

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50135844 ((2S,8S)-2-[({[({[(1S)-1-{[(1S,2R)-2-(benzyloxy)-1-...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad Aut£noma de Madrid Curated by ChEMBL | Assay Description Competitive inhibition against HIV-1 Protease | J Med Chem 46: 5196-207 (2003) Article DOI: 10.1021/jm030871u BindingDB Entry DOI: 10.7270/Q2C53MKZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50135845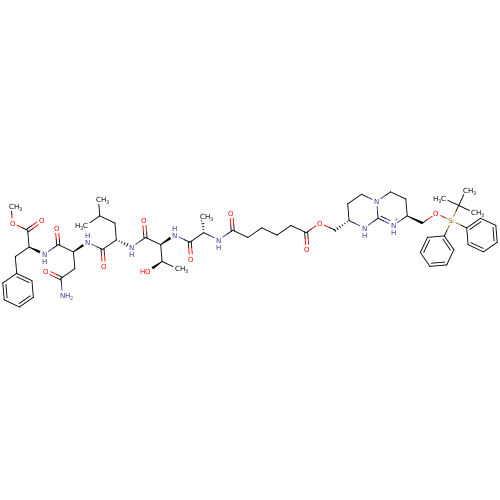 (Bicyclic Guanidinium Subunit | CHEMBL266754) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 5.25E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad Aut£noma de Madrid Curated by ChEMBL | Assay Description Competitive inhibition against HIV-1 Protease | J Med Chem 46: 5196-207 (2003) Article DOI: 10.1021/jm030871u BindingDB Entry DOI: 10.7270/Q2C53MKZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50135842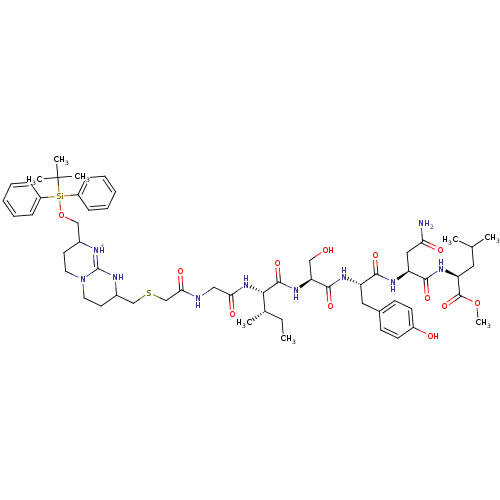 (Bicyclic Guanidinium Subunit | CHEMBL386994) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 6.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad Aut£noma de Madrid Curated by ChEMBL | Assay Description Competitive inhibition against HIV-1 Protease | J Med Chem 46: 5196-207 (2003) Article DOI: 10.1021/jm030871u BindingDB Entry DOI: 10.7270/Q2C53MKZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50135847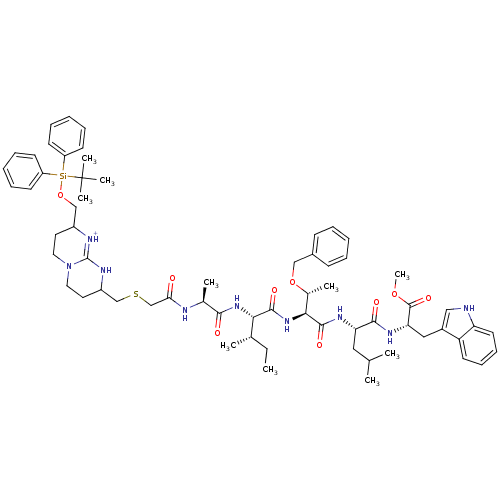 (Bicyclic Guanidinium Subunit | CHEMBL404936) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 7.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad Aut£noma de Madrid Curated by ChEMBL | Assay Description Competitive inhibition against HIV-1 Protease | J Med Chem 46: 5196-207 (2003) Article DOI: 10.1021/jm030871u BindingDB Entry DOI: 10.7270/Q2C53MKZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amyloid-beta precursor protein (Homo sapiens (Human)) | BDBM50583320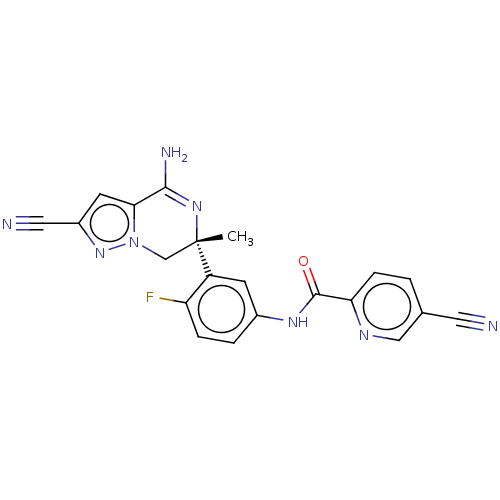 (CHEMBL5084378) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human amyloid beta (1 to 42) in human SKNBE2 cells expressing wild-type amyloid precursor protein hAPP695 incubated for 18 hrs by sandw... | Citation and Details Article DOI: 10.1021/acsmedchemlett.1c00445 BindingDB Entry DOI: 10.7270/Q2GT5S2J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amyloid-beta precursor protein (Homo sapiens (Human)) | BDBM50583322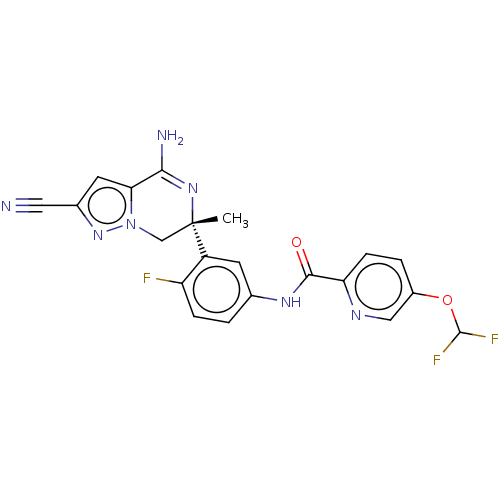 (CHEMBL5093303) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human amyloid beta (1 to 42) in human SKNBE2 cells expressing wild-type amyloid precursor protein hAPP695 incubated for 18 hrs by sandw... | Citation and Details Article DOI: 10.1021/acsmedchemlett.1c00445 BindingDB Entry DOI: 10.7270/Q2GT5S2J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM338837 (US9751886, Compound 2) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Research & Development Curated by ChEMBL | Assay Description Inhibition of BACE1 in human SKNBE2 cells expressing wild type amyloid precursor protein (APP695) assessed as amyloid beta42 level incubated for 18 h... | ACS Med Chem Lett 10: 1159-1165 (2019) Article DOI: 10.1021/acsmedchemlett.9b00181 BindingDB Entry DOI: 10.7270/Q2542RT5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amyloid-beta precursor protein (Homo sapiens (Human)) | BDBM50583324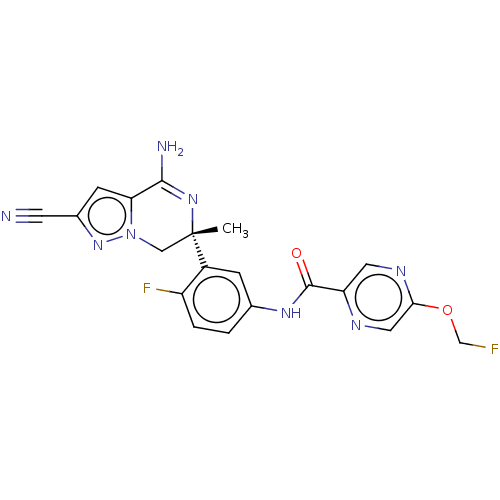 (CHEMBL5082406) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human amyloid beta (1 to 42) in human SKNBE2 cells expressing wild-type amyloid precursor protein hAPP695 incubated for 18 hrs by sandw... | Citation and Details Article DOI: 10.1021/acsmedchemlett.1c00445 BindingDB Entry DOI: 10.7270/Q2GT5S2J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50012662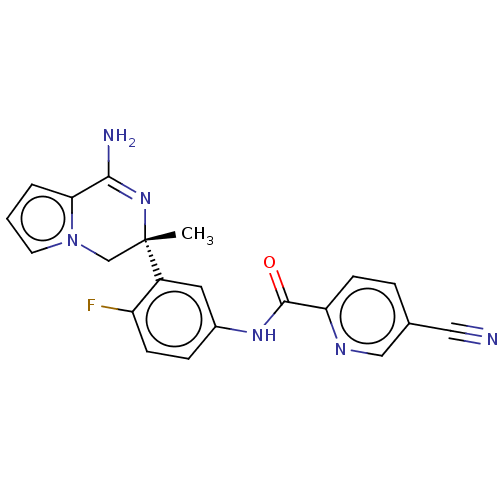 (CHEMBL3261075) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem | Article PubMed | n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Research & Development Curated by ChEMBL | Assay Description Inhibition of BACE1 in human SKNBE2 cells expressing wild type amyloid precursor protein (APP695) assessed as amyloid beta42 level incubated for 18 h... | ACS Med Chem Lett 10: 1159-1165 (2019) Article DOI: 10.1021/acsmedchemlett.9b00181 BindingDB Entry DOI: 10.7270/Q2542RT5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amyloid-beta precursor protein (Homo sapiens (Human)) | BDBM50583315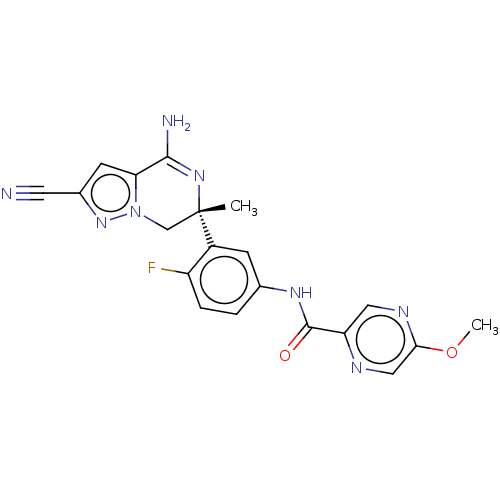 (CHEMBL5074068) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human amyloid beta (1 to 42) in human SKNBE2 cells expressing wild-type amyloid precursor protein hAPP695 incubated for 18 hrs by sandw... | Citation and Details Article DOI: 10.1021/acsmedchemlett.1c00445 BindingDB Entry DOI: 10.7270/Q2GT5S2J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50583322 (CHEMBL5093303) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of BACE1 (1 to 454 residues) (unknown origin) using APP harboring Swedish Lys/Met mutant-derived peptide as substrate by FRET assay | Citation and Details Article DOI: 10.1021/acsmedchemlett.1c00445 BindingDB Entry DOI: 10.7270/Q2GT5S2J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50583320 (CHEMBL5084378) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of BACE1 (1 to 454 residues) (unknown origin) using APP harboring Swedish Lys/Met mutant-derived peptide as substrate by FRET assay | Citation and Details Article DOI: 10.1021/acsmedchemlett.1c00445 BindingDB Entry DOI: 10.7270/Q2GT5S2J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM338837 (US9751886, Compound 2) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Research & Development Curated by ChEMBL | Assay Description Inhibition of BACE1 (unknown origin) | ACS Med Chem Lett 10: 1159-1165 (2019) Article DOI: 10.1021/acsmedchemlett.9b00181 BindingDB Entry DOI: 10.7270/Q2542RT5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50502598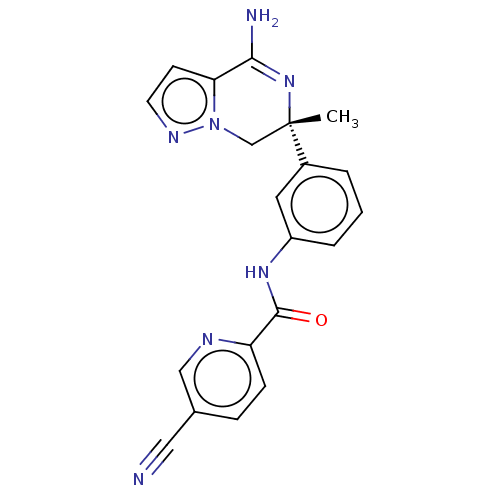 (CHEMBL4521954) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Research & Development Curated by ChEMBL | Assay Description Inhibition of BACE1 in human SKNBE2 cells expressing wild type amyloid precursor protein (APP695) assessed as amyloid beta42 level incubated for 18 h... | ACS Med Chem Lett 10: 1159-1165 (2019) Article DOI: 10.1021/acsmedchemlett.9b00181 BindingDB Entry DOI: 10.7270/Q2542RT5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50583324 (CHEMBL5082406) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of BACE1 (1 to 454 residues) (unknown origin) using APP harboring Swedish Lys/Met mutant-derived peptide as substrate by FRET assay | Citation and Details Article DOI: 10.1021/acsmedchemlett.1c00445 BindingDB Entry DOI: 10.7270/Q2GT5S2J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amyloid-beta precursor protein (Homo sapiens (Human)) | BDBM50583323 (CHEMBL5077699) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human amyloid beta (1 to 42) in human SKNBE2 cells expressing wild-type amyloid precursor protein hAPP695 incubated for 18 hrs by sandw... | Citation and Details Article DOI: 10.1021/acsmedchemlett.1c00445 BindingDB Entry DOI: 10.7270/Q2GT5S2J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50583318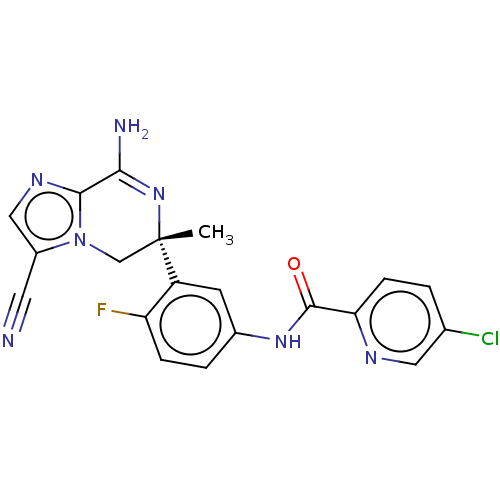 (CHEMBL5075933) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.60 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of BACE1 (1 to 454 residues) (unknown origin) using APP harboring Swedish Lys/Met mutant-derived peptide as substrate by FRET assay | Citation and Details Article DOI: 10.1021/acsmedchemlett.1c00445 BindingDB Entry DOI: 10.7270/Q2GT5S2J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amyloid-beta precursor protein (Homo sapiens (Human)) | BDBM50583321 (CHEMBL5085412) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.60 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human amyloid beta (1 to 42) in human SKNBE2 cells expressing wild-type amyloid precursor protein hAPP695 incubated for 18 hrs by sandw... | Citation and Details Article DOI: 10.1021/acsmedchemlett.1c00445 BindingDB Entry DOI: 10.7270/Q2GT5S2J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50583321 (CHEMBL5085412) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.80 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of BACE1 (1 to 454 residues) (unknown origin) using APP harboring Swedish Lys/Met mutant-derived peptide as substrate by FRET assay | Citation and Details