

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Aldo-keto reductase family 1 member C3 (Homo sapiens (Human)) | BDBM50533570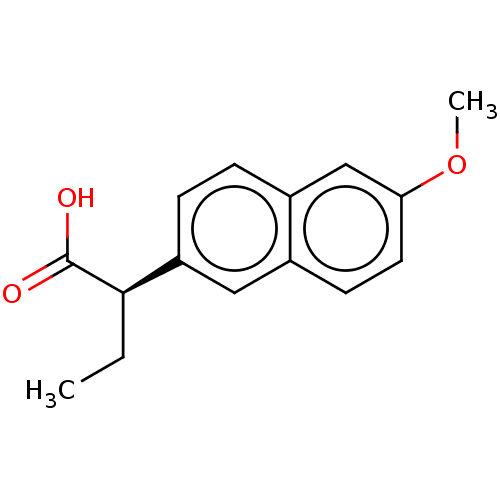 (CHEMBL4435662 | US11459295, Compound LM5750A 8b) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | 31 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pennsylvania Curated by ChEMBL | Assay Description Competitive inhibition of human recombinant AKR1C3 using S-tetralol as substrate assessed as reduction in NADP+-dependent S-tetralol oxidation preinc... | J Med Chem 59: 7431-44 (2016) Article DOI: 10.1021/acs.jmedchem.6b00160 BindingDB Entry DOI: 10.7270/Q21Z47WN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member C3 (Homo sapiens (Human)) | BDBM50533570 (CHEMBL4435662 | US11459295, Compound LM5750A 8b) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | 750 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pennsylvania Curated by ChEMBL | Assay Description Competitive inhibition of human recombinant AKR1C3 using assessed as reduction in NADPH-dependent reduction of delat4-androsten-3,17-dione preincubat... | J Med Chem 59: 7431-44 (2016) Article DOI: 10.1021/acs.jmedchem.6b00160 BindingDB Entry DOI: 10.7270/Q21Z47WN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM17638 (2-{1-[(4-chlorophenyl)carbonyl]-5-methoxy-2-methyl...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank PDB Article PubMed | 1.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University Curated by ChEMBL | Assay Description Binding affinity to wild type human COX2 expressed in insect cells using [1-14C]-arachidonic acid as substrate | ACS Med Chem Lett 4: 486-490 (2013) Article DOI: 10.1021/ml400066a BindingDB Entry DOI: 10.7270/Q28D005G | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50491628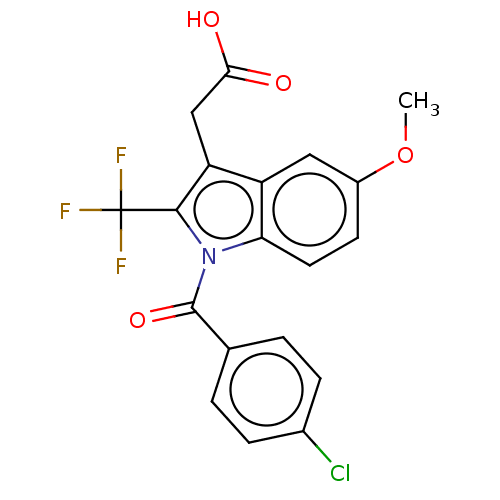 (CHEMBL2386352) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University Curated by ChEMBL | Assay Description Binding affinity to wild type human COX2 expressed in insect cells using [1-14C]-arachidonic acid as substrate | ACS Med Chem Lett 4: 486-490 (2013) Article DOI: 10.1021/ml400066a BindingDB Entry DOI: 10.7270/Q28D005G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Ovis aries (Sheep)) | BDBM50143476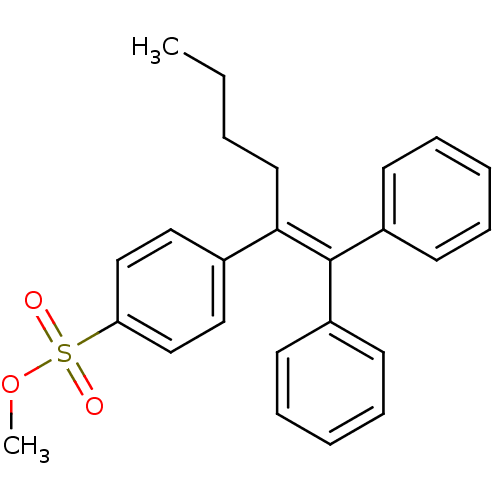 (4-(1-Benzhydrylidene-pentyl)-benzenesulfonic acid ...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Alberta Curated by ChEMBL | Assay Description In vitro inhibitory activity of the compound against prostaglandin G/H synthase 2 from ovine | Bioorg Med Chem Lett 14: 1953-6 (2004) Article DOI: 10.1016/j.bmcl.2004.01.075 BindingDB Entry DOI: 10.7270/Q24F1Q46 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Ovis aries (Sheep)) | BDBM50150726 (3-(4-Methanesulfonyl-phenyl)-6-(4-methoxy-phenyl)-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Alberta Curated by ChEMBL | Assay Description Inhibition of ovine Prostaglandin G/H synthase 2 | J Med Chem 47: 3972-90 (2004) Article DOI: 10.1021/jm049939b BindingDB Entry DOI: 10.7270/Q22F7MXX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Mus musculus (Mouse)) | BDBM50561580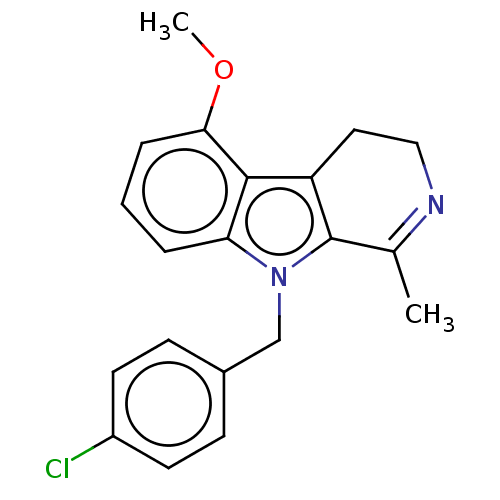 (CHEMBL4777544) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of mouse COX2 using 2-arachidonylglycerol as substrate preincubated for 3 mins followed by substrate addition and measured for 30 sec by L... | Citation and Details Article DOI: 10.1021/acsmedchemlett.9b00555 BindingDB Entry DOI: 10.7270/Q2N58R3Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 1 (Ovis aries (Sheep)) | BDBM17638 (2-{1-[(4-chlorophenyl)carbonyl]-5-methoxy-2-methyl...) