 Found 307 hits with Last Name = 'toki' and Initial = 'h'
Found 307 hits with Last Name = 'toki' and Initial = 'h' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Retinoic acid receptor RXR-alpha
(Homo sapiens (Human)) | BDBM50032675

(4-[1-(3,5,5,8,8-pentamethyl-5,6,7,8-tetrahydronaph...)Show SMILES Cc1cc2c(cc1C(=C)c1ccc(cc1)C(O)=O)C(C)(C)CCC2(C)C Show InChI InChI=1S/C24H28O2/c1-15-13-20-21(24(5,6)12-11-23(20,3)4)14-19(15)16(2)17-7-9-18(10-8-17)22(25)26/h7-10,13-14H,2,11-12H2,1,3-6H3,(H,25,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
MMDB
PDB
Article
PubMed
| 150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Okayama University Graduate School of Medicine
Curated by ChEMBL
| Assay Description
Displacement of 9-cis-[11,12-3H]-retinoic acid from human RXRalpha LBD incubated for overnight by scintillation counting method |
J Med Chem 62: 8809-8818 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00995
BindingDB Entry DOI: 10.7270/Q2G44TRN |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Retinoic acid receptor RXR-alpha
(Homo sapiens (Human)) | BDBM50032675

(4-[1-(3,5,5,8,8-pentamethyl-5,6,7,8-tetrahydronaph...)Show SMILES Cc1cc2c(cc1C(=C)c1ccc(cc1)C(O)=O)C(C)(C)CCC2(C)C Show InChI InChI=1S/C24H28O2/c1-15-13-20-21(24(5,6)12-11-23(20,3)4)14-19(15)16(2)17-7-9-18(10-8-17)22(25)26/h7-10,13-14H,2,11-12H2,1,3-6H3,(H,25,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
MMDB
PDB
Article
PubMed
| 150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Okayama University Graduate School of Medicine
Curated by ChEMBL
| Assay Description
Displacement of 9-cis-[11,12-3H]-retinoic acid from human RXRalpha LBD incubated for overnight by scintillation counting method |
J Med Chem 62: 8809-8818 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00995
BindingDB Entry DOI: 10.7270/Q2G44TRN |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Retinoic acid receptor RXR-alpha
(Homo sapiens (Human)) | BDBM50339081
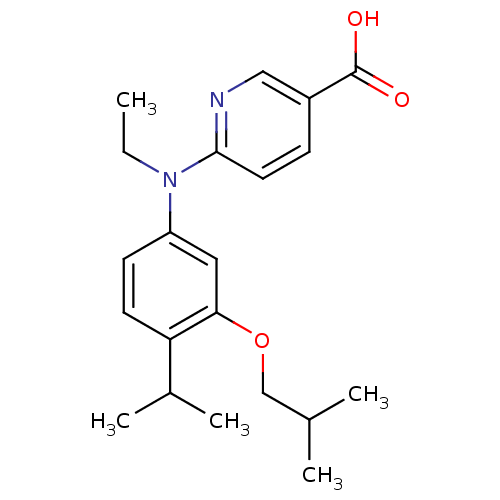
(6-[Ethyl-(3-isobutoxy-4-isopropylphenyl)amino]nico...)Show SMILES CCN(c1ccc(C(C)C)c(OCC(C)C)c1)c1ccc(cn1)C(O)=O Show InChI InChI=1S/C21H28N2O3/c1-6-23(20-10-7-16(12-22-20)21(24)25)17-8-9-18(15(4)5)19(11-17)26-13-14(2)3/h7-12,14-15H,6,13H2,1-5H3,(H,24,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 182 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Okayama University Graduate School of Medicine
Curated by ChEMBL
| Assay Description
Displacement of CU-6PMN from human RXRalpha LBD incubated for 2 hrs by fluorescence based assay |
J Med Chem 62: 8809-8818 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00995
BindingDB Entry DOI: 10.7270/Q2G44TRN |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Retinoic acid receptor RXR-alpha
(Homo sapiens (Human)) | BDBM50339081

(6-[Ethyl-(3-isobutoxy-4-isopropylphenyl)amino]nico...)Show SMILES CCN(c1ccc(C(C)C)c(OCC(C)C)c1)c1ccc(cn1)C(O)=O Show InChI InChI=1S/C21H28N2O3/c1-6-23(20-10-7-16(12-22-20)21(24)25)17-8-9-18(15(4)5)19(11-17)26-13-14(2)3/h7-12,14-15H,6,13H2,1-5H3,(H,24,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 182 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Okayama University Graduate School of Medicine
Curated by ChEMBL
| Assay Description
Displacement of CU-6PMN from human RXRalpha LBD incubated for 2 hrs by fluorescence based assay |
J Med Chem 62: 8809-8818 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00995
BindingDB Entry DOI: 10.7270/Q2G44TRN |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Retinoic acid receptor RXR-alpha
(Homo sapiens (Human)) | BDBM50530499

(CHEMBL4449685)Show SMILES Cc1cc2c(cc1-c1cc3cc(C(O)=O)c(=O)oc3cc1O)C(C)(C)CCC2(C)C Show InChI InChI=1S/C25H26O5/c1-13-8-18-19(25(4,5)7-6-24(18,2)3)11-15(13)16-9-14-10-17(22(27)28)23(29)30-21(14)12-20(16)26/h8-12,26H,6-7H2,1-5H3,(H,27,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| 230 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Okayama University Graduate School of Medicine
Curated by ChEMBL
| Assay Description
Displacement of 9-cis-[11,12-3H]-retinoic acid from human RXRalpha LBD incubated for overnight by scintillation counting method |
J Med Chem 62: 8809-8818 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00995
BindingDB Entry DOI: 10.7270/Q2G44TRN |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Retinoic acid receptor RXR-alpha
(Homo sapiens (Human)) | BDBM50530499

(CHEMBL4449685)Show SMILES Cc1cc2c(cc1-c1cc3cc(C(O)=O)c(=O)oc3cc1O)C(C)(C)CCC2(C)C Show InChI InChI=1S/C25H26O5/c1-13-8-18-19(25(4,5)7-6-24(18,2)3)11-15(13)16-9-14-10-17(22(27)28)23(29)30-21(14)12-20(16)26/h8-12,26H,6-7H2,1-5H3,(H,27,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| 230 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Okayama University Graduate School of Medicine
Curated by ChEMBL
| Assay Description
Displacement of 9-cis-[11,12-3H]-retinoic acid from human RXRalpha LBD incubated for overnight by scintillation counting method |
J Med Chem 62: 8809-8818 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00995
BindingDB Entry DOI: 10.7270/Q2G44TRN |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Retinoic acid receptor RXR-alpha
(Homo sapiens (Human)) | BDBM50032675

(4-[1-(3,5,5,8,8-pentamethyl-5,6,7,8-tetrahydronaph...)Show SMILES Cc1cc2c(cc1C(=C)c1ccc(cc1)C(O)=O)C(C)(C)CCC2(C)C Show InChI InChI=1S/C24H28O2/c1-15-13-20-21(24(5,6)12-11-23(20,3)4)14-19(15)16(2)17-7-9-18(10-8-17)22(25)26/h7-10,13-14H,2,11-12H2,1,3-6H3,(H,25,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
MMDB
PDB
Article
PubMed
| 379 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Okayama University Graduate School of Medicine
Curated by ChEMBL
| Assay Description
Displacement of CU-6PMN from human RXRalpha LBD incubated for 2 hrs by fluorescence based assay |
J Med Chem 62: 8809-8818 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00995
BindingDB Entry DOI: 10.7270/Q2G44TRN |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Retinoic acid receptor RXR-alpha
(Homo sapiens (Human)) | BDBM50032675