Article DOI: 10.1021/acsmedchemlett.1c00445 BindingDB Entry DOI: 10.7270/Q2GT5S2J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50583315 (CHEMBL5074068) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.90 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of BACE1 (1 to 454 residues) (unknown origin) using APP harboring Swedish Lys/Met mutant-derived peptide as substrate by FRET assay | Citation and Details Article DOI: 10.1021/acsmedchemlett.1c00445 BindingDB Entry DOI: 10.7270/Q2GT5S2J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amyloid-beta precursor protein (Homo sapiens (Human)) | BDBM50393084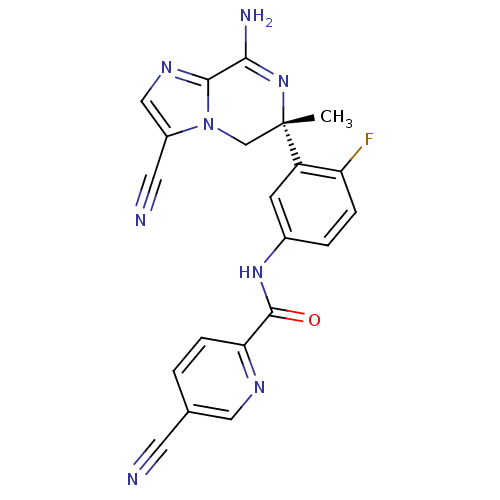 (CHEMBL2152899) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.30 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human amyloid beta (1 to 42) in human SKNBE2 cells expressing wild-type amyloid precursor protein hAPP695 incubated for 18 hrs by sandw... | Citation and Details Article DOI: 10.1021/acsmedchemlett.1c00445 BindingDB Entry DOI: 10.7270/Q2GT5S2J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50131808 (CHEMBL3634341) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Pharmaceutica NV Curated by ChEMBL | Assay Description Inhibition of BACE1 in human SKNBE2 cells expressing wild type human APP695 assessed as reduction in amyloid beta-42 production after 18 hrs by sandw... | J Med Chem 58: 8216-35 (2015) Article DOI: 10.1021/acs.jmedchem.5b01101 BindingDB Entry DOI: 10.7270/Q2ST7RPR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amyloid-beta precursor protein (Homo sapiens (Human)) | BDBM50583318 (CHEMBL5075933) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.60 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human amyloid beta (1 to 42) in human SKNBE2 cells expressing wild-type amyloid precursor protein hAPP695 incubated for 18 hrs by sandw... | Citation and Details Article DOI: 10.1021/acsmedchemlett.1c00445 BindingDB Entry DOI: 10.7270/Q2GT5S2J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50393084 (CHEMBL2152899) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.70 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of BACE1 (1 to 454 residues) (unknown origin) using APP harboring Swedish Lys/Met mutant-derived peptide as substrate by FRET assay | Citation and Details Article DOI: 10.1021/acsmedchemlett.1c00445 BindingDB Entry DOI: 10.7270/Q2GT5S2J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50583323 (CHEMBL5077699) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.70 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of BACE1 (1 to 454 residues) (unknown origin) using APP harboring Swedish Lys/Met mutant-derived peptide as substrate by FRET assay | Citation and Details Article DOI: 10.1021/acsmedchemlett.1c00445 BindingDB Entry