 Found 113 hits with Last Name = 'ueno' and Initial = 'k'
Found 113 hits with Last Name = 'ueno' and Initial = 'k' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Abscisic acid 8'-hydroxylase 3
(Arabidopsis thaliana) | BDBM50174072
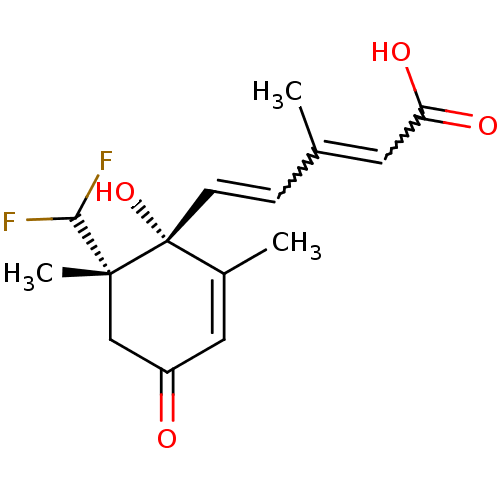
((+)-8',8'-difluoro-ABA | CHEMBL199709)Show SMILES CC(C=C[C@@]1(O)C(C)=CC(=O)C[C@]1(C)C(F)F)=CC(O)=O |w:17.18,2.1,c:7| Show InChI InChI=1S/C15H18F2O4/c1-9(6-12(19)20)4-5-15(21)10(2)7-11(18)8-14(15,3)13(16)17/h4-7,13,21H,8H2,1-3H3,(H,19,20)/t14-,15-/m1/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 170 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Gifu University
Curated by ChEMBL
| Assay Description
Inhibitory activity against recombinant CYP707A3 in Arabidopsis |
Bioorg Med Chem Lett 15: 5226-9 (2005)
Article DOI: 10.1016/j.bmcl.2005.08.042
BindingDB Entry DOI: 10.7270/Q2D21X5T |
More data for this
Ligand-Target Pair | |
Abscisic acid 8'-hydroxylase 3
(Arabidopsis thaliana) | BDBM50174070
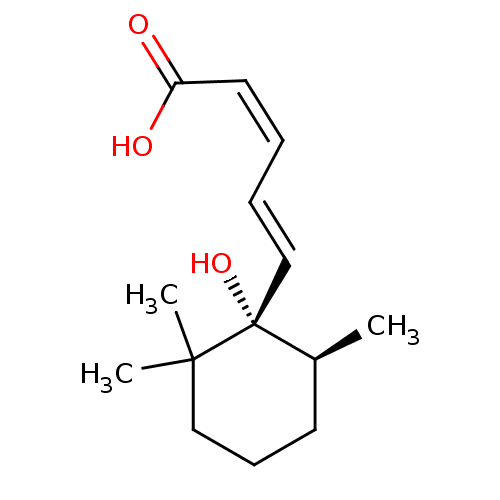
((+)-(2Z,4E)-5-((1S,6S)-1-hydroxy-2,2,6-trimethylcy...)Show InChI InChI=1S/C14H22O3/c1-11-7-6-9-13(2,3)14(11,17)10-5-4-8-12(15)16/h4-5,8,10-11,17H,6-7,9H2,1-3H3,(H,15,16)/b8-4-,10-5+/t11-,14+/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Gifu University
Curated by ChEMBL
| Assay Description
Inhibitory activity against recombinant CYP707A3 in Arabidopsis |
Bioorg Med Chem Lett 15: 5226-9 (2005)
Article DOI: 10.1016/j.bmcl.2005.08.042
BindingDB Entry DOI: 10.7270/Q2D21X5T |
More data for this
Ligand-Target Pair | |
Abscisic acid 8'-hydroxylase 3
(Arabidopsis thaliana) | BDBM50174069
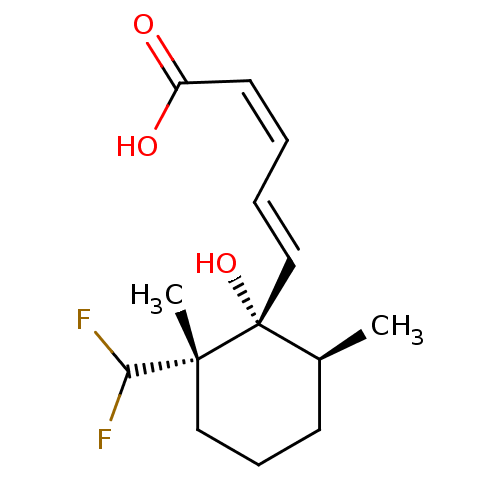
((1'S*,2'S*,6'S*)-(+/-)-6-nor-2',3'-dihydro-4'-deox...)Show SMILES C[C@H]1CCC[C@](C)(C(F)F)[C@@]1(O)\C=C\C=C/C(O)=O Show InChI InChI=1S/C14H20F2O3/c1-10-6-5-8-13(2,12(15)16)14(10,19)9-4-3-7-11(17)18/h3-4,7,9-10,12,19H,5-6,8H2,1-2H3,(H,17,18)/b7-3-,9-4+/t10-,13+,14+/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 410 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Gifu University
