 Found 17 hits with Last Name = 'trower' and Initial = 'm'
Found 17 hits with Last Name = 'trower' and Initial = 'm' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Substance-P receptor
(Rattus norvegicus (rat)) | BDBM50442585
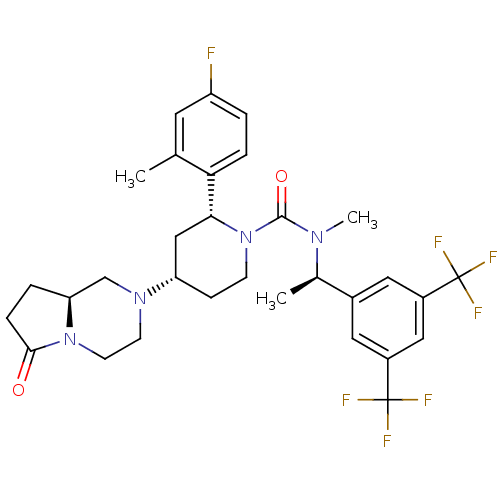
(GW823296X | ORVEPITANT)Show SMILES C[C@@H](N(C)C(=O)N1CC[C@@H](C[C@@H]1c1ccc(F)cc1C)N1CCN2[C@@H](CCC2=O)C1)c1cc(cc(c1)C(F)(F)F)C(F)(F)F |r| Show InChI InChI=1S/C31H35F7N4O2/c1-18-12-23(32)4-6-26(18)27-16-24(40-10-11-41-25(17-40)5-7-28(41)43)8-9-42(27)29(44)39(3)19(2)20-13-21(30(33,34)35)15-22(14-20)31(36,37)38/h4,6,12-15,19,24-25,27H,5,7-11,16-17H2,1-3H3/t19-,24+,25+,27-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0316 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Medicines Research Centre
Curated by ChEMBL
| Assay Description
Displacement of [3H]GR205171 from Mongolian gerbil brain NK1 receptor after 60 mins by scintillation counting analysis |
Bioorg Med Chem 21: 6264-73 (2013)
Article DOI: 10.1016/j.bmc.2013.09.001
BindingDB Entry DOI: 10.7270/Q2D79CWC |
More data for this
Ligand-Target Pair | |
Substance-P receptor
(Homo sapiens (Human)) | BDBM50442585

(GW823296X | ORVEPITANT)Show SMILES C[C@@H](N(C)C(=O)N1CC[C@@H](C[C@@H]1c1ccc(F)cc1C)N1CCN2[C@@H](CCC2=O)C1)c1cc(cc(c1)C(F)(F)F)C(F)(F)F |r| Show InChI InChI=1S/C31H35F7N4O2/c1-18-12-23(32)4-6-26(18)27-16-24(40-10-11-41-25(17-40)5-7-28(41)43)8-9-42(27)29(44)39(3)19(2)20-13-21(30(33,34)35)15-22(14-20)31(36,37)38/h4,6,12-15,19,24-25,27H,5,7-11,16-17H2,1-3H3/t19-,24+,25+,27-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0631 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Medicines Research Centre
Curated by ChEMBL
| Assay Description
Displacement of [3H]SP from human recombinant NK1 receptor expressed in CHO cells after 40 mins by scintillation counting analysis |
Bioorg Med Chem 21: 6264-73 (2013)
Article DOI: 10.1016/j.bmc.2013.09.001
BindingDB Entry DOI: 10.7270/Q2D79CWC |
More data for this
Ligand-Target Pair | |
Substance-P receptor
(Homo sapiens (Human)) | BDBM50442588
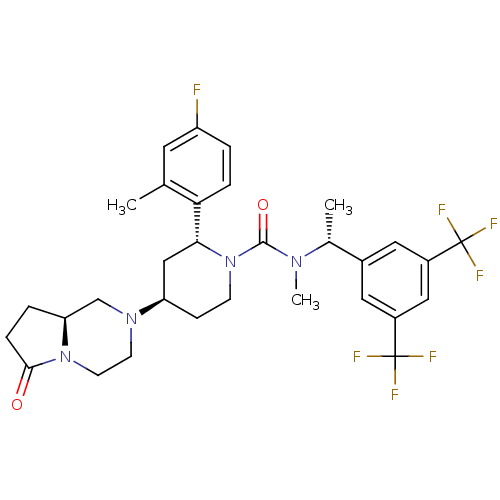