(4-[1-(3,5,5,8,8-pentamethyl-5,6,7,8-tetrahydronaph...)Show SMILES Cc1cc2c(cc1C(=C)c1ccc(cc1)C(O)=O)C(C)(C)CCC2(C)C Show InChI InChI=1S/C24H28O2/c1-15-13-20-21(24(5,6)12-11-23(20,3)4)14-19(15)16(2)17-7-9-18(10-8-17)22(25)26/h7-10,13-14H,2,11-12H2,1,3-6H3,(H,25,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
MMDB
PDB
Article
PubMed
| 379 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Okayama University Graduate School of Medicine
Curated by ChEMBL
| Assay Description
Displacement of CU-6PMN from human RXRalpha LBD incubated for 2 hrs by fluorescence based assay |
J Med Chem 62: 8809-8818 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00995
BindingDB Entry DOI: 10.7270/Q2G44TRN |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Retinoic acid receptor RXR-alpha
(Homo sapiens (Human)) | BDBM31892

(9-cis retinoic acid | 9C-RA | CHEMBL705 | Panretin...)Show SMILES C\C(\C=C\C1=C(C)CCCC1(C)C)=C\C=C\C(\C)=C\C(O)=O |c:4| Show InChI InChI=1S/C20H28O2/c1-15(8-6-9-16(2)14-19(21)22)11-12-18-17(3)10-7-13-20(18,4)5/h6,8-9,11-12,14H,7,10,13H2,1-5H3,(H,21,22)/b9-6+,12-11+,15-8-,16-14+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
MMDB
PDB
Article
PubMed
| 583 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Okayama University Graduate School of Medicine
Curated by ChEMBL
| Assay Description
Displacement of CU-6PMN from human RXRalpha LBD incubated for 2 hrs by fluorescence based assay |
J Med Chem 62: 8809-8818 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00995
BindingDB Entry DOI: 10.7270/Q2G44TRN |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Retinoic acid receptor RXR-alpha
(Homo sapiens (Human)) | BDBM31892

(9-cis retinoic acid | 9C-RA | CHEMBL705 | Panretin...)Show SMILES C\C(\C=C\C1=C(C)CCCC1(C)C)=C\C=C\C(\C)=C\C(O)=O |c:4| Show InChI InChI=1S/C20H28O2/c1-15(8-6-9-16(2)14-19(21)22)11-12-18-17(3)10-7-13-20(18,4)5/h6,8-9,11-12,14H,7,10,13H2,1-5H3,(H,21,22)/b9-6+,12-11+,15-8-,16-14+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
MMDB
PDB
Article
PubMed
| 583 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Okayama University Graduate School of Medicine
Curated by ChEMBL
| Assay Description
Displacement of CU-6PMN from human RXRalpha LBD incubated for 2 hrs by fluorescence based assay |
J Med Chem 62: 8809-8818 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00995
BindingDB Entry DOI: 10.7270/Q2G44TRN |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Retinoic acid receptor RXR-alpha
(Homo sapiens (Human)) | BDBM50210259
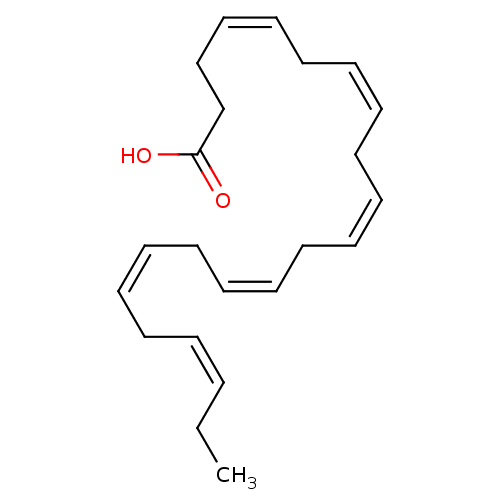
((4Z,7Z,10Z,13Z,16Z,19Z)-docosa-4,7,10,13,16,19-hex...)Show SMILES CC\C=C/C\C=C/C\C=C/C\C=C/C\C=C/C\C=C/CCC(O)=O Show InChI InChI=1S/C22H32O2/c1-2-3-4-5-6-7-8-9-10-11-12-13-14-15-16-17-18-19-20-21-22(23)24/h3-4,6-7,9-10,12-13,15-16,18-19H,2,5,8,11,14,17,20-21H2,1H3,(H,23,24)/b4-3-,7-6-,10-9-,13-12-,16-15-,19-18- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| 6.29E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Okayama University Graduate School of Medicine
Curated by ChEMBL
| Assay Description
Displacement of CU-6PMN from human RXRalpha LBD incubated for 2 hrs by fluorescence based assay |
J Med Chem 62: 8809-8818 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00995
BindingDB Entry DOI: 10.7270/Q2G44TRN |
More data for this
Ligand-Target Pair | |
Retinoic acid receptor RXR-alpha
(Homo sapiens (Human)) | BDBM50210259

((4Z,7Z,10Z,13Z,16Z,19Z)-docosa-4,7,10,13,16,19-hex...)Show SMILES CC\C=C/C\C=C/C\C=C/C\C=C/C\C=C/C\C=C/CCC(O)=O Show InChI InChI=1S/C22H32O2/c1-2-3-4-5-6-7-8-9-10-11-12-13-14-15-16-17-18-19-20-21-22(23)24/h3-4,6-7,9-10,12-13,15-16,18-19H,2,5,8,11,14,17,20-21H2,1H3,(H,23,24)/b4-3-,7-6-,10-9-,13-12-,16-15-,19-18- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| 6.29E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Okayama University Graduate School of Medicine
Curated by ChEMBL
| Assay Description
Displacement of CU-6PMN from human RXRalpha LBD incubated for 2 hrs by fluorescence based assay |
J Med Chem 62: 8809-8818 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00995
BindingDB Entry DOI: 10.7270/Q2G44TRN |
More data for this
Ligand-Target Pair | |
Retinoic acid receptor RXR-alpha
(Homo sapiens (Human)) | BDBM50242349

((5Z,8Z,11Z,14Z,17Z)-5,8,11,14,17-eicosapentaenoic ...)Show InChI InChI=1S/C20H30O2/c1-2-3-4-5-6-7-8-9-10-11-12-13-14-15-16-17-18-19-20(21)22/h3-4,6-7,9-10,12-13,15-16H,2,5,8,11,14,17-19H2,1H3,(H,21,22)/b4-3-,7-6-,10-9-,13-12-,16-15- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 7.63E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Okayama University Graduate School of Medicine
Curated by ChEMBL
| Assay Description
Displacement of CU-6PMN from human RXRalpha LBD incubated for 2 hrs by fluorescence based assay |
J Med Chem 62: 8809-8818 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00995
BindingDB Entry DOI: 10.7270/Q2G44TRN |
More data for this
Ligand-Target Pair | |
Retinoic acid receptor RXR-alpha
(Homo sapiens (Human)) | BDBM50242349