Curated by ChEMBL
| Assay Description
Inhibitory activity against recombinant CYP707A3 in Arabidopsis |
Bioorg Med Chem Lett 15: 5226-9 (2005)
Article DOI: 10.1016/j.bmcl.2005.08.042
BindingDB Entry DOI: 10.7270/Q2D21X5T |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1A
(Homo sapiens (Human)) | BDBM86693
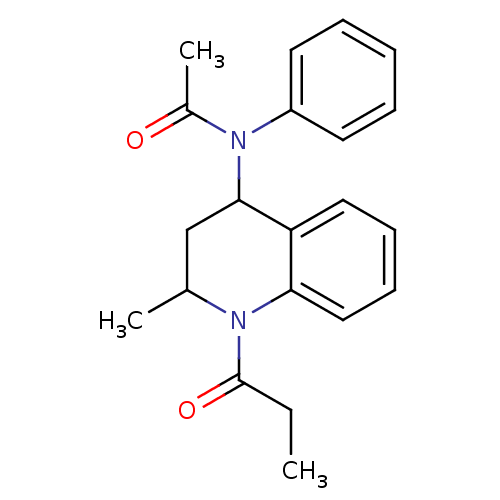
(K376)Show SMILES CCC(=O)N1C(C)CC(N(C(C)=O)c2ccccc2)c2ccccc12 Show InChI InChI=1S/C21H24N2O2/c1-4-21(25)22-15(2)14-20(18-12-8-9-13-19(18)22)23(16(3)24)17-10-6-5-7-11-17/h5-13,15,20H,4,14H2,1-3H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyowa Hakko Kogyo Co., Ltd.
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 314: 244-51 (2005)
Article DOI: 10.1124/jpet.104.081539
BindingDB Entry DOI: 10.7270/Q2RN36FF |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1A
(Homo sapiens (Human)) | BDBM86691
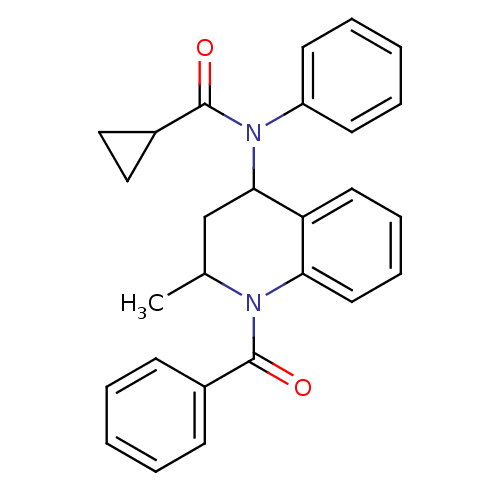
(K604)Show SMILES CC1CC(N(C(=O)C2CC2)c2ccccc2)c2ccccc2N1C(=O)c1ccccc1 Show InChI InChI=1S/C27H26N2O2/c1-19-18-25(29(27(31)21-16-17-21)22-12-6-3-7-13-22)23-14-8-9-15-24(23)28(19)26(30)20-10-4-2-5-11-20/h2-15,19,21,25H,16-18H2,1H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyowa Hakko Kogyo Co., Ltd.
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 314: 244-51 (2005)
Article DOI: 10.1124/jpet.104.081539
BindingDB Entry DOI: 10.7270/Q2RN36FF |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Rattus norvegicus (rat)) | BDBM86692
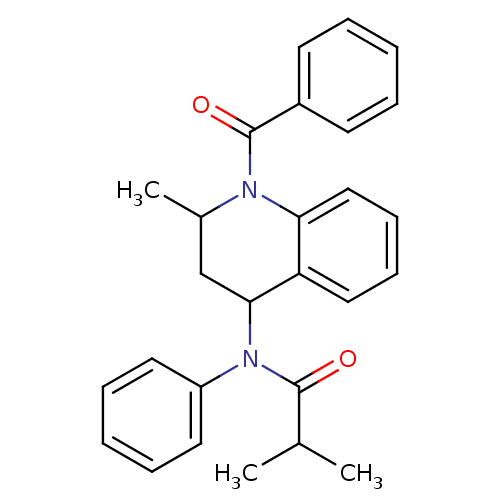
(K117)Show SMILES CC(C)C(=O)N(C1CC(C)N(C(=O)c2ccccc2)c2ccccc12)c1ccccc1 Show InChI InChI=1S/C27H28N2O2/c1-19(2)26(30)29(22-14-8-5-9-15-22)25-18-20(3)28(24-17-11-10-16-23(24)25)27(31)21-12-6-4-7-13-21/h4-17,19-20,25H,18H2,1-3H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyowa Hakko Kogyo Co., Ltd.
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 314: 244-51 (2005)
Article DOI: 10.1124/jpet.104.081539
BindingDB Entry DOI: 10.7270/Q2RN36FF |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Rattus norvegicus (rat)) | BDBM86693