DOI: 10.7270/Q2GT5S2J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50502602 (CHEMBL4557890) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Research & Development Curated by ChEMBL | Assay Description Inhibition of BACE1 (unknown origin) | ACS Med Chem Lett 10: 1159-1165 (2019) Article DOI: 10.1021/acsmedchemlett.9b00181 BindingDB Entry DOI: 10.7270/Q2542RT5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50131774 (CHEMBL2403769) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Pharmaceutica NV Curated by ChEMBL | Assay Description Inhibition of BACE1 in human SKNBE2 cells expressing wild type human APP695 assessed as reduction in amyloid beta-42 production after 18 hrs by sandw... | J Med Chem 58: 8216-35 (2015) Article DOI: 10.1021/acs.jmedchem.5b01101 BindingDB Entry DOI: 10.7270/Q2ST7RPR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Mus musculus (Mouse)) | BDBM50131773 (CHEMBL3634123) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Pharmaceutica NV Curated by ChEMBL | Assay Description Inhibition of BACE1 in mouse Neuro-2a cells expressing wild type human APP695 assessed as reduction in amyloid beta-42 production after 18 hrs by san... | J Med Chem 58: 8216-35 (2015) Article DOI: 10.1021/acs.jmedchem.5b01101 BindingDB Entry DOI: 10.7270/Q2ST7RPR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50580217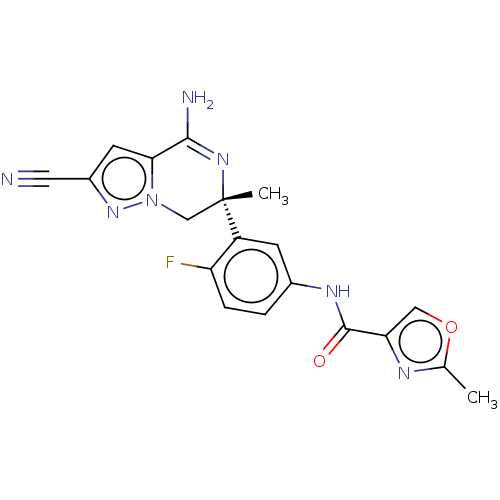 (CHEMBL5093195) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of BACE1 (1 to 454 residues) (unknown origin) using APP harboring Swedish Lys/Met mutant-derived peptide as substrate by FRET assay | Citation and Details Article DOI: 10.1021/acsmedchemlett.1c00445 BindingDB Entry DOI: 10.7270/Q2GT5S2J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Mus musculus (Mouse)) | BDBM50131774 (CHEMBL2403769) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Pharmaceutica NV Curated by ChEMBL | Assay Description Inhibition of BACE1 in mouse Neuro-2a cells expressing wild type human APP695 assessed as reduction in amyloid beta-42 production after 18 hrs by san... | J Med Chem 58: 8216-35 (2015) Article DOI: 10.1021/acs.jmedchem.5b01101 BindingDB Entry DOI: 10.7270/Q2ST7RPR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50502598 (CHEMBL4521954) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Research & Development Curated by ChEMBL | Assay Description Inhibition of BACE1 (unknown origin) | ACS Med Chem Lett 10: 1159-1165 (2019) Article DOI: 10.1021/acsmedchemlett.9b00181 BindingDB Entry DOI: 10.7270/Q2542RT5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50502598 (CHEMBL4521954) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4.60 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of BACE1 (1 to 454 residues) (unknown origin) using APP harboring Swedish Lys/Met