((5Z,8Z,11Z,14Z,17Z)-5,8,11,14,17-eicosapentaenoic ...)Show InChI InChI=1S/C20H30O2/c1-2-3-4-5-6-7-8-9-10-11-12-13-14-15-16-17-18-19-20(21)22/h3-4,6-7,9-10,12-13,15-16H,2,5,8,11,14,17-19H2,1H3,(H,21,22)/b4-3-,7-6-,10-9-,13-12-,16-15- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 7.63E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Okayama University Graduate School of Medicine
Curated by ChEMBL
| Assay Description
Displacement of CU-6PMN from human RXRalpha LBD incubated for 2 hrs by fluorescence based assay |
J Med Chem 62: 8809-8818 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00995
BindingDB Entry DOI: 10.7270/Q2G44TRN |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50606383
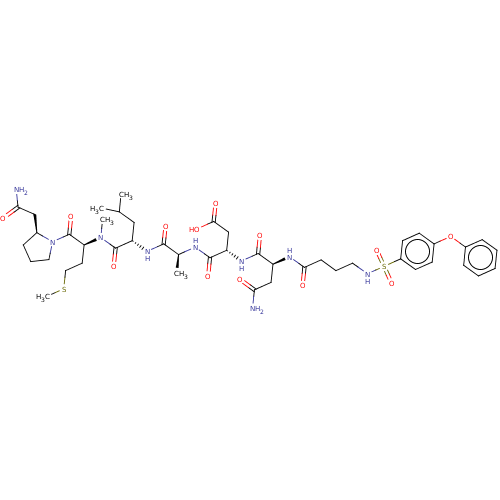
(CHEMBL5209489)Show SMILES CSCC[C@H](N(C)C(=O)[C@H](CC(C)C)NC(=O)[C@H](C)NC(=O)[C@H](CC(O)=O)NC(=O)[C@H](CC(N)=O)NC(=O)CCCNS(=O)(=O)c1ccc(Oc2ccccc2)cc1)C(=O)N1CCC[C@H]1CC(N)=O |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.0440 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00613
BindingDB Entry DOI: 10.7270/Q2KD231G |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50606385
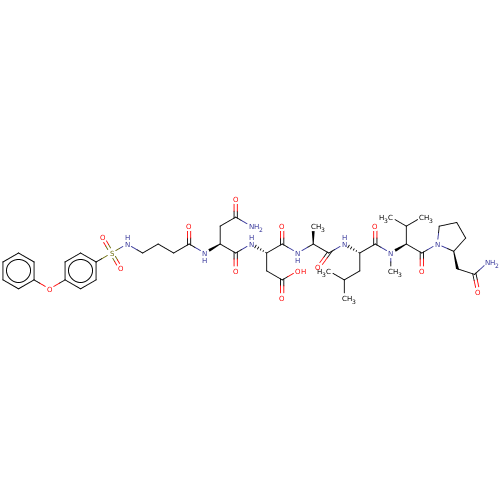
(CHEMBL5203315)Show SMILES CC(C)C[C@H](NC(=O)[C@H](C)NC(=O)[C@H](CC(O)=O)NC(=O)[C@H](CC(N)=O)NC(=O)CCCNS(=O)(=O)c1ccc(Oc2ccccc2)cc1)C(=O)N(C)[C@@H](C(C)C)C(=O)N1CCC[C@H]1CC(N)=O |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.0460 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00613
BindingDB Entry DOI: 10.7270/Q2KD231G |
More data for this
Ligand-Target Pair | |
Vitamin D3 receptor
(Homo sapiens (Human)) | BDBM50200182