(K376)Show SMILES CCC(=O)N1C(C)CC(N(C(C)=O)c2ccccc2)c2ccccc12 Show InChI InChI=1S/C21H24N2O2/c1-4-21(25)22-15(2)14-20(18-12-8-9-13-19(18)22)23(16(3)24)17-10-6-5-7-11-17/h5-13,15,20H,4,14H2,1-3H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyowa Hakko Kogyo Co., Ltd.
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 314: 244-51 (2005)
Article DOI: 10.1124/jpet.104.081539
BindingDB Entry DOI: 10.7270/Q2RN36FF |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Rattus norvegicus (rat)) | BDBM86691

(K604)Show SMILES CC1CC(N(C(=O)C2CC2)c2ccccc2)c2ccccc2N1C(=O)c1ccccc1 Show InChI InChI=1S/C27H26N2O2/c1-19-18-25(29(27(31)21-16-17-21)22-12-6-3-7-13-22)23-14-8-9-15-24(23)28(19)26(30)20-10-4-2-5-11-20/h2-15,19,21,25H,16-18H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyowa Hakko Kogyo Co., Ltd.
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 314: 244-51 (2005)
Article DOI: 10.1124/jpet.104.081539
BindingDB Entry DOI: 10.7270/Q2RN36FF |
More data for this
Ligand-Target Pair | |
Polyunsaturated fatty acid 5-lipoxygenase
(Homo sapiens (Human)) | BDBM86692

(K117)Show SMILES CC(C)C(=O)N(C1CC(C)N(C(=O)c2ccccc2)c2ccccc12)c1ccccc1 Show InChI InChI=1S/C27H28N2O2/c1-19(2)26(30)29(22-14-8-5-9-15-22)25-18-20(3)28(24-17-11-10-16-23(24)25)27(31)21-12-6-4-7-13-21/h4-17,19-20,25H,18H2,1-3H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyowa Hakko Kogyo Co., Ltd.
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 314: 244-51 (2005)
Article DOI: 10.1124/jpet.104.081539
BindingDB Entry DOI: 10.7270/Q2RN36FF |
More data for this
Ligand-Target Pair | |
Polyunsaturated fatty acid 5-lipoxygenase
(Homo sapiens (Human)) | BDBM86693

(K376)Show SMILES CCC(=O)N1C(C)CC(N(C(C)=O)c2ccccc2)c2ccccc12 Show InChI InChI=1S/C21H24N2O2/c1-4-21(25)22-15(2)14-20(18-12-8-9-13-19(18)22)23(16(3)24)17-10-6-5-7-11-17/h5-13,15,20H,4,14H2,1-3H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyowa Hakko Kogyo Co., Ltd.
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 314: 244-51 (2005)
Article DOI: 10.1124/jpet.104.081539
BindingDB Entry DOI: 10.7270/Q2RN36FF |
More data for this
Ligand-Target Pair | |
Polyunsaturated fatty acid 5-lipoxygenase
(Homo sapiens (Human)) | BDBM86691

(K604)Show SMILES CC1CC(N(C(=O)C2CC2)c2ccccc2)c2ccccc2N1C(=O)c1ccccc1 Show InChI InChI=1S/C27H26N2O2/c1-19-18-25(29(27(31)21-16-17-21)22-12-6-3-7-13-22)23-14-8-9-15-24(23)28(19)26(30)20-10-4-2-5-11-20/h2-15,19,21,25H,16-18H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyowa Hakko Kogyo Co., Ltd.
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 314: 244-51 (2005)
Article DOI: 10.1124/jpet.104.081539
BindingDB Entry DOI: 10.7270/Q2RN36FF |
More data for this
Ligand-Target Pair | |
Alpha-1A adrenergic receptor
(Rattus norvegicus (Rat)) | BDBM86692

(K117)Show SMILES CC(C)C(=O)N(C1CC(C)N(C(=O)c2ccccc2)c2ccccc12)c1ccccc1 Show InChI InChI=1S/C27H28N2O2/c1-19(2)26(30)29(22-14-8-5-9-15-22)25-18-20(3)28(24-17-11-10-16-23(24)25)27(31)21-12-6-4-7-13-21/h4-17,19-20,25H,18H2,1-3H3 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyowa Hakko Kogyo Co., Ltd.
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 314: 244-51 (2005)
Article DOI: 10.1124/jpet.104.081539
BindingDB Entry DOI: 10.7270/Q2RN36FF |
More data for this
Ligand-Target Pair | |
Alpha-1A adrenergic receptor
(Rattus norvegicus (Rat)) | BDBM86693

(K376)Show SMILES CCC(=O)N1C(C)CC(N(C(C)=O)c2ccccc2)c2ccccc12 Show InChI InChI=1S/C21H24N2O2/c1-4-21(25)22-15(2)14-20(18-12-8-9-13-19(18)22)23(16(3)24)17-10-6-5-7-11-17/h5-13,15,20H,4,14H2,1-3H3 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyowa Hakko Kogyo Co., Ltd.
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 314: 244-51 (2005)
Article DOI: 10.1124/jpet.104.081539
BindingDB Entry DOI: 10.7270/Q2RN36FF |
More data for this
Ligand-Target Pair | |
Alpha-1A adrenergic receptor
(Rattus norvegicus (Rat)) | BDBM86691

(K604)Show SMILES CC1CC(N(C(=O)C2CC2)c2ccccc2)c2ccccc2N1C(=O)c1ccccc1 Show InChI InChI=1S/C27H26N2O2/c1-19-18-25(29(27(31)21-16-17-21)22-12-6-3-7-13-22)23-14-8-9-15-24(23)28(19)26(30)20-10-4-2-5-11-20/h2-15,19,21,25H,16-18H2,1H3 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyowa Hakko Kogyo Co., Ltd.
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 314: 244-51 (2005)
Article DOI: 10.1124/jpet.104.081539
BindingDB Entry DOI: 10.7270/Q2RN36FF |
More data for this
Ligand-Target Pair | |
Alpha-1A adrenergic receptor
(Rattus norvegicus (Rat)) | BDBM86692