(CHEMBL2441373)Show SMILES C[C@@H](N(C)C(=O)N1CC[C@H](C[C@@H]1c1ccc(F)cc1C)N1CCN2[C@@H](CCC2=O)C1)c1cc(cc(c1)C(F)(F)F)C(F)(F)F |r| Show InChI InChI=1S/C31H35F7N4O2/c1-18-12-23(32)4-6-26(18)27-16-24(40-10-11-41-25(17-40)5-7-28(41)43)8-9-42(27)29(44)39(3)19(2)20-13-21(30(33,34)35)15-22(14-20)31(36,37)38/h4,6,12-15,19,24-25,27H,5,7-11,16-17H2,1-3H3/t19-,24-,25+,27-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0646 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Medicines Research Centre
Curated by ChEMBL
| Assay Description
Displacement of [3H]SP from human recombinant NK1 receptor expressed in CHO cells after 40 mins by scintillation counting analysis |
Bioorg Med Chem 21: 6264-73 (2013)
Article DOI: 10.1016/j.bmc.2013.09.001
BindingDB Entry DOI: 10.7270/Q2D79CWC |
More data for this
Ligand-Target Pair | |
Substance-P receptor
(Homo sapiens (Human)) | BDBM50442589
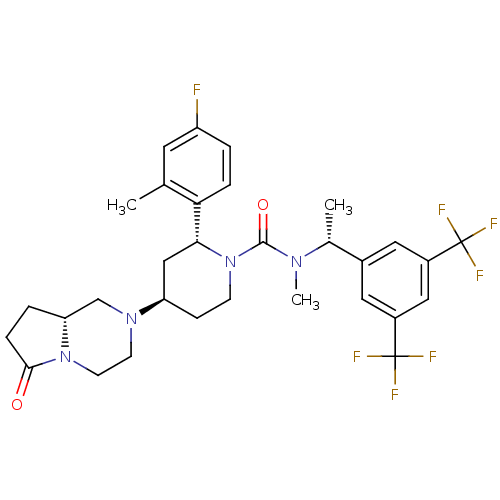
(CHEMBL2441371)Show SMILES C[C@@H](N(C)C(=O)N1CC[C@H](C[C@@H]1c1ccc(F)cc1C)N1CCN2[C@H](CCC2=O)C1)c1cc(cc(c1)C(F)(F)F)C(F)(F)F |r| Show InChI InChI=1S/C31H35F7N4O2/c1-18-12-23(32)4-6-26(18)27-16-24(40-10-11-41-25(17-40)5-7-28(41)43)8-9-42(27)29(44)39(3)19(2)20-13-21(30(33,34)35)15-22(14-20)31(36,37)38/h4,6,12-15,19,24-25,27H,5,7-11,16-17H2,1-3H3/t19-,24-,25-,27-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.138 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Medicines Research Centre
Curated by ChEMBL
| Assay Description
Displacement of [3H]SP from human recombinant NK1 receptor expressed in CHO cells after 40 mins by scintillation counting analysis |
Bioorg Med Chem 21: 6264-73 (2013)
Article DOI: 10.1016/j.bmc.2013.09.001
BindingDB Entry DOI: 10.7270/Q2D79CWC |
More data for this
Ligand-Target Pair | |
Substance-P receptor
(Homo sapiens (Human)) | BDBM50442590
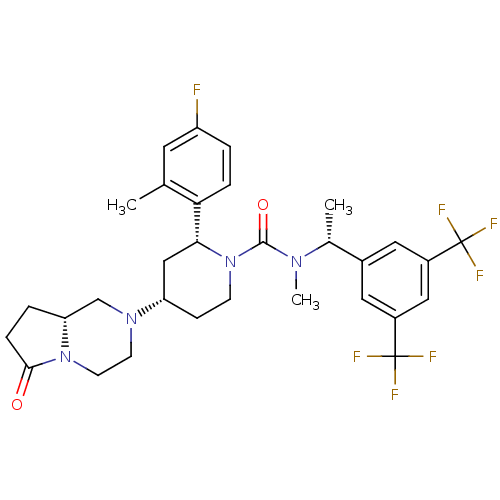
(CHEMBL2441372)Show SMILES C[C@@H](N(C)C(=O)N1CC[C@@H](C[C@@H]1c1ccc(F)cc1C)N1CCN2[C@H](CCC2=O)C1)c1cc(cc(c1)C(F)(F)F)C(F)(F)F |r| Show InChI InChI=1S/C31H35F7N4O2/c1-18-12-23(32)4-6-26(18)27-16-24(40-10-11-41-25(17-40)5-7-28(41)43)8-9-42(27)29(44)39(3)19(2)20-13-21(30(33,34)35)15-22(14-20)31(36,37)38/h4,6,12-15,19,24-25,27H,5,7-11,16-17H2,1-3H3/t19-,24+,25-,27-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Medicines Research Centre