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 27 | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University Curated by ChEMBL | Assay Description Inhibition of wild type ovine COX1 expressed in ram seminal vesicles using [1-14C]-arachidonic acid as substrate incubated for 17 mins at 25 degC fol... | ACS Med Chem Lett 4: 486-490 (2013) Article DOI: 10.1021/ml400066a BindingDB Entry DOI: 10.7270/Q28D005G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Mus musculus (Mouse)) | BDBM50336971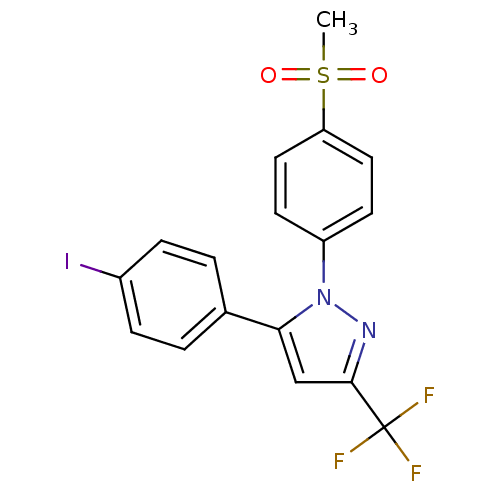 (5-(4-iodophenyl)-1-(4-(methylsulfonyl)phenyl)-3-(t...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of COX2 in mouse LPS-stimulated RAW264.7 cells after 30 mins | ACS Med Chem Lett 2: 160-164 (2011) Article DOI: 10.1021/ml100232q BindingDB Entry DOI: 10.7270/Q25T3MG9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Mus musculus (Mouse)) | BDBM11639 (4-[5-(4-methylphenyl)-3-(trifluoromethyl)-1H-pyraz...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of mouse purified COX2 after 20 mins | ACS Med Chem Lett 2: 160-164 (2011) Article DOI: 10.1021/ml100232q BindingDB Entry DOI: 10.7270/Q25T3MG9 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Prostaglandin G/H synthase 1 (Ovis aries (Sheep)) | BDBM50152545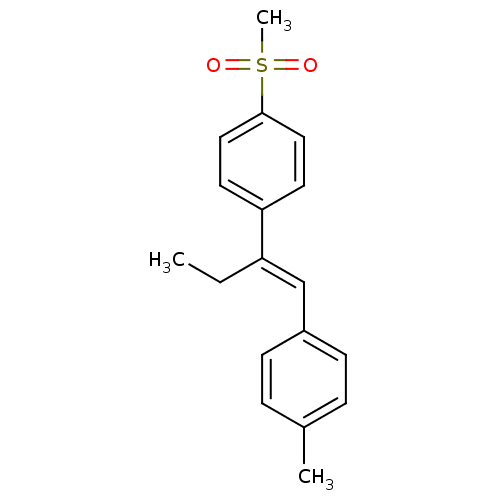 (1-[(E)-1-ethyl-2-(4-methylphenyl)vinyl]-4-(methyls...) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Alberta Curated by ChEMBL | Assay Description In vitro inhibitory concentration against ovine Prostaglandin G/H synthase 1 | Bioorg Med Chem Lett 14: 4911-4 (2004) Article DOI: 10.1016/j.bmcl.2004.07.027 BindingDB Entry DOI: 10.7270/Q2348JVS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Ovis aries (Sheep)) | BDBM50150735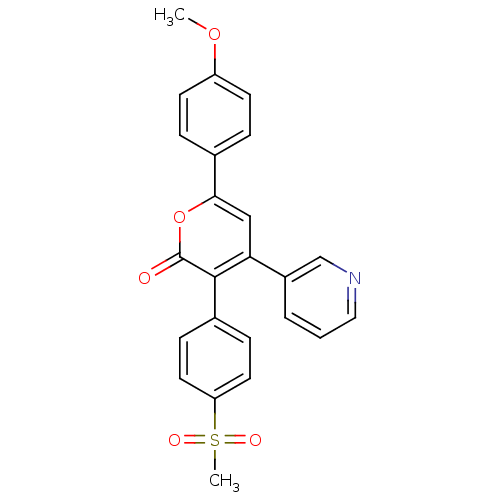 (3-(4-Methanesulfonyl-phenyl)-6-(4-methoxy-phenyl)-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Alberta Curated by ChEMBL | Assay Description Inhibition of ovine Prostaglandin G/H synthase 2 | J Med Chem 47: 3972-90 (2004) Article DOI: 10.1021/jm049939b BindingDB Entry DOI: 10.7270/Q22F7MXX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Ovis aries (Sheep)) | BDBM50143475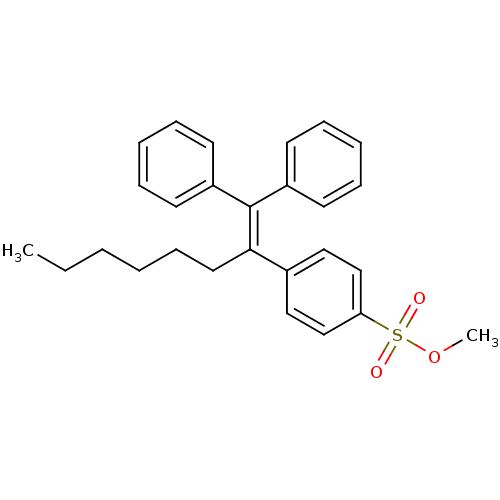 (4-(1-Benzhydrylidene-heptyl)-benzenesulfonic acid ...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 31 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Alberta Curated by ChEMBL | Assay Description In vitro inhibitory activity of the compound against prostaglandin G/H synthase 2 from ovine | Bioorg Med Chem Lett 14: 1953-6 (2004) Article DOI: 10.1016/j.bmcl.2004.01.075 BindingDB Entry DOI: 10.7270/Q24F1Q46 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Ovis aries (Sheep)) | BDBM50158187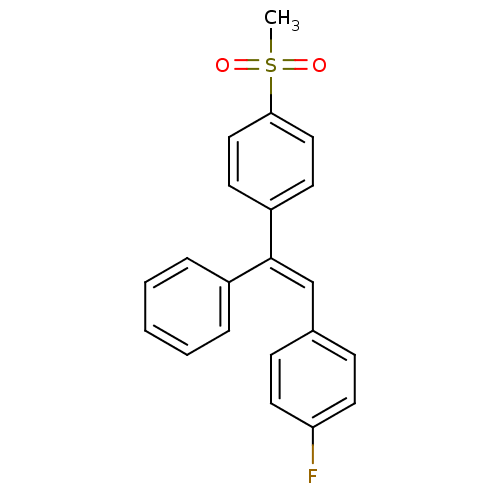 (1-fluoro-4-{(E)-2-[4-(methylsulfonyl)phenyl]-2-phe...