(K117)Show SMILES CC(C)C(=O)N(C1CC(C)N(C(=O)c2ccccc2)c2ccccc12)c1ccccc1 Show InChI InChI=1S/C27H28N2O2/c1-19(2)26(30)29(22-14-8-5-9-15-22)25-18-20(3)28(24-17-11-10-16-23(24)25)27(31)21-12-6-4-7-13-21/h4-17,19-20,25H,18H2,1-3H3 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyowa Hakko Kogyo Co., Ltd.
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 314: 244-51 (2005)
Article DOI: 10.1124/jpet.104.081539
BindingDB Entry DOI: 10.7270/Q2RN36FF |
More data for this
Ligand-Target Pair | |
Alpha-1A adrenergic receptor
(Rattus norvegicus (Rat)) | BDBM86693

(K376)Show SMILES CCC(=O)N1C(C)CC(N(C(C)=O)c2ccccc2)c2ccccc12 Show InChI InChI=1S/C21H24N2O2/c1-4-21(25)22-15(2)14-20(18-12-8-9-13-19(18)22)23(16(3)24)17-10-6-5-7-11-17/h5-13,15,20H,4,14H2,1-3H3 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyowa Hakko Kogyo Co., Ltd.
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 314: 244-51 (2005)
Article DOI: 10.1124/jpet.104.081539
BindingDB Entry DOI: 10.7270/Q2RN36FF |
More data for this
Ligand-Target Pair | |
Alpha-1A adrenergic receptor
(Rattus norvegicus (Rat)) | BDBM86691

(K604)Show SMILES CC1CC(N(C(=O)C2CC2)c2ccccc2)c2ccccc2N1C(=O)c1ccccc1 Show InChI InChI=1S/C27H26N2O2/c1-19-18-25(29(27(31)21-16-17-21)22-12-6-3-7-13-22)23-14-8-9-15-24(23)28(19)26(30)20-10-4-2-5-11-20/h2-15,19,21,25H,16-18H2,1H3 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyowa Hakko Kogyo Co., Ltd.
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 314: 244-51 (2005)
Article DOI: 10.1124/jpet.104.081539
BindingDB Entry DOI: 10.7270/Q2RN36FF |
More data for this
Ligand-Target Pair | |
Alpha-2A adrenergic receptor [16-465]
(Rattus norvegicus (rat)) | BDBM86692

(K117)Show SMILES CC(C)C(=O)N(C1CC(C)N(C(=O)c2ccccc2)c2ccccc12)c1ccccc1 Show InChI InChI=1S/C27H28N2O2/c1-19(2)26(30)29(22-14-8-5-9-15-22)25-18-20(3)28(24-17-11-10-16-23(24)25)27(31)21-12-6-4-7-13-21/h4-17,19-20,25H,18H2,1-3H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyowa Hakko Kogyo Co., Ltd.
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 314: 244-51 (2005)
Article DOI: 10.1124/jpet.104.081539
BindingDB Entry DOI: 10.7270/Q2RN36FF |
More data for this
Ligand-Target Pair | |
Alpha-2A adrenergic receptor [16-465]
(Rattus norvegicus (rat)) | BDBM86693

(K376)Show SMILES CCC(=O)N1C(C)CC(N(C(C)=O)c2ccccc2)c2ccccc12 Show InChI InChI=1S/C21H24N2O2/c1-4-21(25)22-15(2)14-20(18-12-8-9-13-19(18)22)23(16(3)24)17-10-6-5-7-11-17/h5-13,15,20H,4,14H2,1-3H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyowa Hakko Kogyo Co., Ltd.
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 314: 244-51 (2005)
Article DOI: 10.1124/jpet.104.081539
BindingDB Entry DOI: 10.7270/Q2RN36FF |
More data for this
Ligand-Target Pair | |
Alpha-2A adrenergic receptor [16-465]
(Rattus norvegicus (rat)) | BDBM86691

(K604)Show SMILES CC1CC(N(C(=O)C2CC2)c2ccccc2)c2ccccc2N1C(=O)c1ccccc1 Show InChI InChI=1S/C27H26N2O2/c1-19-18-25(29(27(31)21-16-17-21)22-12-6-3-7-13-22)23-14-8-9-15-24(23)28(19)26(30)20-10-4-2-5-11-20/h2-15,19,21,25H,16-18H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyowa Hakko Kogyo Co., Ltd.
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 314: 244-51 (2005)
Article DOI: 10.1124/jpet.104.081539
BindingDB Entry DOI: 10.7270/Q2RN36FF |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1
(Homo sapiens (Human)) | BDBM86692

(K117)Show SMILES CC(C)C(=O)N(C1CC(C)N(C(=O)c2ccccc2)c2ccccc12)c1ccccc1 Show InChI InChI=1S/C27H28N2O2/c1-19(2)26(30)29(22-14-8-5-9-15-22)25-18-20(3)28(24-17-11-10-16-23(24)25)27(31)21-12-6-4-7-13-21/h4-17,19-20,25H,18H2,1-3H3 | UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyowa Hakko Kogyo Co., Ltd.
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 314: 244-51 (2005)
Article DOI: 10.1124/jpet.104.081539
BindingDB Entry DOI: 10.7270/Q2RN36FF |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1
(Homo sapiens (Human)) | BDBM86693