((1S,3R,5Z,7E)-9,10-secocholesta-5,7,10(19)-triene-...)Show SMILES C[C@H](CCCC(C)(C)O)[C@H]1CC[C@H]2\C(CCC[C@]12C)=C\C=C1\C[C@@H](O)C[C@H](O)C1=C |r| Show InChI InChI=1S/C27H44O3/c1-18(8-6-14-26(3,4)30)23-12-13-24-20(9-7-15-27(23,24)5)10-11-21-16-22(28)17-25(29)19(21)2/h10-11,18,22-25,28-30H,2,6-9,12-17H2,1,3-5H3/b20-10+,21-11-/t18-,22-,23-,24+,25+,27-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| n/a | n/a | 0.0500 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad de Santiago de Compostela
Curated by ChEMBL
| Assay Description
Transrepression of VP16-tagged VDR (unknown origin) expressed in HEK293 cells harboring pCMX-GAL4-NCoR and MH100(UAS) X 4tk-LUC reporter plasmid asse... |
J Med Chem 61: 6658-6673 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00427
BindingDB Entry DOI: 10.7270/Q2M04801 |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50606395
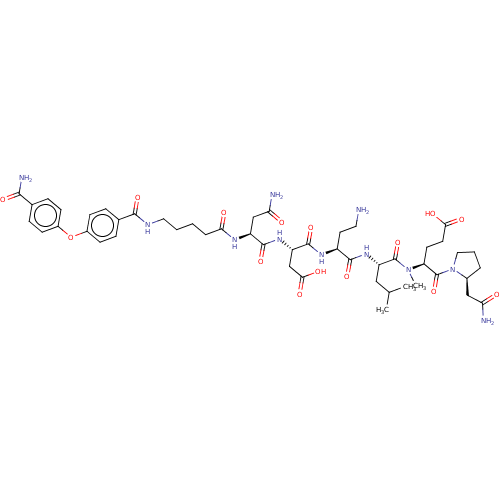
(CHEMBL5207094)Show SMILES CC(C)C[C@H](NC(=O)[C@H](CCN)NC(=O)[C@H](CC(O)=O)NC(=O)[C@H](CC(N)=O)NC(=O)CCCCNC(=O)c1ccc(Oc2ccc(cc2)C(N)=O)cc1)C(=O)N(C)[C@@H](CCC(O)=O)C(=O)N1CCC[C@H]1CC(N)=O |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.0510 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00613
BindingDB Entry DOI: 10.7270/Q2KD231G |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50606386
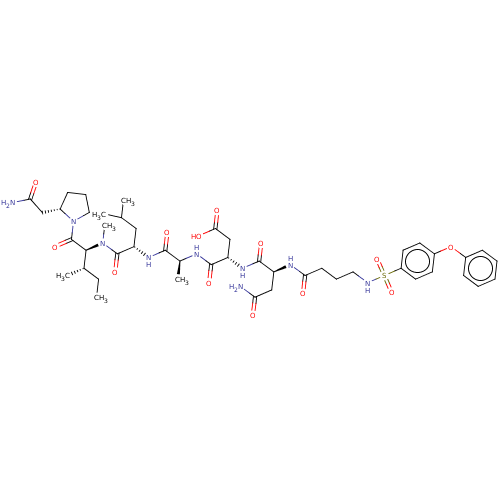
(CHEMBL5182207)Show SMILES CC[C@H](C)[C@H](N(C)C(=O)[C@H](CC(C)C)NC(=O)[C@H](C)NC(=O)[C@H](CC(O)=O)NC(=O)[C@H](CC(N)=O)NC(=O)CCCNS(=O)(=O)c1ccc(Oc2ccccc2)cc1)C(=O)N1CCC[C@H]1CC(N)=O |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.0550 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00613
BindingDB Entry DOI: 10.7270/Q2KD231G |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50606384
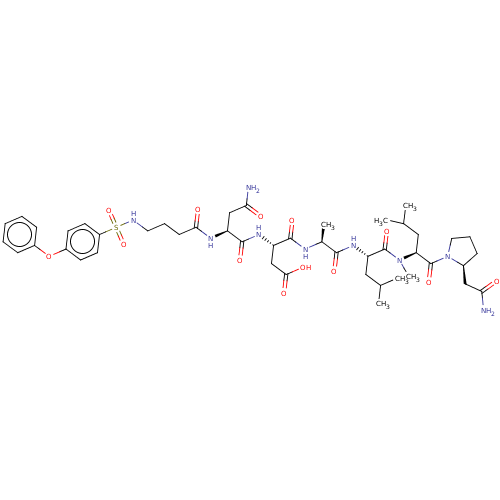
(CHEMBL5183245)Show SMILES CC(C)C[C@H](NC(=O)[C@H](C)NC(=O)[C@H](CC(O)=O)NC(=O)[C@H](CC(N)=O)NC(=O)CCCNS(=O)(=O)c1ccc(Oc2ccccc2)cc1)C(=O)N(C)[C@@H](CC(C)C)C(=O)N1CCC[C@H]1CC(N)=O |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.0550 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00613
BindingDB Entry DOI: 10.7270/Q2KD231G |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50606380
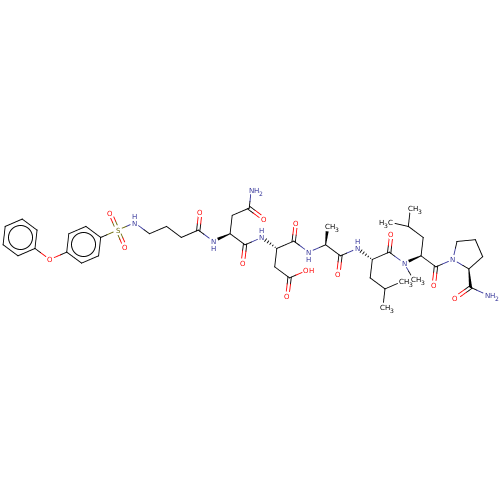
(CHEMBL5191373)Show SMILES CC(C)C[C@H](NC(=O)[C@H](C)NC(=O)[C@H](CC(O)=O)NC(=O)[C@H](CC(N)=O)NC(=O)CCCNS(=O)(=O)c1ccc(Oc2ccccc2)cc1)C(=O)N(C)[C@@H](CC(C)C)C(=O)N1CCC[C@H]1C(N)=O |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.0660 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00613
BindingDB Entry DOI: 10.7270/Q2KD231G |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50606390
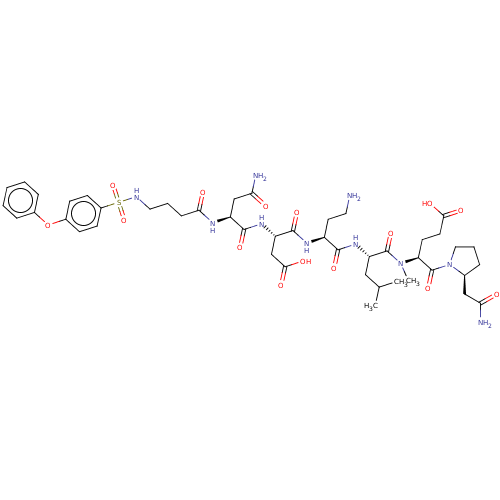
(CHEMBL5185612)Show SMILES CC(C)C[C@H](NC(=O)[C@H](CCN)NC(=O)[C@H](CC(O)=O)NC(=O)[C@H](CC(N)=O)NC(=O)CCCNS(=O)(=O)c1ccc(Oc2ccccc2)cc1)C(=O)N(C)[C@@H](CCC(O)=O)C(=O)N1CCC[C@H]1CC(N)=O |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.0680 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00613
BindingDB Entry DOI: 10.7270/Q2KD231G |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50606381
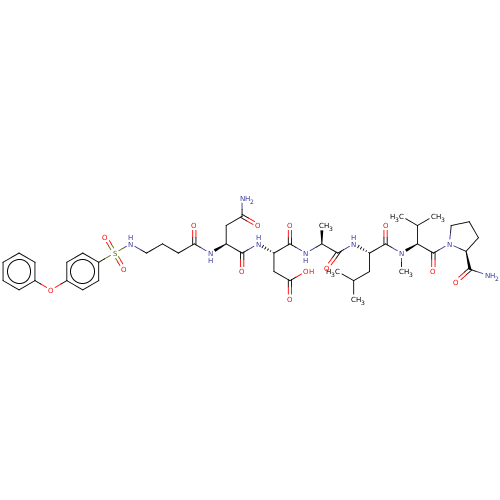
(CHEMBL5198135)Show SMILES CC(C)C[C@H](NC(=O)[C@H](C)NC(=O)[C@H](CC(O)=O)NC(=O)[C@H](CC(N)=O)NC(=O)CCCNS(=O)(=O)c1ccc(Oc2ccccc2)cc1)C(=O)N(C)[C@@H](C(C)C)C(=O)N1CCC[C@H]1C(N)=O |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.0780 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00613
BindingDB Entry DOI: 10.7270/Q2KD231G |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50606377
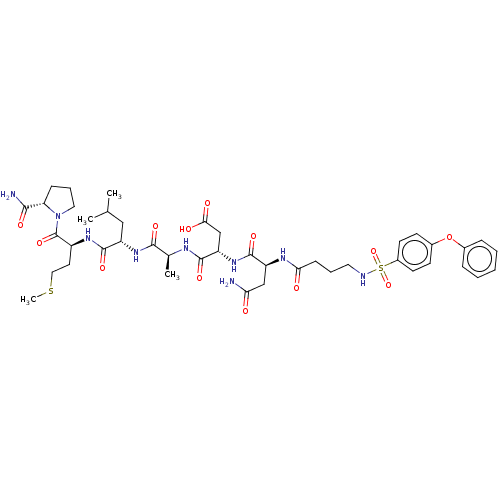
(CHEMBL5186594)Show SMILES CSCC[C@H](NC(=O)[C@H](CC(C)C)NC(=O)[C@H](C)NC(=O)[C@H](CC(O)=O)NC(=O)[C@H](CC(N)=O)NC(=O)CCCNS(=O)(=O)c1ccc(Oc2ccccc2)cc1)C(=O)N1CCC[C@H]1C(N)=O |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.0830 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00613
BindingDB Entry DOI: 10.7270/Q2KD231G |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50606378
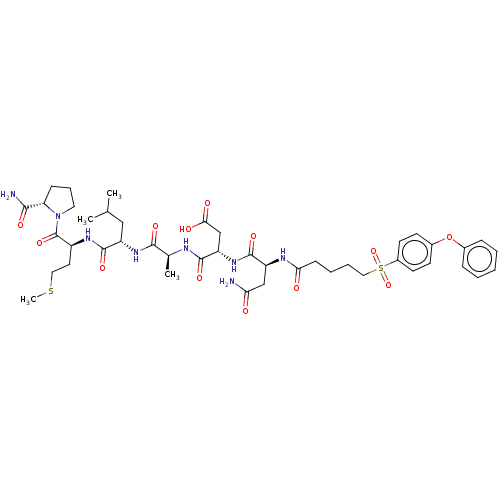
(CHEMBL5170274)Show SMILES CSCC[C@H](NC(=O)[C@H](CC(C)C)NC(=O)[C@H](C)NC(=O)[C@H](CC(O)=O)NC(=O)[C@H](CC(N)=O)NC(=O)CCCCS(=O)(=O)c1ccc(Oc2ccccc2)cc1)C(=O)N1CCC[C@H]1C(N)=O |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.0890 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00613
BindingDB Entry DOI: 10.7270/Q2KD231G |
More data for this
Ligand-Target Pair | |
Vitamin D3 receptor
(Homo sapiens (Human)) | BDBM50135039
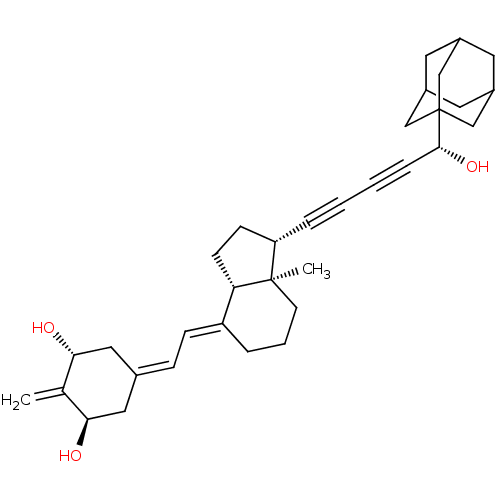
(CHEMBL3745849)Show SMILES [H][C@@]12[#6]-[#6]-[#6@H](C#CC#C[#6@@H](-[#8])C34[#6]-[#6]-5-[#6]-[#6](-[#6]-[#6](-[#6]-5)-[#6]3)-[#6]4)[C@@]1([#6])[#6]-[#6]-[#6]\[#6]2=[#6]/[#6]=[#6]-1/[#6]-[#6@@H](-[#8])-[#6](=[#6])-[#6@H](-[#8])-[#6]-1 |r,TLB:18:13:20:17.19.16,18:17:20:12.13.14,THB:16:15:12:18.17.19,16:17:12:20.14.15| Show InChI InChI=1S/C34H44O3/c1-22-30(35)17-23(18-31(22)36)9-10-27-6-5-13-33(2)28(11-12-29(27)33)7-3-4-8-32(37)34-19-24-14-25(20-34)16-26(15-24)21-34/h9-10,24-26,28-32,35-37H,1,5-6,11-21H2,2H3/b27-10+/t24?,25?,26?,28-,29-,30+,31+,32+,33+,34?/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.0900 | n/a | n/a | n/a | n/a | n/a | n/a |
Rikkyo University
Curated by ChEMBL
| Assay Description
Agonist activity at VDR (unknown origin) expressed in HEK293 cells cotransfected with NCoR assessed as decrease in NCoR recruitment by two-hybrid ass... |
J Med Chem 58: 9510-21 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00792
BindingDB Entry DOI: 10.7270/Q22V2HZC |
More data for this
Ligand-Target Pair | |
Vitamin D3 receptor
(Homo sapiens (Human)) | BDBM50281390
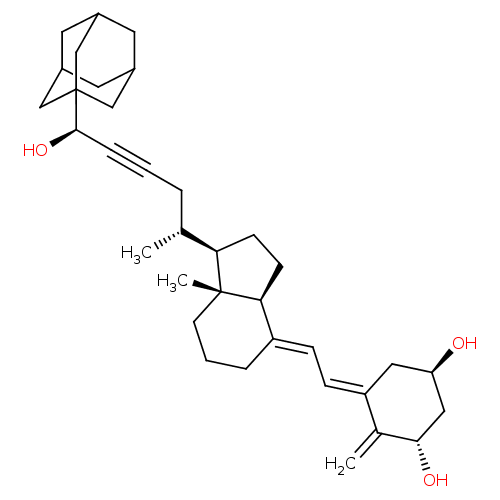
(CHEMBL4159525)Show SMILES [H][C@@]1(CC[C@@]2([H])\C(CCC[C@]12C)=C\C=C1\C[C@@H](O)C[C@H](O)C1=C)[C@H](C)CC#C[C@@H](O)C12CC3CC(CC(C3)C1)C2 |r,TLB:33:34:32.31.37:38,THB:35:34:31:37.36.38,35:36:33.34.39:31,33:32:34.39.35:38| Show InChI InChI=1S/C35H50O3/c1-22(6-4-8-33(38)35-19-24-14-25(20-35)16-26(15-24)21-35)30-11-12-31-27(7-5-13-34(30,31)3)9-10-28-17-29(36)18-32(37)23(28)2/h9-10,22,24-26,29-33,36-38H,2,5-7,11-21H2,1,3H3/b27-9+,28-10-/t22-,24?,25?,26?,29-,30-,31+,32+,33-,34-,35?/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.0900 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad de Santiago de Compostela
Curated by ChEMBL
| Assay Description
Transrepression of VP16-tagged VDR (unknown origin) expressed in HEK293 cells harboring pCMX-GAL4-NCoR and MH100(UAS) X 4tk-LUC reporter plasmid asse... |
J Med Chem 61: 6658-6673 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00427
BindingDB Entry DOI: 10.7270/Q2M04801 |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50606388
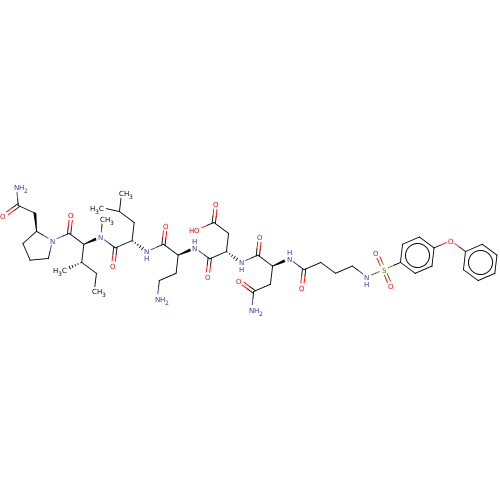
(CHEMBL5203492)Show SMILES CC[C@H](C)[C@H](N(C)C(=O)[C@H](CC(C)C)NC(=O)[C@H](CCN)NC(=O)[C@H](CC(O)=O)NC(=O)[C@H](CC(N)=O)NC(=O)CCCNS(=O)(=O)c1ccc(Oc2ccccc2)cc1)C(=O)N1CCC[C@H]1CC(N)=O |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.0900 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00613
BindingDB Entry DOI: 10.7270/Q2KD231G |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50606382
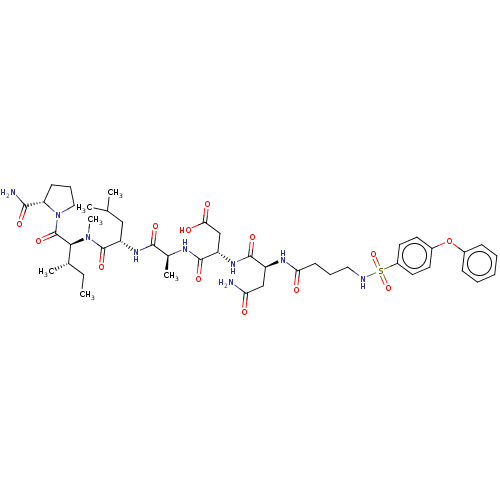
(CHEMBL5191820)Show SMILES CC[C@H](C)[C@H](N(C)C(=O)[C@H](CC(C)C)NC(=O)[C@H](C)NC(=O)[C@H](CC(O)=O)NC(=O)[C@H](CC(N)=O)NC(=O)CCCNS(=O)(=O)c1ccc(Oc2ccccc2)cc1)C(=O)N1CCC[C@H]1C(N)=O |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.0970 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00613
BindingDB Entry DOI: 10.7270/Q2KD231G |
More data for this
Ligand-Target Pair | |
Vitamin D3 receptor
(Homo sapiens (Human)) | BDBM50200182