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 32 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Alberta Curated by ChEMBL | Assay Description In vitro inhibitory concentration against ovine Cyclooxygenase-2 | Bioorg Med Chem Lett 15: 439-42 (2004) Article DOI: 10.1016/j.bmcl.2004.10.050 BindingDB Entry DOI: 10.7270/Q2GF0T0C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Ovis aries (Sheep)) | BDBM50158187 (1-fluoro-4-{(E)-2-[4-(methylsulfonyl)phenyl]-2-phe...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 32 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Alberta Curated by ChEMBL | Assay Description In vitro inhibitory concentration against ovine Cyclooxygenase-2 | Bioorg Med Chem Lett 15: 439-42 (2004) Article DOI: 10.1016/j.bmcl.2004.10.050 BindingDB Entry DOI: 10.7270/Q2GF0T0C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 1 (Ovis aries (Sheep)) | BDBM17638 (2-{1-[(4-chlorophenyl)carbonyl]-5-methoxy-2-methyl...) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt Institute of Chemical Biology, Vanderbilt University School of Medicine | Assay Description We evaluated the ability of test compounds to inhibit purified ovine COX-1 or murine COX-2 utilizing previously published protocols. [Uddin et al, Ca... | ACS Chem Biol 11: 3052-3060 (2016) Article DOI: 10.1021/acschembio.6b00560 BindingDB Entry DOI: 10.7270/Q23777JP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Mus musculus (Mouse)) | BDBM50336964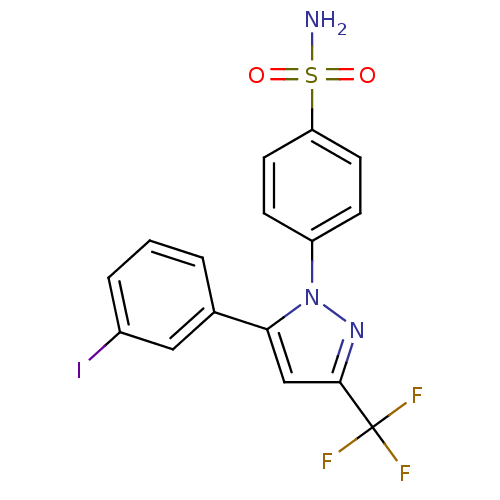 (4-[5-(3-Iodophenyl)-3-(trifluoromethyl)-1H-pyrazol...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of COX2 in mouse LPS-stimulated RAW264.7 cells after 30 mins | ACS Med Chem Lett 2: 160-164 (2011) Article DOI: 10.1021/ml100232q BindingDB Entry DOI: 10.7270/Q25T3MG9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 1 (Homo sapiens (Human)) | BDBM50561529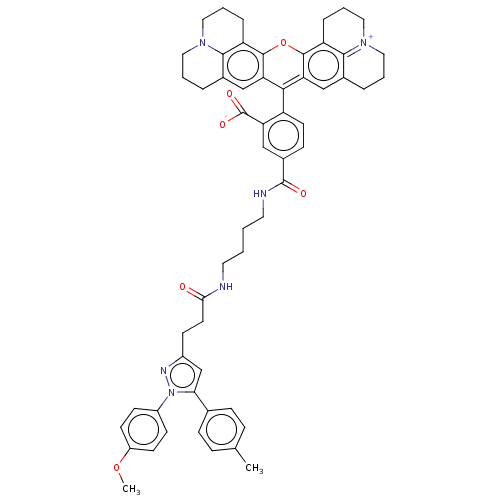 (CHEMBL4792985) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 44 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of COX-1 in human OVCAR3 cells assessed as [14C] arachidonic acid remaining using [14C] arachidonic acid as substrate preincubated for 30 ... | Citation and Details Article DOI: 10.1021/acsmedchemlett.9b00280 BindingDB Entry DOI: 10.7270/Q21C21J2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Mus musculus (Mouse)) | BDBM50336971 (5-(4-iodophenyl)-1-(4-(methylsulfonyl)phenyl)-3-(t...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of mouse purified COX2 after 20 mins | ACS Med Chem Lett 2: 160-164 (2011) Article DOI: 10.1021/ml100232q BindingDB Entry DOI: 10.7270/Q25T3MG9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Ovis aries (Sheep)) | BDBM50150734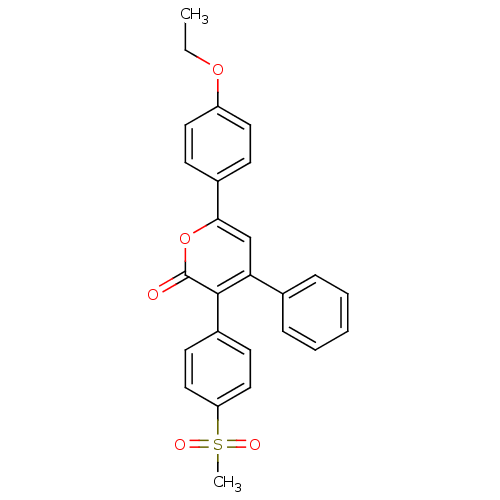 (6-(4-Ethoxy-phenyl)-3-(4-methanesulfonyl-phenyl)-4...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Alberta Curated by ChEMBL | Assay Description Inhibition of ovine Prostaglandin G/H synthase 2 | J Med Chem 47: 3972-90 (2004) Article DOI: 10.1021/jm049939b BindingDB Entry DOI: 10.7270/Q22F7MXX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 1 (Ovis aries (Sheep)) | BDBM17638 (2-{1-[(4-chlorophenyl)carbonyl]-5-methoxy-2-methyl...) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt Institute of Chemical Biology, Vanderbilt University School of Medicine | Assay Description Each molecule was evaluated for its ability to inhibit purified mouse COX-2 or ovine COX-1 using a previously described assay. [Kalgutkar et al, J. M... | ACS Chem Biol 11: 3052-3060 (2016) Article DOI: 10.1021/acschembio.6b00560 BindingDB Entry DOI: 10.7270/Q23777JP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member C3 (Homo sapiens (Human)) | BDBM50533571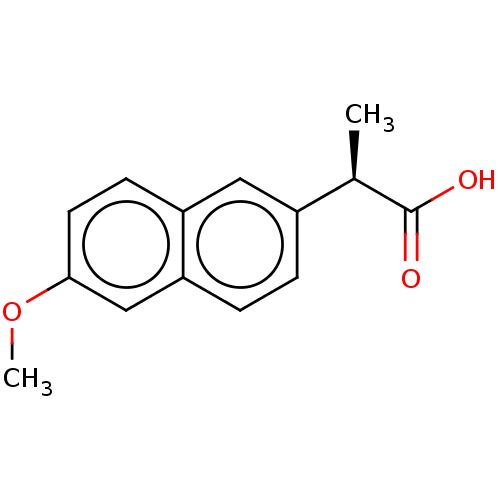 (CHEMBL1618254 | US11459295, Compound R-Naproxen (1...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE KEGG PC cid PC sid PDB UniChem | PDB Article PubMed | n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pennsylvania Curated by ChEMBL | Assay Description Inhibition of human recombinant AKR1C3 using S-tetralol as substrate assessed as reduction in NADP+-dependent S-tetralol oxidation preincubated for 1... | J Med Chem 59: 7431-44 (2016) Article DOI: 10.1021/acs.jmedchem.6b00160 BindingDB Entry DOI: 10.7270/Q21Z47WN | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Prostaglandin G/H synthase 1 (Ovis aries (Sheep)) | BDBM17638 (2-{1-[(4-chlorophenyl)carbonyl]-5-methoxy-2-methyl...) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University School of Medicine Curated by ChEMBL | Assay Description Inhibition of ovine COX1 | Bioorg Med Chem Lett 20: 1787-91 (2010) Article DOI: 10.1016/j.bmcl.2010.01.009 BindingDB Entry DOI: 10.7270/Q2J966H5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member C3 (Homo sapiens (Human)) | BDBM50533573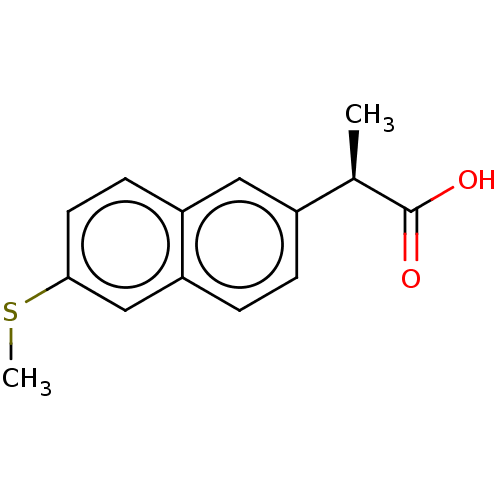 (CHEMBL4437291 | US11459295, Compound (R) 4a) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pennsylvania Curated by ChEMBL | Assay Description Inhibition of human recombinant AKR1C3 using S-tetralol as substrate assessed as reduction in NADP+-dependent S-tetralol oxidation preincubated for 1... | J Med Chem 59: 7431-44 (2016) Article DOI: 10.1021/acs.jmedchem.6b00160 BindingDB Entry DOI: 10.7270/Q21Z47WN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Ovis aries (Sheep)) | BDBM50156244 (Acetic acid 4-{1-[1-(4-methanesulfonyl-phenyl)-1-p...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Alberta Curated by ChEMBL | Assay Description In vitro inhibition of ovine prostaglandin G/H synthase 2 | J Med Chem 47: 6108-11 (2004) Article DOI: 10.1021/jm049523y BindingDB Entry DOI: 10.7270/Q2MG7P0Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Ovis aries (Sheep)) | BDBM11639 (4-[5-(4-methylphenyl)-3-(trifluoromethyl)-1H-pyraz...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 57 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Alberta Curated by ChEMBL | Assay Description In vitro inhibitory activity of the compound against prostaglandin G/H synthase 2 from ovine | Bioorg Med Chem Lett 14: 1953-6 (2004) Article DOI: 10.1016/j.bmcl.2004.01.075 BindingDB Entry DOI: 10.7270/Q24F1Q46 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Prostaglandin G/H synthase 2 (Mus musculus (Mouse)) | BDBM22369 (4-(4-methanesulfonylphenyl)-3-phenyl-2,5-dihydrofu...