mutant-derived peptide as substrate by FRET assay | Citation and Details Article DOI: 10.1021/acsmedchemlett.1c00445 BindingDB Entry DOI: 10.7270/Q2GT5S2J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amyloid-beta precursor protein (Homo sapiens (Human)) | BDBM50580217 (CHEMBL5093195) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4.80 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human amyloid beta (1 to 42) in human SKNBE2 cells expressing wild-type amyloid precursor protein hAPP695 incubated for 18 hrs by sandw... | Citation and Details Article DOI: 10.1021/acsmedchemlett.1c00445 BindingDB Entry DOI: 10.7270/Q2GT5S2J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50280451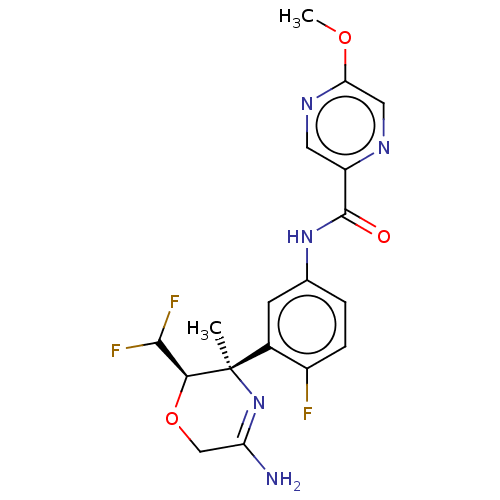 (CHEMBL4162197) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 4.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Pharmaceutica NV Curated by ChEMBL | Assay Description In vivo inhibition of leutenising hormone in rats. | J Med Chem 61: 5292-5303 (2018) Article DOI: 10.1021/acs.jmedchem.8b00304 BindingDB Entry DOI: 10.7270/Q2J38W46 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50131807 (CHEMBL3634340) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Pharmaceutica NV Curated by ChEMBL | Assay Description Inhibition of BACE1 in human SKNBE2 cells expressing wild type human APP695 assessed as reduction in amyloid beta-42 production after 18 hrs by sandw... | J Med Chem 58: 8216-35 (2015) Article DOI: 10.1021/acs.jmedchem.5b01101 BindingDB Entry DOI: 10.7270/Q2ST7RPR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50131773 (CHEMBL3634123) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Pharmaceutica NV Curated by ChEMBL | Assay Description Inhibition of BACE1 in human SKNBE2 cells expressing wild type human APP695 assessed as reduction in amyloid beta-42 production after 18 hrs by sandw... | J Med Chem 58: 8216-35 (2015) Article DOI: 10.1021/acs.jmedchem.5b01101 BindingDB Entry DOI: 10.7270/Q2ST7RPR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50583319 (CHEMBL5073381) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 5.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of BACE1 (1 to 454 residues) (unknown origin) using APP harboring Swedish Lys/Met mutant-derived peptide as substrate by FRET assay | Citation and Details Article DOI: 10.1021/acsmedchemlett.1c00445 BindingDB Entry DOI: 10.7270/Q2GT5S2J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 2 (Homo sapiens (Human)) | BDBM338837 (US9751886, Compound 2) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 5.