((1S,3R,5Z,7E)-9,10-secocholesta-5,7,10(19)-triene-...)Show SMILES C[C@H](CCCC(C)(C)O)[C@H]1CC[C@H]2\C(CCC[C@]12C)=C\C=C1\C[C@@H](O)C[C@H](O)C1=C |r| Show InChI InChI=1S/C27H44O3/c1-18(8-6-14-26(3,4)30)23-12-13-24-20(9-7-15-27(23,24)5)10-11-21-16-22(28)17-25(29)19(21)2/h10-11,18,22-25,28-30H,2,6-9,12-17H2,1,3-5H3/b20-10+,21-11-/t18-,22-,23-,24+,25+,27-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
Rikkyo University
Curated by ChEMBL
| Assay Description
Agonist activity at VDR (unknown origin) expressed in HEK293 cells cotransfected with NCoR assessed as decrease in NCoR recruitment by two-hybrid ass... |
J Med Chem 58: 9510-21 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00792
BindingDB Entry DOI: 10.7270/Q22V2HZC |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50606392
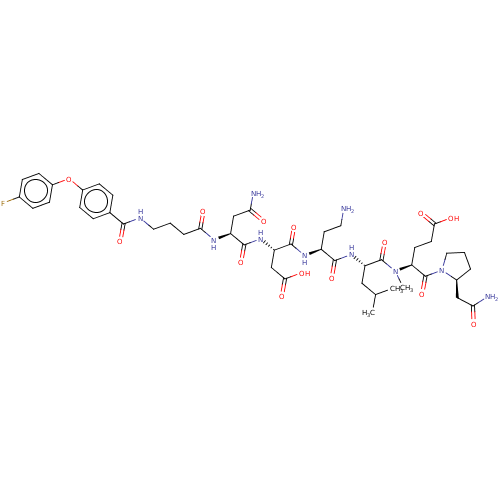
(CHEMBL5178802)Show SMILES CC(C)C[C@H](NC(=O)[C@H](CCN)NC(=O)[C@H](CC(O)=O)NC(=O)[C@H](CC(N)=O)NC(=O)CCCNC(=O)c1ccc(Oc2ccc(F)cc2)cc1)C(=O)N(C)[C@@H](CCC(O)=O)C(=O)N1CCC[C@H]1CC(N)=O |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.110 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00613
BindingDB Entry DOI: 10.7270/Q2KD231G |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50606379