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 60 | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University School of Medicine Curated by ChEMBL | Assay Description Inhibition of mouse COX-2 assessed as inhibition of [14C]arachidonic acid to radiolabeled prostaglandins preincubated for 15 mins by TLC-based assay | ACS Med Chem Lett 5: 1254-8 (2014) Article DOI: 10.1021/ml500344j BindingDB Entry DOI: 10.7270/Q2BK1DZG | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Aldo-keto reductase family 1 member C3 (Homo sapiens (Human)) | BDBM50533576 (CHEMBL4551048 | US11459295, Compound LM5752(+-) 4) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | Article PubMed | n/a | n/a | 60 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pennsylvania Curated by ChEMBL | Assay Description Inhibition of human recombinant AKR1C3 using S-tetralol as substrate assessed as reduction in NADP+-dependent S-tetralol oxidation preincubated for 1... | J Med Chem 59: 7431-44 (2016) Article DOI: 10.1021/acs.jmedchem.6b00160 BindingDB Entry DOI: 10.7270/Q21Z47WN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 1 (Ovis aries (Sheep)) | BDBM50339185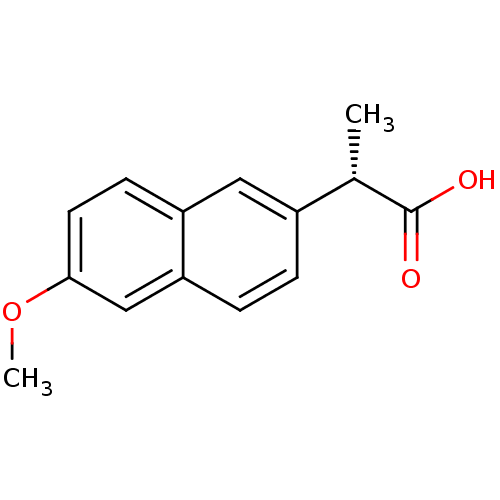 ((2S)-2-(6-methoxynaphthalen-2-yl)propanoic acid | ...) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 61 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pennsylvania Curated by ChEMBL | Assay Description Inhibition of COX1 in ram seminal vesicles using arachidonic acid as substrate assessed as reduction in PGH2 conversion to PGG2 by measuring TMPD oxi... | J Med Chem 59: 7431-44 (2016) Article DOI: 10.1021/acs.jmedchem.6b00160 BindingDB Entry DOI: 10.7270/Q21Z47WN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Ovis aries (Sheep)) | BDBM11639 (4-[5-(4-methylphenyl)-3-(trifluoromethyl)-1H-pyraz...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 70 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Alberta Curated by ChEMBL | Assay Description In vitro inhibitory concentration against ovine Cyclooxygenase-2 | Bioorg Med Chem Lett 15: 439-42 (2004) Article DOI: 10.1016/j.bmcl.2004.10.050 BindingDB Entry DOI: 10.7270/Q2GF0T0C | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Prostaglandin G/H synthase 2 (Ovis aries (Sheep)) | BDBM11639 (4-[5-(4-methylphenyl)-3-(trifluoromethyl)-1H-pyraz...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 70 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Alberta Curated by ChEMBL | Assay Description In vitro inhibitory concentration against ovine Cyclooxygenase-2 | Bioorg Med Chem Lett 15: 439-42 (2004) Article DOI: 10.1016/j.bmcl.2004.10.050 BindingDB Entry DOI: 10.7270/Q2GF0T0C | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Aldo-keto reductase family 1 member C3 (Homo sapiens (Human)) | BDBM50533577 (CHEMBL1236131 | US11459295, Compound (S) 4b) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | Article PubMed | n/a | n/a | 70 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pennsylvania Curated by ChEMBL | Assay Description Inhibition of human recombinant AKR1C3 using S-tetralol as substrate assessed as reduction in NADP+-dependent S-tetralol oxidation preincubated for 1... | J Med Chem 59: 7431-44 (2016) Article DOI: 10.1021/acs.jmedchem.6b00160 BindingDB Entry DOI: 10.7270/Q21Z47WN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Ovis aries (Sheep)) | BDBM50150751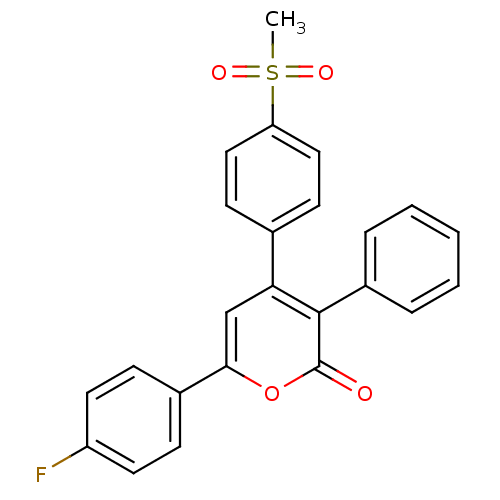 (6-(4-Fluoro-phenyl)-4-(4-methanesulfonyl-phenyl)-3...