Curated by ChEMBL
| Assay Description
Displacement of [3H]SP from human recombinant NK1 receptor expressed in CHO cells after 40 mins by scintillation counting analysis |
Bioorg Med Chem 21: 6264-73 (2013)
Article DOI: 10.1016/j.bmc.2013.09.001
BindingDB Entry DOI: 10.7270/Q2D79CWC |
More data for this
Ligand-Target Pair | |
Sigma non-opioid intracellular receptor 1
(Homo sapiens (Human)) | BDBM50442585

(GW823296X | ORVEPITANT)Show SMILES C[C@@H](N(C)C(=O)N1CC[C@@H](C[C@@H]1c1ccc(F)cc1C)N1CCN2[C@@H](CCC2=O)C1)c1cc(cc(c1)C(F)(F)F)C(F)(F)F |r| Show InChI InChI=1S/C31H35F7N4O2/c1-18-12-23(32)4-6-26(18)27-16-24(40-10-11-41-25(17-40)5-7-28(41)43)8-9-42(27)29(44)39(3)19(2)20-13-21(30(33,34)35)15-22(14-20)31(36,37)38/h4,6,12-15,19,24-25,27H,5,7-11,16-17H2,1-3H3/t19-,24+,25+,27-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 940 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Medicines Research Centre
Curated by ChEMBL
| Assay Description
Inhibition of sigma1 receptor (unknown origin) |
Bioorg Med Chem 21: 6264-73 (2013)
Article DOI: 10.1016/j.bmc.2013.09.001
BindingDB Entry DOI: 10.7270/Q2D79CWC |
More data for this
Ligand-Target Pair | |
Platelet-activating factor receptor
(Homo sapiens (Human)) | BDBM50442585

(GW823296X | ORVEPITANT)Show SMILES C[C@@H](N(C)C(=O)N1CC[C@@H](C[C@@H]1c1ccc(F)cc1C)N1CCN2[C@@H](CCC2=O)C1)c1cc(cc(c1)C(F)(F)F)C(F)(F)F |r| Show InChI InChI=1S/C31H35F7N4O2/c1-18-12-23(32)4-6-26(18)27-16-24(40-10-11-41-25(17-40)5-7-28(41)43)8-9-42(27)29(44)39(3)19(2)20-13-21(30(33,34)35)15-22(14-20)31(36,37)38/h4,6,12-15,19,24-25,27H,5,7-11,16-17H2,1-3H3/t19-,24+,25+,27-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.27E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Medicines Research Centre
Curated by ChEMBL
| Assay Description
Inhibition of platelet activating factor receptor (unknown origin) |
Bioorg Med Chem 21: 6264-73 (2013)
Article DOI: 10.1016/j.bmc.2013.09.001
BindingDB Entry DOI: 10.7270/Q2D79CWC |
More data for this
Ligand-Target Pair | |
Substance-P receptor
(Homo sapiens (Human)) | BDBM50442587
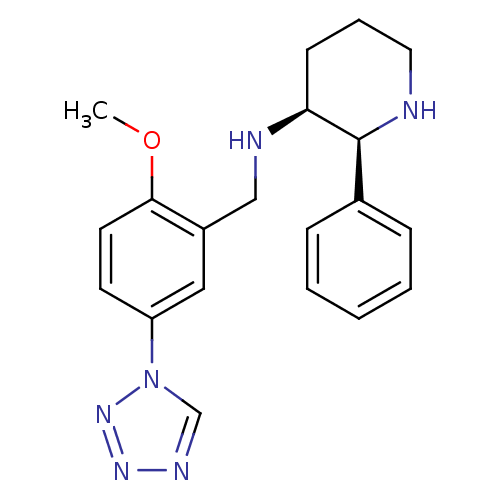
