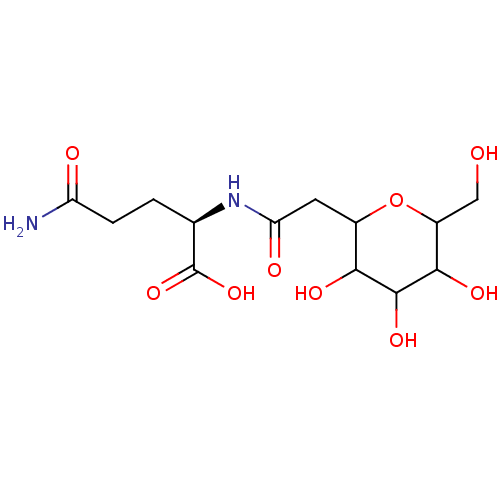 (4-Carbamoyl-2-[2-(3,4,5-trihydroxy-6-hydroxymethyl...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | >1.00E+7 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research Curated by ChEMBL | Assay Description In vitro inhibitory activity against Selectin E binding to human recombinant AGP (alpha-1 acid glycoprotein) | Bioorg Med Chem Lett 11: 151-5 (2001) BindingDB Entry DOI: 10.7270/Q2G73F71 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P-selectin (Homo sapiens (Human)) | BDBM50169053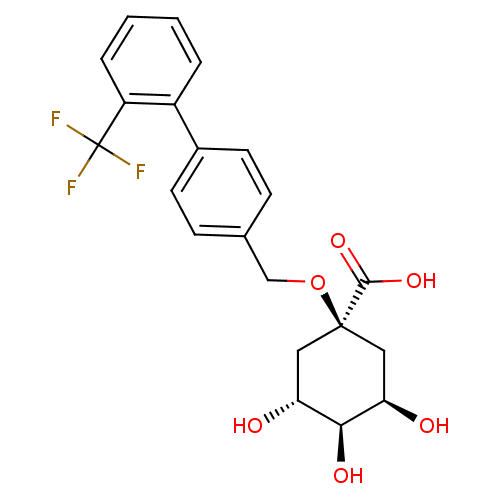 ((3R,5R)-3,4,5-Trihydroxy-1-(2'-trifluoromethyl-bip...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.00E+7 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Curated by ChEMBL | Assay Description Inhibitory concentration against selectin P in Biacore assay | J Med Chem 48: 4346-57 (2005) Article DOI: 10.1021/jm050049l BindingDB Entry DOI: 10.7270/Q2W095GF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| E-selectin (Homo sapiens (Human)) | BDBM50096285 (3-{4-[2-(3,4,5-Trihydroxy-6-hydroxymethyl-tetrahyd...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.00E+7 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research Curated by ChEMBL | Assay Description In vitro inhibitory activity against Selectin E binding to human recombinant AGP (alpha-1 acid glycoprotein) | Bioorg Med Chem Lett 11: 151-5 (2001) BindingDB Entry DOI: 10.7270/Q2G73F71 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| E-selectin (Homo sapiens (Human)) | BDBM50096288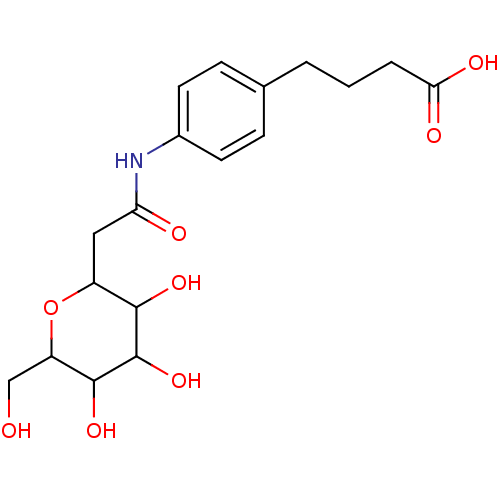 (4-{4-[2-(3,4,5-Trihydroxy-6-hydroxymethyl-tetrahyd...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | >1.00E+7 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research Curated by ChEMBL | Assay Description In vitro inhibitory activity against Selectin E binding to human recombinant AGP (alpha-1 acid glycoprotein) | Bioorg Med Chem Lett 11: 151-5 (2001) BindingDB Entry DOI: 10.7270/Q2G73F71 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P-selectin glycoprotein ligand 1 (Homo sapiens (Human)) | BDBM50111452 ((4R,5S)-5-[({[6-({[(1S)-1,3-dicarboxypropyl]carbam...) | NCI pathway Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2.50E+8 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research Curated by ChEMBL | Assay Description Ability to inhibit the binding of P-selectin glycoprotein ligand 1 (PSGL-1) fusion protein to immobilized soluble P-selectin in a P-selectin assay. | J Med Chem 45: 1563-6 (2002) BindingDB Entry DOI: 10.7270/Q27W6CZ4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||