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 70 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Alberta Curated by ChEMBL | Assay Description Inhibition of ovine Prostaglandin G/H synthase 2 | J Med Chem 47: 3972-90 (2004) Article DOI: 10.1021/jm049939b BindingDB Entry DOI: 10.7270/Q22F7MXX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Ovis aries (Sheep)) | BDBM11639 (4-[5-(4-methylphenyl)-3-(trifluoromethyl)-1H-pyraz...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 70 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Alberta Curated by ChEMBL | Assay Description In vitro inhibitory concentration against ovine Prostaglandin G/H synthase 2 | Bioorg Med Chem Lett 14: 4911-4 (2004) Article DOI: 10.1016/j.bmcl.2004.07.027 BindingDB Entry DOI: 10.7270/Q2348JVS | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Prostaglandin G/H synthase 2 (Ovis aries (Sheep)) | BDBM11639 (4-[5-(4-methylphenyl)-3-(trifluoromethyl)-1H-pyraz...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 70 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Alberta Curated by ChEMBL | Assay Description In vitro inhibition of ovine prostaglandin G/H synthase 2 | J Med Chem 47: 6108-11 (2004) Article DOI: 10.1021/jm049523y BindingDB Entry DOI: 10.7270/Q2MG7P0Z | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Prostaglandin G/H synthase 2 (Ovis aries (Sheep)) | BDBM11639 (4-[5-(4-methylphenyl)-3-(trifluoromethyl)-1H-pyraz...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 70 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Alberta Curated by ChEMBL | Assay Description Inhibition of ovine Prostaglandin G/H synthase 2 | J Med Chem 47: 3972-90 (2004) Article DOI: 10.1021/jm049939b BindingDB Entry DOI: 10.7270/Q22F7MXX | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Prostaglandin G/H synthase 2 (Mus musculus (Mouse)) | BDBM50336964 (4-[5-(3-Iodophenyl)-3-(trifluoromethyl)-1H-pyrazol...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 80 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of mouse purified COX2 after 20 mins | ACS Med Chem Lett 2: 160-164 (2011) Article DOI: 10.1021/ml100232q BindingDB Entry DOI: 10.7270/Q25T3MG9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 1 (Ovis aries (Sheep)) | BDBM50033168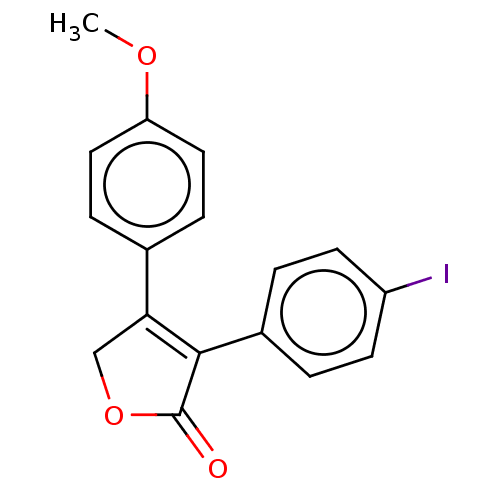 (CHEMBL3357106) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 90 | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University School of Medicine Curated by ChEMBL | Assay Description Inhibition of ovine COX-1 assessed as inhibition of [14C]arachidonic acid to radiolabeled prostaglandins preincubated for 15 mins by TLC-based assay | ACS Med Chem Lett 5: 1254-8 (2014) Article DOI: 10.1021/ml500344j BindingDB Entry DOI: 10.7270/Q2BK1DZG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 [18-604,R106Q] (Mus musculus (Mouse)) | BDBM50312668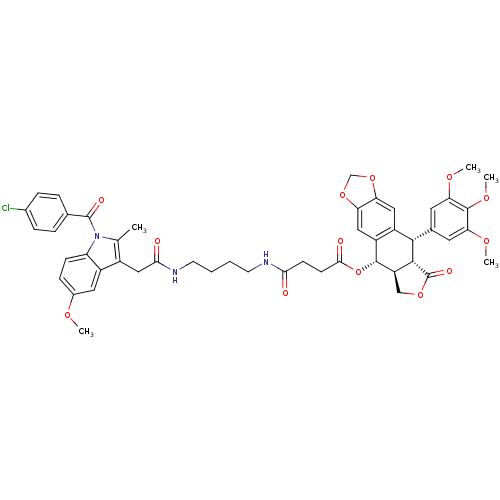 (CHEMBL1076638 | Chemocoxib A (12) | N-{(Succinylpo...