(CHEMBL2441370)Show SMILES COc1ccc(cc1CN[C@H]1CCCN[C@H]1c1ccccc1)-n1cnnn1 |r| Show InChI InChI=1S/C20H24N6O/c1-27-19-10-9-17(26-14-23-24-25-26)12-16(19)13-22-18-8-5-11-21-20(18)15-6-3-2-4-7-15/h2-4,6-7,9-10,12,14,18,20-22H,5,8,11,13H2,1H3/t18-,20-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.0400 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Medicines Research Centre
Curated by ChEMBL
| Assay Description
Antagonist activity at NK1 receptor (unknown origin) |
Bioorg Med Chem 21: 6264-73 (2013)
Article DOI: 10.1016/j.bmc.2013.09.001
BindingDB Entry DOI: 10.7270/Q2D79CWC |
More data for this
Ligand-Target Pair | |
Substance-P receptor
(Homo sapiens (Human)) | BDBM50220136
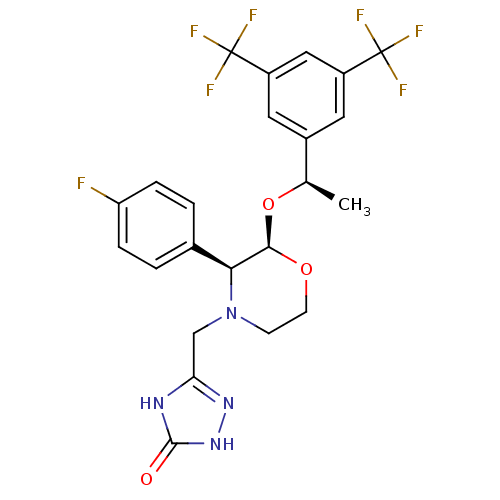
(3-[2-{1-[3,5-di(trifluoromethyl)phenyl]ethoxy}-3-(...)Show SMILES C[C@@H](O[C@H]1OCCN(Cc2n[nH]c(=O)[nH]2)[C@H]1c1ccc(F)cc1)c1cc(cc(c1)C(F)(F)F)C(F)(F)F |r| Show InChI InChI=1S/C23H21F7N4O3/c1-12(14-8-15(22(25,26)27)10-16(9-14)23(28,29)30)37-20-19(13-2-4-17(24)5-3-13)34(6-7-36-20)11-18-31-21(35)33-32-18/h2-5,8-10,12,19-20H,6-7,11H2,1H3,(H2,31,32,33,35)/t12-,19+,20-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| n/a | n/a | 0.0900 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Medicines Research Centre
Curated by ChEMBL
| Assay Description
Antagonist activity at NK1 receptor (unknown origin) |
Bioorg Med Chem 21: 6264-73 (2013)
Article DOI: 10.1016/j.bmc.2013.09.001
BindingDB Entry DOI: 10.7270/Q2D79CWC |
More data for this
Ligand-Target Pair | |
Substance-P receptor
(Homo sapiens (Human)) | BDBM50185071

((5S,6S,7S)-5-((R)-1-(3,5-bis(trifluoromethyl)pheny...)Show SMILES C[C@@H](O[C@H]1CCC(=O)N[C@@H](C)[C@@H]1c1ccc(F)cc1)c1cc(cc(c1)C(F)(F)F)C(F)(F)F Show InChI InChI=1S/C23H22F7NO2/c1-12-21(14-3-5-18(24)6-4-14)19(7-8-20(32)31-12)33-13(2)15-9-16(22(25,26)27)11-17(10-15)23(28,29)30/h3-6,9-13,19,21H,7-8H2,1-2H3,(H,31,32)/t12-,13+,19-,21+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.0900 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Medicines Research Centre
Curated by ChEMBL
| Assay Description
Antagonist activity at NK1 receptor (unknown origin) |
Bioorg Med Chem 21: 6264-73 (2013)