(CHEMBL5180149)Show SMILES CSCC[C@H](N(C)C(=O)[C@H](CC(C)C)NC(=O)[C@H](C)NC(=O)[C@H](CC(O)=O)NC(=O)[C@H](CC(N)=O)NC(=O)CCCNS(=O)(=O)c1ccc(Oc2ccccc2)cc1)C(=O)N1CCC[C@H]1C(N)=O |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.128 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00613
BindingDB Entry DOI: 10.7270/Q2KD231G |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50606387
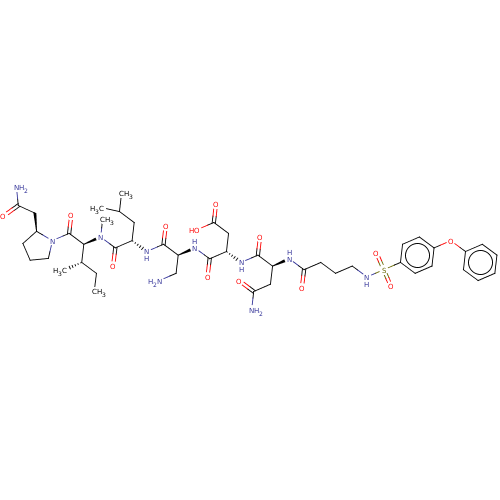
(CHEMBL5206852)Show SMILES CC[C@H](C)[C@H](N(C)C(=O)[C@H](CC(C)C)NC(=O)[C@H](CN)NC(=O)[C@H](CC(O)=O)NC(=O)[C@H](CC(N)=O)NC(=O)CCCNS(=O)(=O)c1ccc(Oc2ccccc2)cc1)C(=O)N1CCC[C@H]1CC(N)=O |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.130 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00613
BindingDB Entry DOI: 10.7270/Q2KD231G |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50606396
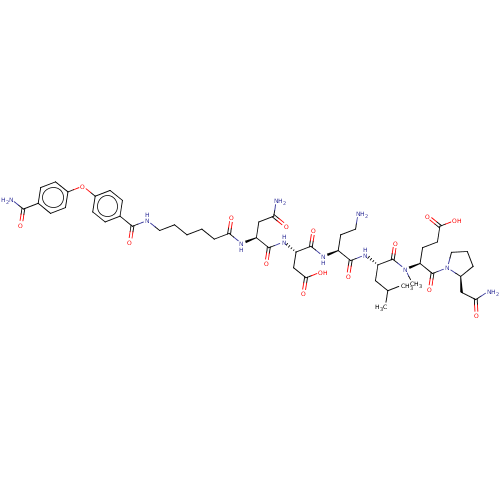
(CHEMBL5206743)Show SMILES CC(C)C[C@H](NC(=O)[C@H](CCN)NC(=O)[C@H](CC(O)=O)NC(=O)[C@H](CC(N)=O)NC(=O)CCCCCNC(=O)c1ccc(Oc2ccc(cc2)C(N)=O)cc1)C(=O)N(C)[C@@H](CCC(O)=O)C(=O)N1CCC[C@H]1CC(N)=O |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.130 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00613
BindingDB Entry DOI: 10.7270/Q2KD231G |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50606393

(CHEMBL5190160)Show SMILES CC(C)C[C@H](NC(=O)[C@H](CCN)NC(=O)[C@H](CC(O)=O)NC(=O)[C@H](CC(N)=O)NC(=O)CCCNC(=O)c1ccc(Oc2ccc(cc2)C(N)=O)cc1)C(=O)N(C)[C@@H](CCC(O)=O)C(=O)N1CCC[C@H]1CC(N)=O |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.170 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00613
BindingDB Entry DOI: 10.7270/Q2KD231G |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50062351