(K376)Show SMILES CCC(=O)N1C(C)CC(N(C(C)=O)c2ccccc2)c2ccccc12 Show InChI InChI=1S/C21H24N2O2/c1-4-21(25)22-15(2)14-20(18-12-8-9-13-19(18)22)23(16(3)24)17-10-6-5-7-11-17/h5-13,15,20H,4,14H2,1-3H3 | UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyowa Hakko Kogyo Co., Ltd.
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 314: 244-51 (2005)
Article DOI: 10.1124/jpet.104.081539
BindingDB Entry DOI: 10.7270/Q2RN36FF |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1
(Homo sapiens (Human)) | BDBM86691

(K604)Show SMILES CC1CC(N(C(=O)C2CC2)c2ccccc2)c2ccccc2N1C(=O)c1ccccc1 Show InChI InChI=1S/C27H26N2O2/c1-19-18-25(29(27(31)21-16-17-21)22-12-6-3-7-13-22)23-14-8-9-15-24(23)28(19)26(30)20-10-4-2-5-11-20/h2-15,19,21,25H,16-18H2,1H3 | UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyowa Hakko Kogyo Co., Ltd.
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 314: 244-51 (2005)
Article DOI: 10.1124/jpet.104.081539
BindingDB Entry DOI: 10.7270/Q2RN36FF |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 1
(Homo sapiens (Human)) | BDBM86692

(K117)Show SMILES CC(C)C(=O)N(C1CC(C)N(C(=O)c2ccccc2)c2ccccc12)c1ccccc1 Show InChI InChI=1S/C27H28N2O2/c1-19(2)26(30)29(22-14-8-5-9-15-22)25-18-20(3)28(24-17-11-10-16-23(24)25)27(31)21-12-6-4-7-13-21/h4-17,19-20,25H,18H2,1-3H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyowa Hakko Kogyo Co., Ltd.
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 314: 244-51 (2005)
Article DOI: 10.1124/jpet.104.081539
BindingDB Entry DOI: 10.7270/Q2RN36FF |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 1
(Homo sapiens (Human)) | BDBM86693

(K376)Show SMILES CCC(=O)N1C(C)CC(N(C(C)=O)c2ccccc2)c2ccccc12 Show InChI InChI=1S/C21H24N2O2/c1-4-21(25)22-15(2)14-20(18-12-8-9-13-19(18)22)23(16(3)24)17-10-6-5-7-11-17/h5-13,15,20H,4,14H2,1-3H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyowa Hakko Kogyo Co., Ltd.
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 314: 244-51 (2005)
Article DOI: 10.1124/jpet.104.081539
BindingDB Entry DOI: 10.7270/Q2RN36FF |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 1
(Homo sapiens (Human)) | BDBM86691

(K604)Show SMILES CC1CC(N(C(=O)C2CC2)c2ccccc2)c2ccccc2N1C(=O)c1ccccc1 Show InChI InChI=1S/C27H26N2O2/c1-19-18-25(29(27(31)21-16-17-21)22-12-6-3-7-13-22)23-14-8-9-15-24(23)28(19)26(30)20-10-4-2-5-11-20/h2-15,19,21,25H,16-18H2,1H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyowa Hakko Kogyo Co., Ltd.
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 314: 244-51 (2005)
Article DOI: 10.1124/jpet.104.081539
BindingDB Entry DOI: 10.7270/Q2RN36FF |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM86692

(K117)Show SMILES CC(C)C(=O)N(C1CC(C)N(C(=O)c2ccccc2)c2ccccc12)c1ccccc1 Show InChI InChI=1S/C27H28N2O2/c1-19(2)26(30)29(22-14-8-5-9-15-22)25-18-20(3)28(24-17-11-10-16-23(24)25)27(31)21-12-6-4-7-13-21/h4-17,19-20,25H,18H2,1-3H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyowa Hakko Kogyo Co., Ltd.
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 314: 244-51 (2005)
Article DOI: 10.1124/jpet.104.081539
BindingDB Entry DOI: 10.7270/Q2RN36FF |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM86693

(K376)Show SMILES CCC(=O)N1C(C)CC(N(C(C)=O)c2ccccc2)c2ccccc12 Show InChI InChI=1S/C21H24N2O2/c1-4-21(25)22-15(2)14-20(18-12-8-9-13-19(18)22)23(16(3)24)17-10-6-5-7-11-17/h5-13,15,20H,4,14H2,1-3H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyowa Hakko Kogyo Co., Ltd.
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 314: 244-51 (2005)
Article DOI: 10.1124/jpet.104.081539
BindingDB Entry DOI: 10.7270/Q2RN36FF |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM86691

(K604)Show SMILES CC1CC(N(C(=O)C2CC2)c2ccccc2)c2ccccc2N1C(=O)c1ccccc1 Show InChI InChI=1S/C27H26N2O2/c1-19-18-25(29(27(31)21-16-17-21)22-12-6-3-7-13-22)23-14-8-9-15-24(23)28(19)26(30)20-10-4-2-5-11-20/h2-15,19,21,25H,16-18H2,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyowa Hakko Kogyo Co., Ltd.
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 314: 244-51 (2005)
Article DOI: 10.1124/jpet.104.081539
BindingDB Entry DOI: 10.7270/Q2RN36FF |
More data for this
Ligand-Target Pair | |
D(1A) dopamine receptor
(Homo sapiens (Human)) | BDBM86692