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Research & Development Curated by ChEMBL | Assay Description Inhibition of BACE2 (unknown origin) | ACS Med Chem Lett 10: 1159-1165 (2019) Article DOI: 10.1021/acsmedchemlett.9b00181 BindingDB Entry DOI: 10.7270/Q2542RT5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50280423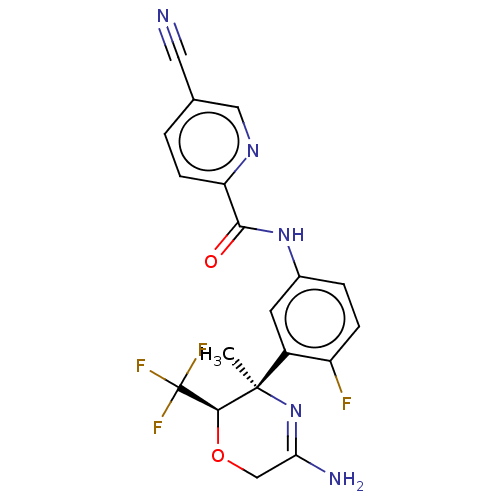 (CHEMBL4165225) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Pharmaceutica NV Curated by ChEMBL | Assay Description Inhibition of human WIL2/dihydrofolate reductase activity | J Med Chem 61: 5292-5303 (2018) Article DOI: 10.1021/acs.jmedchem.8b00304 BindingDB Entry DOI: 10.7270/Q2J38W46 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50502599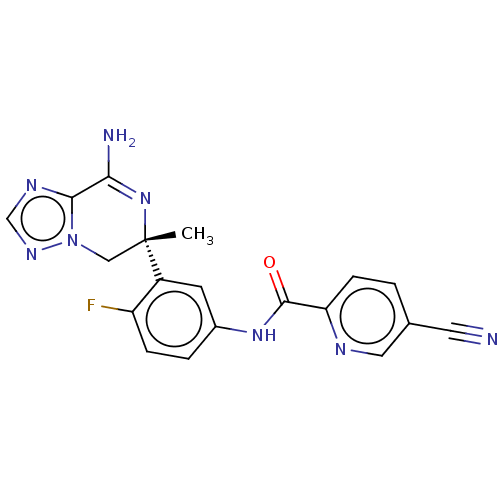 (CHEMBL4438397) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Research & Development Curated by ChEMBL | Assay Description Inhibition of BACE1 (unknown origin) | ACS Med Chem Lett 10: 1159-1165 (2019) Article DOI: 10.1021/acsmedchemlett.9b00181 BindingDB Entry DOI: 10.7270/Q2542RT5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50012662 (CHEMBL3261075) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem | Article PubMed | n/a | n/a | 6.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Research & Development Curated by ChEMBL | Assay Description Inhibition of BACE1 (unknown origin) | ACS Med Chem Lett 10: 1159-1165 (2019) Article DOI: 10.1021/acsmedchemlett.9b00181 BindingDB Entry DOI: 10.7270/Q2542RT5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amyloid-beta precursor protein (Homo sapiens (Human)) | BDBM50583319 (CHEMBL5073381) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 6.30 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human amyloid beta (1 to 42) in human SKNBE2 cells expressing wild-type amyloid precursor protein hAPP695 incubated for 18 hrs by sandw... | Citation and Details Article DOI: 10.1021/acsmedchemlett.1c00445 BindingDB Entry DOI: 10.7270/Q2GT5S2J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50502601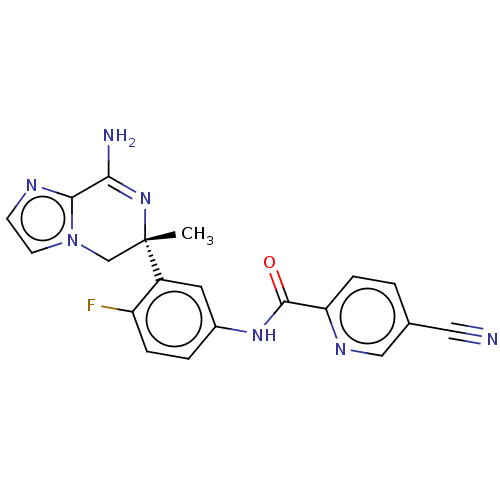 (CHEMBL4525199) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 6.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Research & Development Curated by ChEMBL | Assay Description Inhibition of BACE1 in human SKNBE2 cells expressing wild type amyloid precursor protein (APP695) assessed as amyloid beta42 level incubated for 18 h... | ACS Med Chem Lett 10: 1159-1165 (2019) Article DOI: 10.1021/acsmedchemlett.9b00181 BindingDB Entry DOI: 10.7270/Q2542RT5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50280473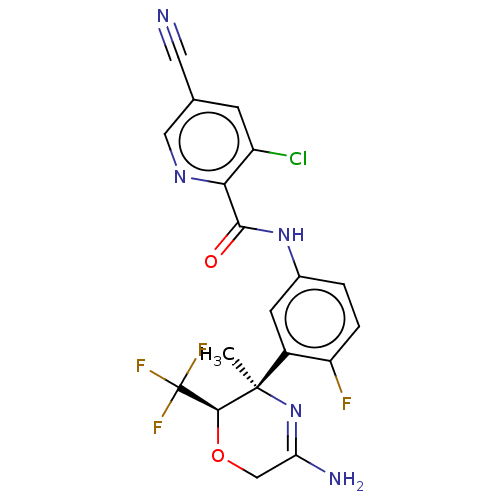 (CHEMBL4169060) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Pharmaceutica NV Curated by ChEMBL | Assay Description In vivo inhibition of leutenising hormone in rats. | J Med Chem 61: 5292-5303 (2018) Article DOI: 10.1021/acs.jmedchem.8b00304 BindingDB Entry DOI: 10.7270/Q2J38W46 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50502596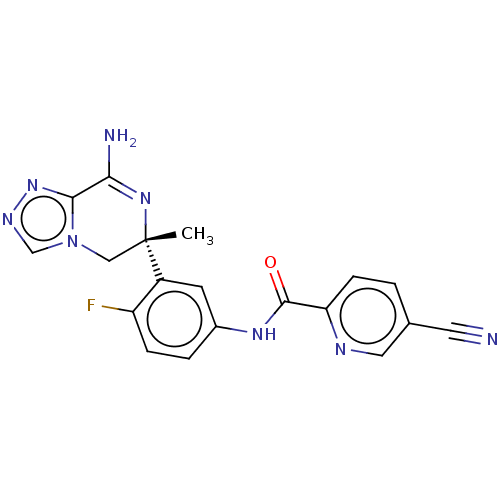 (CHEMBL4473647) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 6.