Article DOI: 10.1016/j.bmc.2013.09.001
BindingDB Entry DOI: 10.7270/Q2D79CWC |
More data for this
Ligand-Target Pair | |
Substance-P receptor
(Homo sapiens (Human)) | BDBM50442586
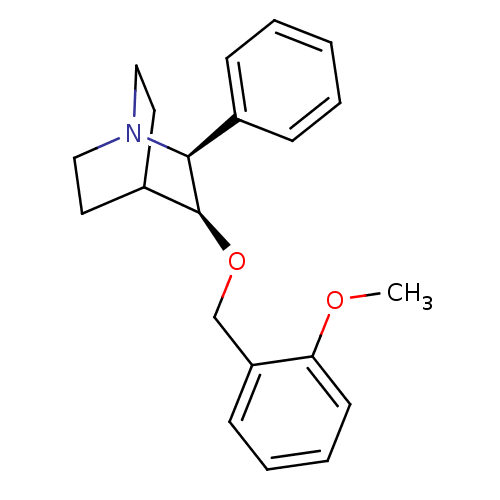
(CHEMBL2441368)Show SMILES COc1ccccc1CO[C@H]1C2CCN(CC2)[C@H]1c1ccccc1 |r,wD:17.20,10.10,(10.25,-5.1,;10.25,-6.64,;11.58,-7.42,;12.91,-6.65,;14.25,-7.43,;14.24,-8.97,;12.9,-9.73,;11.58,-8.95,;10.24,-9.71,;10.23,-11.25,;8.89,-12.01,;7.58,-11.23,;6.23,-12.01,;6.23,-13.55,;7.56,-14.31,;8.31,-12.97,;6.82,-12.57,;8.89,-13.55,;10.22,-14.32,;10.21,-15.86,;11.54,-16.63,;12.88,-15.86,;12.88,-14.32,;11.55,-13.55,)| Show InChI InChI=1S/C21H25NO2/c1-23-19-10-6-5-9-18(19)15-24-21-17-11-13-22(14-12-17)20(21)16-7-3-2-4-8-16/h2-10,17,20-21H,11-15H2,1H3/t20-,21-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.170 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Medicines Research Centre
Curated by ChEMBL
| Assay Description
Antagonist activity at NK1 receptor (unknown origin) |
Bioorg Med Chem 21: 6264-73 (2013)
Article DOI: 10.1016/j.bmc.2013.09.001
BindingDB Entry DOI: 10.7270/Q2D79CWC |
More data for this
Ligand-Target Pair | |
Substance-P receptor
(Homo sapiens (Human)) | BDBM50029884
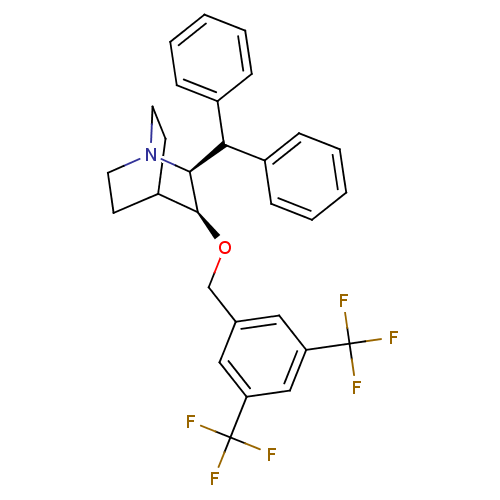
((2S,3S)-2-Benzhydryl-3-(3,5-bis-trifluoromethyl-be...)Show SMILES FC(F)(F)c1cc(CO[C@H]2C3CCN(CC3)[C@H]2C(c2ccccc2)c2ccccc2)cc(c1)C(F)(F)F |wD:16.18,9.8,(12.7,-1.59,;11.46,-2.62,;10.22,-1.61,;11.46,-1.22,;11.48,-4.16,;10.15,-4.95,;10.15,-6.49,;8.8,-7.26,;8.8,-8.8,;7.47,-9.57,;6.14,-8.78,;4.81,-9.55,;4.81,-11.09,;6.14,-11.86,;5.61,-10.88,;6.77,-9.78,;7.47,-11.11,;8.82,-11.88,;10.15,-11.11,;11.48,-11.88,;12.82,-11.11,;12.82,-9.57,;11.48,-8.8,;10.15,-9.57,;8.8,-13.42,;10.13,-14.17,;10.15,-15.71,;8.8,-16.48,;7.47,-15.71,;7.47,-14.17,;11.48,-7.26,;12.82,-6.49,;12.82,-4.93,;14.15,-7.26,;15.38,-7.94,;14.42,-8.84,;15.66,-6.67,)| Show InChI InChI=1S/C29H27F6NO/c30-28(31,32)23-15-19(16-24(17-23)29(33,34)35)18-37-27-22-11-13-36(14-12-22)26(27)25(20-7-3-1-4-8-20)21-9-5-2-6-10-21/h1-10,15-17,22,25-27H,11-14,18H2/t26-,27-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Medicines Research Centre
Curated by ChEMBL