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 90 | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt Institute of Chemical Biology, Vanderbilt University School of Medicine | Assay Description We evaluated the ability of test compounds to inhibit purified ovine COX-1 or murine COX-2 utilizing previously published protocols. [Uddin et al, Ca... | ACS Chem Biol 11: 3052-3060 (2016) Article DOI: 10.1021/acschembio.6b00560 BindingDB Entry DOI: 10.7270/Q23777JP | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Prostaglandin G/H synthase 1 (Ovis aries (Sheep)) | BDBM50561529 (CHEMBL4792985) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 94 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of ovine COX-1 assessed as [14C] arachidonic acid remaining using [14C] arachidonic acid as substrate preincubated for 20 mins followed by... | Citation and Details Article DOI: 10.1021/acsmedchemlett.9b00280 BindingDB Entry DOI: 10.7270/Q21C21J2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member C3 (Homo sapiens (Human)) | BDBM50533572 (CHEMBL4457933 | US11459295, Compound LM5753(+-) 3) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pennsylvania Curated by ChEMBL | Assay Description Inhibition of human recombinant AKR1C3 using S-tetralol as substrate assessed as reduction in NADP+-dependent S-tetralol oxidation preincubated for 1... | J Med Chem 59: 7431-44 (2016) Article DOI: 10.1021/acs.jmedchem.6b00160 BindingDB Entry DOI: 10.7270/Q21Z47WN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Mus musculus (Mouse)) | BDBM205489 (Cytotoxic conjugates, 6) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt Institute of Chemical Biology, Vanderbilt University School of Medicine | Assay Description Each molecule was evaluated for its ability to inhibit purified mouse COX-2 or ovine COX-1 using a previously described assay. [Kalgutkar et al, J. M... | ACS Chem Biol 11: 3052-3060 (2016) Article DOI: 10.1021/acschembio.6b00560 BindingDB Entry DOI: 10.7270/Q23777JP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member C3 (Homo sapiens (Human)) | BDBM50533570 (CHEMBL4435662 | US11459295, Compound LM5750A 8b) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | n/a | n/a | 110 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pennsylvania Curated by ChEMBL | Assay Description Inhibition of human recombinant AKR1C3 using S-tetralol as substrate assessed as reduction in NADP+-dependent S-tetralol oxidation preincubated for 1... | J Med Chem 59: 7431-44 (2016) Article DOI: 10.1021/acs.jmedchem.6b00160 BindingDB Entry DOI: 10.7270/Q21Z47WN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Ovis aries (Sheep)) | BDBM50158193 (1-chloro-4-{(E)-2-[4-(methylsulfonyl)phenyl]-2-phe...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 114 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Alberta Curated by ChEMBL | Assay Description In vitro inhibitory concentration against ovine Cyclooxygenase-2 | Bioorg Med Chem Lett 15: 439-42 (2004) Article DOI: 10.1016/j.bmcl.2004.10.050 BindingDB Entry DOI: 10.7270/Q2GF0T0C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Ovis aries (Sheep)) | BDBM50158193 (1-chloro-4-{(E)-2-[4-(methylsulfonyl)phenyl]-2-phe...