(K117)Show SMILES CC(C)C(=O)N(C1CC(C)N(C(=O)c2ccccc2)c2ccccc12)c1ccccc1 Show InChI InChI=1S/C27H28N2O2/c1-19(2)26(30)29(22-14-8-5-9-15-22)25-18-20(3)28(24-17-11-10-16-23(24)25)27(31)21-12-6-4-7-13-21/h4-17,19-20,25H,18H2,1-3H3 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyowa Hakko Kogyo Co., Ltd.
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 314: 244-51 (2005)
Article DOI: 10.1124/jpet.104.081539
BindingDB Entry DOI: 10.7270/Q2RN36FF |
More data for this
Ligand-Target Pair | |
D(1A) dopamine receptor
(Homo sapiens (Human)) | BDBM86693

(K376)Show SMILES CCC(=O)N1C(C)CC(N(C(C)=O)c2ccccc2)c2ccccc12 Show InChI InChI=1S/C21H24N2O2/c1-4-21(25)22-15(2)14-20(18-12-8-9-13-19(18)22)23(16(3)24)17-10-6-5-7-11-17/h5-13,15,20H,4,14H2,1-3H3 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyowa Hakko Kogyo Co., Ltd.
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 314: 244-51 (2005)
Article DOI: 10.1124/jpet.104.081539
BindingDB Entry DOI: 10.7270/Q2RN36FF |
More data for this
Ligand-Target Pair | |
D(1A) dopamine receptor
(Homo sapiens (Human)) | BDBM86691

(K604)Show SMILES CC1CC(N(C(=O)C2CC2)c2ccccc2)c2ccccc2N1C(=O)c1ccccc1 Show InChI InChI=1S/C27H26N2O2/c1-19-18-25(29(27(31)21-16-17-21)22-12-6-3-7-13-22)23-14-8-9-15-24(23)28(19)26(30)20-10-4-2-5-11-20/h2-15,19,21,25H,16-18H2,1H3 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyowa Hakko Kogyo Co., Ltd.
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 314: 244-51 (2005)
Article DOI: 10.1124/jpet.104.081539
BindingDB Entry DOI: 10.7270/Q2RN36FF |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Homo sapiens (Human)) | BDBM86692

(K117)Show SMILES CC(C)C(=O)N(C1CC(C)N(C(=O)c2ccccc2)c2ccccc12)c1ccccc1 Show InChI InChI=1S/C27H28N2O2/c1-19(2)26(30)29(22-14-8-5-9-15-22)25-18-20(3)28(24-17-11-10-16-23(24)25)27(31)21-12-6-4-7-13-21/h4-17,19-20,25H,18H2,1-3H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyowa Hakko Kogyo Co., Ltd.
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 314: 244-51 (2005)
Article DOI: 10.1124/jpet.104.081539
BindingDB Entry DOI: 10.7270/Q2RN36FF |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Homo sapiens (Human)) | BDBM86693

(K376)Show SMILES CCC(=O)N1C(C)CC(N(C(C)=O)c2ccccc2)c2ccccc12 Show InChI InChI=1S/C21H24N2O2/c1-4-21(25)22-15(2)14-20(18-12-8-9-13-19(18)22)23(16(3)24)17-10-6-5-7-11-17/h5-13,15,20H,4,14H2,1-3H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyowa Hakko Kogyo Co., Ltd.
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 314: 244-51 (2005)
Article DOI: 10.1124/jpet.104.081539
BindingDB Entry DOI: 10.7270/Q2RN36FF |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Homo sapiens (Human)) | BDBM86691

(K604)Show SMILES CC1CC(N(C(=O)C2CC2)c2ccccc2)c2ccccc2N1C(=O)c1ccccc1 Show InChI InChI=1S/C27H26N2O2/c1-19-18-25(29(27(31)21-16-17-21)22-12-6-3-7-13-22)23-14-8-9-15-24(23)28(19)26(30)20-10-4-2-5-11-20/h2-15,19,21,25H,16-18H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyowa Hakko Kogyo Co., Ltd.
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 314: 244-51 (2005)
Article DOI: 10.1124/jpet.104.081539
BindingDB Entry DOI: 10.7270/Q2RN36FF |
More data for this
Ligand-Target Pair | |
Histamine H1 receptor
(Homo sapiens (Human)) | BDBM86692

(K117)Show SMILES CC(C)C(=O)N(C1CC(C)N(C(=O)c2ccccc2)c2ccccc12)c1ccccc1 Show InChI InChI=1S/C27H28N2O2/c1-19(2)26(30)29(22-14-8-5-9-15-22)25-18-20(3)28(24-17-11-10-16-23(24)25)27(31)21-12-6-4-7-13-21/h4-17,19-20,25H,18H2,1-3H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyowa Hakko Kogyo Co., Ltd.
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 314: 244-51 (2005)
Article DOI: 10.1124/jpet.104.081539
BindingDB Entry DOI: 10.7270/Q2RN36FF |
More data for this
Ligand-Target Pair | |
Histamine H1 receptor
(Homo sapiens (Human)) | BDBM86693