| Assay Description
Antagonist activity at NK1 receptor (unknown origin) |
Bioorg Med Chem 21: 6264-73 (2013)
Article DOI: 10.1016/j.bmc.2013.09.001
BindingDB Entry DOI: 10.7270/Q2D79CWC |
More data for this
Ligand-Target Pair | |
Substance-P receptor
(Homo sapiens (Human)) | BDBM50413891

(VESTIPITANT)Show SMILES C[C@@H](N(C)C(=O)N1CCNC[C@@H]1c1ccc(F)cc1C)c1cc(cc(c1)C(F)(F)F)C(F)(F)F |r| Show InChI InChI=1S/C23H24F7N3O/c1-13-8-18(24)4-5-19(13)20-12-31-6-7-33(20)21(34)32(3)14(2)15-9-16(22(25,26)27)11-17(10-15)23(28,29)30/h4-5,8-11,14,20,31H,6-7,12H2,1-3H3/t14-,20-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.25 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Medicines Research Centre
Curated by ChEMBL
| Assay Description
Antagonist activity at NK1 receptor (unknown origin) |
Bioorg Med Chem 21: 6264-73 (2013)
Article DOI: 10.1016/j.bmc.2013.09.001
BindingDB Entry DOI: 10.7270/Q2D79CWC |
More data for this
Ligand-Target Pair | |
Substance-P receptor
(Homo sapiens (Human)) | BDBM50408458
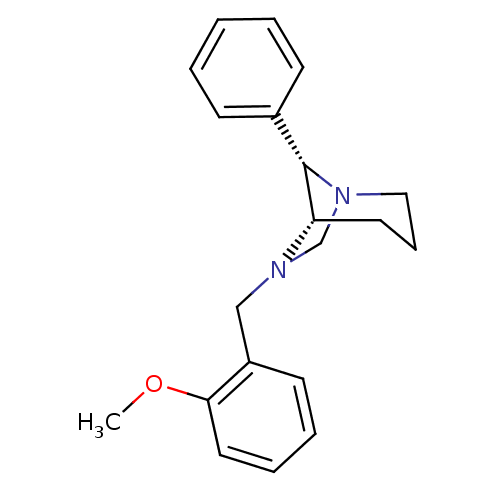
(CHEMBL340559)Show SMILES COc1ccccc1CN1CN2CCC[C@H]1[C@@H]2c1ccccc1 |THB:8:9:16:12.14.13| Show InChI InChI=1S/C20H24N2O/c1-23-19-12-6-5-10-17(19)14-22-15-21-13-7-11-18(22)20(21)16-8-3-2-4-9-16/h2-6,8-10,12,18,20H,7,11,13-15H2,1H3/t18-,20-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.610 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Medicines Research Centre
Curated by ChEMBL
| Assay Description
Antagonist activity at NK1 receptor (unknown origin) |
Bioorg Med Chem 21: 6264-73 (2013)
Article DOI: 10.1016/j.bmc.2013.09.001
BindingDB Entry DOI: 10.7270/Q2D79CWC |
More data for this
Ligand-Target Pair | |
Substance-P receptor
(Homo sapiens (Human)) | BDBM50000040

(((2S,3S)-2-Benzhydryl-1-aza-bicyclo[2.2.2]oct-3-yl...)Show SMILES COc1ccccc1CN[C@H]1C2CCN(CC2)[C@H]1C(c1ccccc1)c1ccccc1 |wD:10.10,17.20,(11.79,-2.71,;10.31,-3.13,;9.22,-2.04,;10.55,-1.27,;10.57,.27,;9.22,1.04,;7.89,.27,;7.89,-1.26,;6.56,-2.01,;6.56,-3.55,;5.21,-4.32,;3.92,-3.55,;3.16,-4.88,;4.65,-5.3,;3.88,-6.63,;2.57,-5.86,;2.57,-4.32,;5.21,-5.86,;6.56,-6.63,;6.54,-8.17,;5.21,-8.94,;5.21,-10.48,;6.54,-11.25,;7.89,-10.48,;7.87,-8.94,;7.89,-5.86,;9.22,-6.63,;10.55,-5.88,;10.55,-4.34,;9.22,-3.55,;7.89,-4.34,)| Show