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 114 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Alberta Curated by ChEMBL | Assay Description In vitro inhibitory concentration against ovine Cyclooxygenase-2 | Bioorg Med Chem Lett 15: 439-42 (2004) Article DOI: 10.1016/j.bmcl.2004.10.050 BindingDB Entry DOI: 10.7270/Q2GF0T0C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 1 (Ovis aries (Sheep)) | BDBM50033167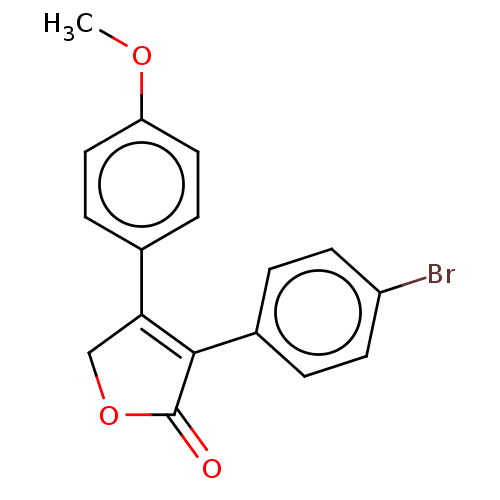 (CHEMBL3357105) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 120 | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University School of Medicine Curated by ChEMBL | Assay Description Inhibition of ovine COX-1 assessed as inhibition of [14C]arachidonic acid to radiolabeled prostaglandins preincubated for 15 mins by TLC-based assay | ACS Med Chem Lett 5: 1254-8 (2014) Article DOI: 10.1021/ml500344j BindingDB Entry DOI: 10.7270/Q2BK1DZG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member C3 (Homo sapiens (Human)) | BDBM50533574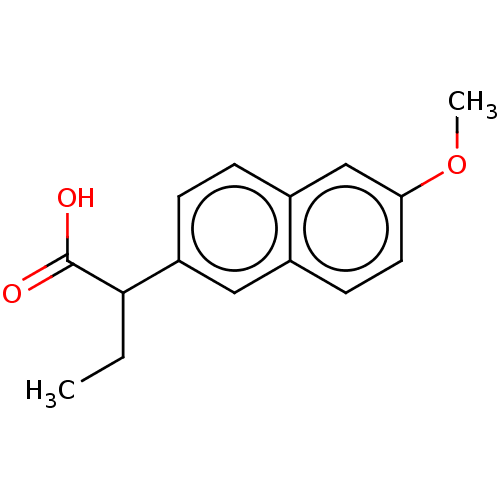 (CHEMBL4437071 | US11459295, Compound LM5750(+-) 8) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | n/a | n/a | 120 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pennsylvania Curated by ChEMBL | Assay Description Inhibition of human recombinant AKR1C3 using S-tetralol as substrate assessed as reduction in NADP+-dependent S-tetralol oxidation preincubated for 1... | J Med Chem 59: 7431-44 (2016) Article DOI: 10.1021/acs.jmedchem.6b00160 BindingDB Entry DOI: 10.7270/Q21Z47WN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member C3 (Homo sapiens (Human)) | BDBM50533568 (CHEMBL4466610 | US11459295, Compound LM5750B 8a) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | n/a | n/a | 120 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pennsylvania Curated by ChEMBL | Assay Description Inhibition of human recombinant AKR1C3 using S-tetralol as substrate assessed as reduction in NADP+-dependent S-tetralol oxidation preincubated for 1... | J Med Chem 59: 7431-44 (2016) Article DOI: 10.1021/acs.jmedchem.6b00160 BindingDB Entry DOI: 10.7270/Q21Z47WN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member C3 (Homo sapiens (Human)) | BDBM50533569 (CHEMBL4446810 | US11459295, Compound LM5751(+-) 2) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 120 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pennsylvania Curated by ChEMBL | Assay Description Inhibition of human recombinant AKR1C3 using S-tetralol as substrate assessed as reduction in NADP+-dependent S-tetralol oxidation preincubated for 1... | J Med Chem 59: 7431-44 (2016) Article DOI: 10.1021/acs.jmedchem.6b00160 BindingDB Entry DOI: 10.7270/Q21Z47WN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Mus musculus (Mouse)) | BDBM17638 (2-{1-[(4-chlorophenyl)carbonyl]-5-methoxy-2-methyl...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 127 | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University Curated by ChEMBL | Assay Description Inhibition of wild type mouse COX2 expressed in insect cells using [1-14C]-arachidonic acid as substrate incubated for 17 mins at 25 degC followed by... | ACS Med Chem Lett 4: 486-490 (2013) Article DOI: 10.1021/ml400066a BindingDB Entry DOI: 10.7270/Q28D005G | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Displayed 1 to 50 (of 464 total ) | Next | Last >> |