(K376)Show SMILES CCC(=O)N1C(C)CC(N(C(C)=O)c2ccccc2)c2ccccc12 Show InChI InChI=1S/C21H24N2O2/c1-4-21(25)22-15(2)14-20(18-12-8-9-13-19(18)22)23(16(3)24)17-10-6-5-7-11-17/h5-13,15,20H,4,14H2,1-3H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyowa Hakko Kogyo Co., Ltd.
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 314: 244-51 (2005)
Article DOI: 10.1124/jpet.104.081539
BindingDB Entry DOI: 10.7270/Q2RN36FF |
More data for this
Ligand-Target Pair | |
Histamine H1 receptor
(Homo sapiens (Human)) | BDBM86691

(K604)Show SMILES CC1CC(N(C(=O)C2CC2)c2ccccc2)c2ccccc2N1C(=O)c1ccccc1 Show InChI InChI=1S/C27H26N2O2/c1-19-18-25(29(27(31)21-16-17-21)22-12-6-3-7-13-22)23-14-8-9-15-24(23)28(19)26(30)20-10-4-2-5-11-20/h2-15,19,21,25H,16-18H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyowa Hakko Kogyo Co., Ltd.
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 314: 244-51 (2005)
Article DOI: 10.1124/jpet.104.081539
BindingDB Entry DOI: 10.7270/Q2RN36FF |
More data for this
Ligand-Target Pair | |
Histamine H2 receptor
(Homo sapiens (Human)) | BDBM86692

(K117)Show SMILES CC(C)C(=O)N(C1CC(C)N(C(=O)c2ccccc2)c2ccccc12)c1ccccc1 Show InChI InChI=1S/C27H28N2O2/c1-19(2)26(30)29(22-14-8-5-9-15-22)25-18-20(3)28(24-17-11-10-16-23(24)25)27(31)21-12-6-4-7-13-21/h4-17,19-20,25H,18H2,1-3H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyowa Hakko Kogyo Co., Ltd.
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 314: 244-51 (2005)
Article DOI: 10.1124/jpet.104.081539
BindingDB Entry DOI: 10.7270/Q2RN36FF |
More data for this
Ligand-Target Pair | |
Histamine H2 receptor
(Homo sapiens (Human)) | BDBM86693

(K376)Show SMILES CCC(=O)N1C(C)CC(N(C(C)=O)c2ccccc2)c2ccccc12 Show InChI InChI=1S/C21H24N2O2/c1-4-21(25)22-15(2)14-20(18-12-8-9-13-19(18)22)23(16(3)24)17-10-6-5-7-11-17/h5-13,15,20H,4,14H2,1-3H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyowa Hakko Kogyo Co., Ltd.
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 314: 244-51 (2005)
Article DOI: 10.1124/jpet.104.081539
BindingDB Entry DOI: 10.7270/Q2RN36FF |
More data for this
Ligand-Target Pair | |
Histamine H2 receptor
(Homo sapiens (Human)) | BDBM86691

(K604)Show SMILES CC1CC(N(C(=O)C2CC2)c2ccccc2)c2ccccc2N1C(=O)c1ccccc1 Show InChI InChI=1S/C27H26N2O2/c1-19-18-25(29(27(31)21-16-17-21)22-12-6-3-7-13-22)23-14-8-9-15-24(23)28(19)26(30)20-10-4-2-5-11-20/h2-15,19,21,25H,16-18H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyowa Hakko Kogyo Co., Ltd.
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 314: 244-51 (2005)
Article DOI: 10.1124/jpet.104.081539
BindingDB Entry DOI: 10.7270/Q2RN36FF |
More data for this
Ligand-Target Pair | |
Leukotriene B4 receptor 2
(Homo sapiens (Human)) | BDBM86692

(K117)Show SMILES CC(C)C(=O)N(C1CC(C)N(C(=O)c2ccccc2)c2ccccc12)c1ccccc1 Show InChI InChI=1S/C27H28N2O2/c1-19(2)26(30)29(22-14-8-5-9-15-22)25-18-20(3)28(24-17-11-10-16-23(24)25)27(31)21-12-6-4-7-13-21/h4-17,19-20,25H,18H2,1-3H3 | UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyowa Hakko Kogyo Co., Ltd.
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 314: 244-51 (2005)
Article DOI: 10.1124/jpet.104.081539
BindingDB Entry DOI: 10.7270/Q2RN36FF |
More data for this
Ligand-Target Pair | |
Leukotriene B4 receptor 2
(Homo sapiens (Human)) | BDBM86693

(K376)Show SMILES CCC(=O)N1C(C)CC(N(C(C)=O)c2ccccc2)c2ccccc12 Show InChI InChI=1S/C21H24N2O2/c1-4-21(25)22-15(2)14-20(18-12-8-9-13-19(18)22)23(16(3)24)17-10-6-5-7-11-17/h5-13,15,20H,4,14H2,1-3H3 | UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyowa Hakko Kogyo Co., Ltd.
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 314: 244-51 (2005)
Article DOI: 10.1124/jpet.104.081539
BindingDB Entry DOI: 10.7270/Q2RN36FF |
More data for this
Ligand-Target Pair | |
Leukotriene B4 receptor 2
(Homo sapiens (Human)) | BDBM86691