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Research & Development Curated by ChEMBL | Assay Description Inhibition of BACE1 in human SKNBE2 cells expressing wild type amyloid precursor protein (APP695) assessed as amyloid beta42 level incubated for 18 h... | ACS Med Chem Lett 10: 1159-1165 (2019) Article DOI: 10.1021/acsmedchemlett.9b00181 BindingDB Entry DOI: 10.7270/Q2542RT5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50131808 (CHEMBL3634341) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Pharmaceutica NV Curated by ChEMBL | Assay Description Inhibition of BACE1 (unknown origin) using APP Swedish mutant harboring Lys-Met/Asn-Leu mutation in beta-secretase cleavage site as substrate by FRET... | J Med Chem 58: 8216-35 (2015) Article DOI: 10.1021/acs.jmedchem.5b01101 BindingDB Entry DOI: 10.7270/Q2ST7RPR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50012669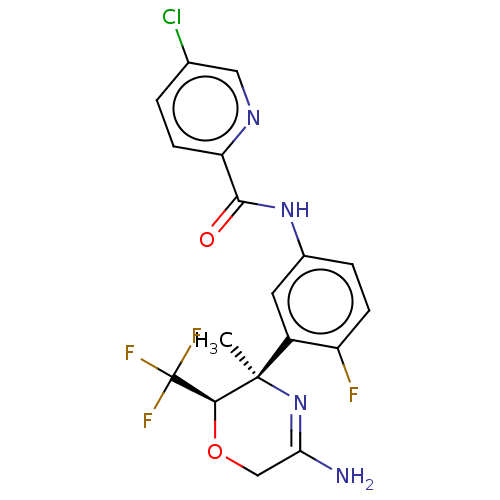 (CHEMBL3261072) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Pharmaceutica NV Curated by ChEMBL | Assay Description Inhibition of L1210 leukemia thymidylate synthase (TS) enzyme | J Med Chem 61: 5292-5303 (2018) Article DOI: 10.1021/acs.jmedchem.8b00304 BindingDB Entry DOI: 10.7270/Q2J38W46 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50131809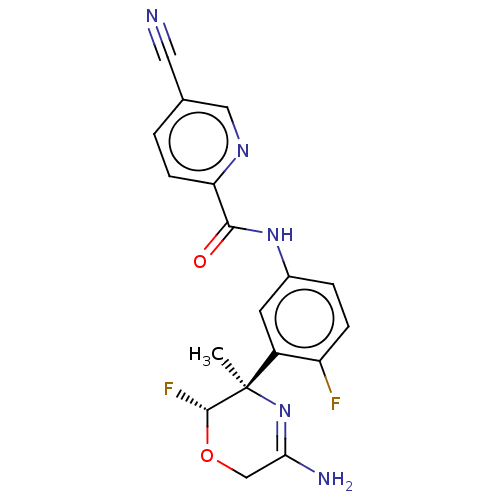 (CHEMBL3634342) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Pharmaceutica NV Curated by ChEMBL | Assay Description Inhibition of L1210 leukemia thymidylate synthase (TS) enzyme | J Med Chem 61: 5292-5303 (2018) Article DOI: 10.1021/acs.jmedchem.8b00304 BindingDB Entry DOI: 10.7270/Q2J38W46 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50131807 (CHEMBL3634340) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Pharmaceutica NV Curated by ChEMBL | Assay Description Inhibition of BACE1 (unknown origin) using APP Swedish mutant harboring Lys-Met/Asn-Leu mutation in beta-secretase cleavage site as substrate by FRET... | J Med Chem 58: 8216-35 (2015) Article DOI: 10.1021/acs.jmedchem.5b01101 BindingDB Entry DOI: 10.7270/Q2ST7RPR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Mus musculus (Mouse)) | BDBM50131775 (CHEMBL3634122) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 7.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Pharmaceutica NV Curated by ChEMBL | Assay Description Inhibition of BACE1 in mouse Neuro-2a cells expressing wild type human APP695 assessed as reduction in amyloid beta-42 production after 18 hrs by san... | J Med Chem 58: 8216-35 (2015) Article DOI: 10.1021/acs.jmedchem.5b01101 BindingDB Entry DOI: 10.7270/Q2ST7RPR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 253 total ) | Next | Last >> |