((R)-N*4*-Hydroxy-N*1*-[(S)-2-(1H-indol-3-yl)-1-met...)Show SMILES CNC(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)[C@H](CC(C)C)CC(=O)NO |r| Show InChI InChI=1S/C20H28N4O4/c1-12(2)8-13(10-18(25)24-28)19(26)23-17(20(27)21-3)9-14-11-22-16-7-5-4-6-15(14)16/h4-7,11-13,17,22,28H,8-10H2,1-3H3,(H,21,27)(H,23,26)(H,24,25)/t13-,17+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.190 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00613
BindingDB Entry DOI: 10.7270/Q2KD231G |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50606391
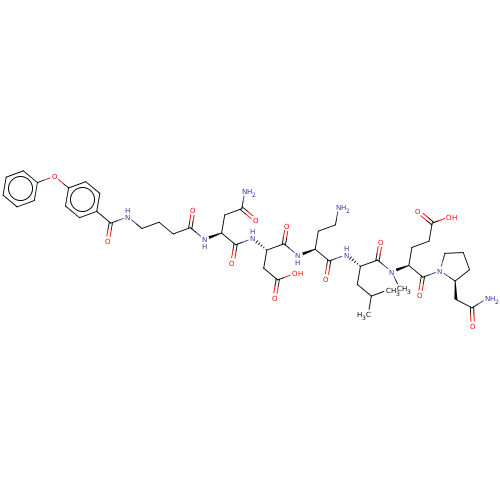
(CHEMBL5207603)Show SMILES CC(C)C[C@H](NC(=O)[C@H](CCN)NC(=O)[C@H](CC(O)=O)NC(=O)[C@H](CC(N)=O)NC(=O)CCCNC(=O)c1ccc(Oc2ccccc2)cc1)C(=O)N(C)[C@@H](CCC(O)=O)C(=O)N1CCC[C@H]1CC(N)=O |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.190 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00613
BindingDB Entry DOI: 10.7270/Q2KD231G |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50606397

(CHEMBL5169565)Show SMILES CC(C)C[C@H](NC(=O)[C@H](CCN)NC(=O)[C@H](CC(O)=O)NC(=O)[C@H](CCC(O)=O)NC(=O)CCCCNC(=O)c1ccc(Oc2ccc(cc2)C(N)=O)cc1)C(=O)N(C)[C@@H](CCC(O)=O)C(=O)N1CCC[C@H]1CC(N)=O |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00613
BindingDB Entry DOI: 10.7270/Q2KD231G |
More data for this
Ligand-Target Pair | |
Vitamin D3 receptor
(Homo sapiens (Human)) | BDBM50281390

(CHEMBL4159525)Show SMILES [H][C@@]1(CC[C@@]2([H])\C(CCC[C@]12C)=C\C=C1\C[C@@H](O)C[C@H](O)C1=C)[C@H](C)CC#C[C@@H](O)C12CC3CC(CC(C3)C1)C2 |r,TLB:33:34:32.31.37:38,THB:35:34:31:37.36.38,35:36:33.34.39:31,33:32:34.39.35:38| Show InChI InChI=1S/C35H50O3/c1-22(6-4-8-33(38)35-19-24-14-25(20-35)16-26(15-24)21-35)30-11-12-31-27(7-5-13-34(30,31)3)9-10-28-17-29(36)18-32(37)23(28)2/h9-10,22,24-26,29-33,36-38H,2,5-7,11-21H2,1,3H3/b27-9+,28-10-/t22-,24?,25?,26?,29-,30-,31+,32+,33-,34-,35?/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.240 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad de Santiago de Compostela
Curated by ChEMBL
| Assay Description
Displacement of [26,27-Methyl-3H]-1,25(OH)2D3 from recombinant human GST-tagged VDR LBD (140 to 427 residues) expressed in Escherichia coli BL21 prei... |
J Med Chem 61: 6658-6673 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00427
BindingDB Entry DOI: 10.7270/Q2M04801 |
More data for this
Ligand-Target Pair | |
Collagenase 3
(Homo sapiens (Human)) | BDBM50062351

((R)-N*4*-Hydroxy-N*1*-[(S)-2-(1H-indol-3-yl)-1-met...)Show SMILES CNC(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)[C@H](CC(C)C)CC(=O)NO |r| Show InChI InChI=1S/C20H28N4O4/c1-12(2)8-13(10-18(25)24-28)19(26)23-17(20(27)21-3)9-14-11-22-16-7-5-4-6-15(14)16/h4-7,11-13,17,22,28H,8-10H2,1-3H3,(H,21,27)(H,23,26)(H,24,25)/t13-,17+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.280 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00613
BindingDB Entry DOI: 10.7270/Q2KD231G |
More data for this
Ligand-Target Pair | |
Neuraminidase
(Influenza A virus) | BDBM50182249

(CHEMBL3818159)Show SMILES OP(O)(O)=O.CCC(CC)OC[C@@H]1CC(=C[C@H](N)[C@H]1NC(C)=O)C(O)=O |r,c:13| Show InChI InChI=1S/C15H26N2O4.H3O4P/c1-4-12(5-2)21-8-11-6-10(15(19)20)7-13(16)14(11)17-9(3)18;1-5(2,3)4/h7,11-14H,4-6,8,16H2,1-3H3,(H,17,18)(H,19,20);(H3,1,2,3,4)/t11-,13-,14-;/m0./s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.350 | n/a | n/a | n/a | n/a | n/a | n/a |
Thammasat University
Curated by ChEMBL
| Assay Description
Inhibition of Influenza A virus (A/duck/Tsukuba/28/2005(H6N2)) neuraminidase N2 activity using 4MU-Neu5Ac as substrate preincubated for 15 mins follo... |
J Med Chem 59: 4563-77 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01863
BindingDB Entry DOI: 10.7270/Q2QN68QP |
More data for this
Ligand-Target Pair | |
Vitamin D3 receptor
(Homo sapiens (Human)) | BDBM50200182

((1S,3R,5Z,7E)-9,10-secocholesta-5,7,10(19)-triene-...)Show SMILES C[C@H](CCCC(C)(C)O)[C@H]1CC[C@H]2\C(CCC[C@]12C)=C\C=C1\C[C@@H](O)C[C@H](O)C1=C |r| Show InChI InChI=1S/C27H44O3/c1-18(8-6-14-26(3,4)30)23-12-13-24-20(9-7-15-27(23,24)5)10-11-21-16-22(28)17-25(29)19(21)2/h10-11,18,22-25,28-30H,2,6-9,12-17H2,1,3-5H3/b20-10+,21-11-/t18-,22-,23-,24+,25+,27-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| n/a | n/a | 0.350 | n/a | n/a | n/a | n/a | n/a | n/a |
Rikkyo University
Curated by ChEMBL
| Assay Description
Displacement of [3H]-1,25(OH)2D3 from recombinant human VDR ligand binding domain |
J Med Chem 58: 9510-21 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00792
BindingDB Entry DOI: 10.7270/Q22V2HZC |
More data for this
Ligand-Target Pair | |
Macrophage metalloelastase
(Homo sapiens (Human)) | BDBM50062351

((R)-N*4*-Hydroxy-N*1*-[(S)-2-(1H-indol-3-yl)-1-met...)Show SMILES CNC(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)[C@H](CC(C)C)CC(=O)NO |r| Show InChI InChI=1S/C20H28N4O4/c1-12(2)8-13(10-18(25)24-28)19(26)23-17(20(27)21-3)9-14-11-22-16-7-5-4-6-15(14)16/h4-7,11-13,17,22,28H,8-10H2,1-3H3,(H,21,27)(H,23,26)(H,24,25)/t13-,17+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.460 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00613
BindingDB Entry DOI: 10.7270/Q2KD231G |
More data for this
Ligand-Target Pair | |
Neuraminidase
(Influenza A virus) | BDBM50330326