(K604)Show SMILES CC1CC(N(C(=O)C2CC2)c2ccccc2)c2ccccc2N1C(=O)c1ccccc1 Show InChI InChI=1S/C27H26N2O2/c1-19-18-25(29(27(31)21-16-17-21)22-12-6-3-7-13-22)23-14-8-9-15-24(23)28(19)26(30)20-10-4-2-5-11-20/h2-15,19,21,25H,16-18H2,1H3 | UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyowa Hakko Kogyo Co., Ltd.
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 314: 244-51 (2005)
Article DOI: 10.1124/jpet.104.081539
BindingDB Entry DOI: 10.7270/Q2RN36FF |
More data for this
Ligand-Target Pair | |
Cysteinyl leukotriene receptor 1
(Homo sapiens (Human)) | BDBM86692

(K117)Show SMILES CC(C)C(=O)N(C1CC(C)N(C(=O)c2ccccc2)c2ccccc12)c1ccccc1 Show InChI InChI=1S/C27H28N2O2/c1-19(2)26(30)29(22-14-8-5-9-15-22)25-18-20(3)28(24-17-11-10-16-23(24)25)27(31)21-12-6-4-7-13-21/h4-17,19-20,25H,18H2,1-3H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyowa Hakko Kogyo Co., Ltd.
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 314: 244-51 (2005)
Article DOI: 10.1124/jpet.104.081539
BindingDB Entry DOI: 10.7270/Q2RN36FF |
More data for this
Ligand-Target Pair | |
Cysteinyl leukotriene receptor 1
(Homo sapiens (Human)) | BDBM86691

(K604)Show SMILES CC1CC(N(C(=O)C2CC2)c2ccccc2)c2ccccc2N1C(=O)c1ccccc1 Show InChI InChI=1S/C27H26N2O2/c1-19-18-25(29(27(31)21-16-17-21)22-12-6-3-7-13-22)23-14-8-9-15-24(23)28(19)26(30)20-10-4-2-5-11-20/h2-15,19,21,25H,16-18H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyowa Hakko Kogyo Co., Ltd.
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 314: 244-51 (2005)
Article DOI: 10.1124/jpet.104.081539
BindingDB Entry DOI: 10.7270/Q2RN36FF |
More data for this
Ligand-Target Pair | |
Platelet-activating factor receptor
(Homo sapiens (Human)) | BDBM86692

(K117)Show SMILES CC(C)C(=O)N(C1CC(C)N(C(=O)c2ccccc2)c2ccccc12)c1ccccc1 Show InChI InChI=1S/C27H28N2O2/c1-19(2)26(30)29(22-14-8-5-9-15-22)25-18-20(3)28(24-17-11-10-16-23(24)25)27(31)21-12-6-4-7-13-21/h4-17,19-20,25H,18H2,1-3H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyowa Hakko Kogyo Co., Ltd.
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 314: 244-51 (2005)
Article DOI: 10.1124/jpet.104.081539
BindingDB Entry DOI: 10.7270/Q2RN36FF |
More data for this
Ligand-Target Pair | |
Platelet-activating factor receptor
(Homo sapiens (Human)) | BDBM86693

(K376)Show SMILES CCC(=O)N1C(C)CC(N(C(C)=O)c2ccccc2)c2ccccc12 Show InChI InChI=1S/C21H24N2O2/c1-4-21(25)22-15(2)14-20(18-12-8-9-13-19(18)22)23(16(3)24)17-10-6-5-7-11-17/h5-13,15,20H,4,14H2,1-3H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyowa Hakko Kogyo Co., Ltd.
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 314: 244-51 (2005)
Article DOI: 10.1124/jpet.104.081539
BindingDB Entry DOI: 10.7270/Q2RN36FF |
More data for this
Ligand-Target Pair | |
Platelet-activating factor receptor
(Homo sapiens (Human)) | BDBM86691

(K604)Show SMILES CC1CC(N(C(=O)C2CC2)c2ccccc2)c2ccccc2N1C(=O)c1ccccc1 Show InChI InChI=1S/C27H26N2O2/c1-19-18-25(29(27(31)21-16-17-21)22-12-6-3-7-13-22)23-14-8-9-15-24(23)28(19)26(30)20-10-4-2-5-11-20/h2-15,19,21,25H,16-18H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyowa Hakko Kogyo Co., Ltd.
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 314: 244-51 (2005)
Article DOI: 10.1124/jpet.104.081539
BindingDB Entry DOI: 10.7270/Q2RN36FF |
More data for this
Ligand-Target Pair | |
Prostaglandin D2 receptor
(Homo sapiens (Human)) | BDBM86692

(K117)Show SMILES CC(C)C(=O)N(C1CC(C)N(C(=O)c2ccccc2)c2ccccc12)c1ccccc1 Show InChI InChI=1S/C27H28N2O2/c1-19(2)26(30)29(22-14-8-5-9-15-22)25-18-20(3)28(24-17-11-10-16-23(24)25)27(31)21-12-6-4-7-13-21/h4-17,19-20,25H,18H2,1-3H3 | UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyowa Hakko Kogyo Co., Ltd.
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 314: 244-51 (2005)
Article DOI: 10.1124/jpet.104.081539
BindingDB Entry DOI: 10.7270/Q2RN36FF |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data