InChI InChI=1S/C28H32N2O/c1-31-25-15-9-8-14-24(25)20-29-27-23-16-18-30(19-17-23)28(27)26(21-10-4-2-5-11-21)22-12-6-3-7-13-22/h2-15,23,26-29H,16-20H2,1H3/t27-,28-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.660 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Medicines Research Centre
Curated by ChEMBL
| Assay Description
Antagonist activity at NK1 receptor (unknown origin) |
Bioorg Med Chem 21: 6264-73 (2013)
Article DOI: 10.1016/j.bmc.2013.09.001
BindingDB Entry DOI: 10.7270/Q2D79CWC |
More data for this
Ligand-Target Pair | |
Substance-P receptor
(Homo sapiens (Human)) | BDBM50178574

(2-(3,5-bis(trifluoromethyl)phenyl)-N,2-dimethyl-N-...)Show SMILES CN(C(=O)C(C)(C)c1cc(cc(c1)C(F)(F)F)C(F)(F)F)c1cnc(cc1-c1ccccc1C)N1CCN(C)CC1 Show InChI InChI=1S/C30H32F6N4O/c1-19-8-6-7-9-23(19)24-17-26(40-12-10-38(4)11-13-40)37-18-25(24)39(5)27(41)28(2,3)20-14-21(29(31,32)33)16-22(15-20)30(34,35)36/h6-9,14-18H,10-13H2,1-5H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| n/a | n/a | 0.950 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Medicines Research Centre
Curated by ChEMBL
| Assay Description
Antagonist activity at NK1 receptor (unknown origin) |
Bioorg Med Chem 21: 6264-73 (2013)
Article DOI: 10.1016/j.bmc.2013.09.001
BindingDB Entry DOI: 10.7270/Q2D79CWC |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Substance-P receptor
(Homo sapiens (Human)) | BDBM50049468
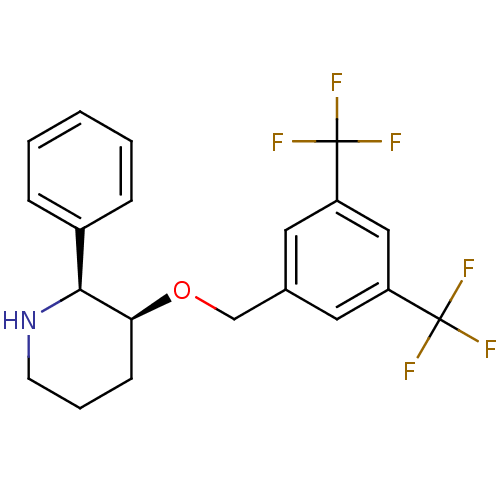
((2S,3S)-3-(3,5-Bis-trifluoromethyl-benzyloxy)-2-ph...)Show SMILES FC(F)(F)c1cc(CO[C@H]2CCCN[C@H]2c2ccccc2)cc(c1)C(F)(F)F |r| Show InChI InChI=1S/C20H19F6NO/c21-19(22,23)15-9-13(10-16(11-15)20(24,25)26)12-28-17-7-4-8-27-18(17)14-5-2-1-3-6-14/h1-3,5-6,9-11,17-18,27H,4,7-8,12H2/t17-,18-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Medicines Research Centre
Curated by ChEMBL
| Assay Description
Antagonist activity at NK1 receptor (unknown origin) |
Bioorg Med Chem 21: 6264-73 (2013)
Article DOI: 10.1016/j.bmc.2013.09.001
BindingDB Entry DOI: 10.7270/Q2D79CWC |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data