((4S,5R,6R)-5-Acetylamino-4-guanidino-6-((1R,3R)-1,...)Show SMILES [#6]-[#6](=O)-[#7]-[#6@@H]-1-[#6@H](-[#6]=[#6](-[#8]-[#6@H]-1-[#6@H](-[#8])-[#6@H](-[#8])-[#6]-[#8])-[#6](-[#8])=O)\[#7]=[#6](\[#7])-[#7] |r,c:6| Show InChI InChI=1S/C12H20N4O7/c1-4(18)15-8-5(16-12(13)14)2-7(11(21)22)23-10(8)9(20)6(19)3-17/h2,5-6,8-10,17,19-20H,3H2,1H3,(H,15,18)(H,21,22)(H4,13,14,16)/t5-,6+,8+,9+,10+/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 0.470 | n/a | n/a | n/a | n/a | n/a | n/a |
Thammasat University
Curated by ChEMBL
| Assay Description
Inhibition of Influenza A virus (A/duck/Tsukuba/67/2005(H1N1)) neuraminidase N1 activity using 4MU-Neu5Ac as substrate preincubated for 15 mins follo... |
J Med Chem 59: 4563-77 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01863
BindingDB Entry DOI: 10.7270/Q2QN68QP |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50606376
(CHEMBL5202634)Show SMILES CSCC[C@H](NC(=O)[C@H](CC(C)C)NC(=O)[C@H](C)NC(=O)[C@H](CC(O)=O)NC(=O)[C@H](CC(N)=O)NC(=O)CCNS(=O)(=O)c1ccc(Oc2ccccc2)cc1)C(=O)N1CCC[C@H]1C(N)=O |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.490 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00613
BindingDB Entry DOI: 10.7270/Q2KD231G |
More data for this
Ligand-Target Pair | |
Vitamin D3 receptor
(Homo sapiens (Human)) | BDBM50015318
(CHEMBL3263871)Show SMILES [H][C@@]1([#6]-[#6][C@@]2([H])\[#6](-[#6]-[#6]-[#6][C@]12[#6])=[#6]\[#6]=[#6]-1\[#6]-[#6@@H](-[#8])-[#6](=[#6])-[#6@H](-[#8])-[#6]-1)[#6@H](-[#6])-[#6]C#C[#6@@H](-[#8])C12[#6]-[#6]-3-[#6]-[#6](-[#6]-[#6](-[#6]-3)-[#6]1)-[#6]2 |r,THB:35:34:31:37.36.38,35:36:33.34.39:31,38:36:33:39.30.31,38:30:33:37.35.36| Show InChI InChI=1S/C35H50O3/c1-22(6-4-8-33(38)35-19-25-14-26(20-35)16-27(15-25)21-35)29-11-12-30-28(7-5-13-34(29,30)3)10-9-24-17-31(36)23(2)32(37)18-24/h9-10,22,25-27,29-33,36-38H,2,5-7,11-21H2,1,3H3/b28-10+/t22-,25?,26?,27?,29-,30+,31-,32-,33-,34-,35?/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Rikkyo University
Curated by ChEMBL
| Assay Description
Displacement of [3H]-1,25(OH)2D3 from GST-fused human VDR-LBD expressed in Escherichia coli BL21 |
J Med Chem 57: 4073-87 (2014)
Article DOI: 10.1021/jm401989c
BindingDB Entry DOI: 10.7270/Q2ZS2Z2G |
More data for this
Ligand-Target Pair | |
Neuraminidase
(Influenza A virus) | BDBM50182249

(CHEMBL3818159)Show SMILES OP(O)(O)=O.CCC(CC)OC[C@@H]1CC(=C[C@H](N)[C@H]1NC(C)=O)C(O)=O |r,c:13| Show InChI InChI=1S/C15H26N2O4.H3O4P/c1-4-12(5-2)21-8-11-6-10(15(19)20)7-13(16)14(11)17-9(3)18;1-5(2,3)4/h7,11-14H,4-6,8,16H2,1-3H3,(H,17,18)(H,19,20);(H3,1,2,3,4)/t11-,13-,14-;/m0./s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.530 | n/a | n/a | n/a | n/a | n/a | n/a |
Thammasat University
Curated by ChEMBL
| Assay Description
Inhibition of Influenza A virus (A/duck/Tsukuba/394/2005(H5N3)) neuraminidase N3 activity using 4MU-Neu5Ac as substrate preincubated for 15 mins foll... |
J Med Chem 59: 4563-77 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01863
BindingDB Entry DOI: 10.7270/Q2QN68QP |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50606394

(CHEMBL5177058)Show SMILES CC(C)C[C@H](NC(=O)[C@H](CCN)NC(=O)[C@H](CC(O)=O)NC(=O)[C@H](CC(N)=O)NC(=O)CCNC(=O)c1ccc(Oc2ccc(cc2)C(N)=O)cc1)C(=O)N(C)[C@@H](CCC(O)=O)C(=O)N1CCC[C@H]1CC(N)=O |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.530 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00613
BindingDB Entry DOI: 10.7270/Q2KD231G |
More data for this
Ligand-Target Pair | |
Interstitial collagenase
(Homo sapiens (Human)) | BDBM50062351

((R)-N*4*-Hydroxy-N*1*-[(S)-2-(1H-indol-3-yl)-1-met...)Show SMILES CNC(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)[C@H](CC(C)C)CC(=O)NO |r| Show InChI InChI=1S/C20H28N4O4/c1-12(2)8-13(10-18(25)24-28)19(26)23-17(20(27)21-3)9-14-11-22-16-7-5-4-6-15(14)16/h4-7,11-13,17,22,28H,8-10H2,1-3H3,(H,21,27)(H,23,26)(H,24,25)/t13-,17+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.560 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00613
BindingDB Entry DOI: 10.7270/Q2KD231G |
More data for this
Ligand-Target Pair | |
Neuraminidase
(Influenza A virus) | BDBM50330326

((4S,5R,6R)-5-Acetylamino-4-guanidino-6-((1R,3R)-1,...)Show SMILES [#6]-[#6](=O)-[#7]-[#6@@H]-1-[#6@H](-[#6]=[#6](-[#8]-[#6@H]-1-[#6@H](-[#8])-[#6@H](-[#8])-[#6]-[#8])-[#6](-[#8])=O)\[#7]=[#6](\[#7])-[#7] |r,c:6| Show InChI InChI=1S/C12H20N4O7/c1-4(18)15-8-5(16-12(13)14)2-7(11(21)22)23-10(8)9(20)6(19)3-17/h2,5-6,8-10,17,19-20H,3H2,1H3,(H,15,18)(H,21,22)(H4,13,14,16)/t5-,6+,8+,9+,10+/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 0.580 | n/a | n/a | n/a | n/a | n/a | n/a |
Thammasat University
Curated by ChEMBL
| Assay Description
Inhibition of Influenza A virus (A/duck/Tsukuba/20/2007(H8N4)) neuraminidase N4 activity using 4MU-Neu5Ac as substrate preincubated for 15 mins follo... |
J Med Chem 59: 4563-77 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01863
BindingDB Entry DOI: 10.7270/Q2QN68QP |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data