 Found 39 hits with Last Name = 'vella-rountree' and Initial = 'l'
Found 39 hits with Last Name = 'vella-rountree' and Initial = 'l' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Muscarinic acetylcholine receptor M1
(RAT) | BDBM86231

(ATR | ATROPINE | Atropine,(-) | CAS_51-55-8 | CHEM...)Show SMILES CN1C2CCC1CC(C2)OC(=O)C(CO)c1ccccc1 |THB:9:7:1:3.4| Show InChI InChI=1S/C17H23NO3/c1-18-13-7-8-14(18)10-15(9-13)21-17(20)16(11-19)12-5-3-2-4-6-12/h2-6,13-16,19H,7-11H2,1H3 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| 0.520 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Mayo Clinic Jacksonville
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 257: 1121-9 (1991)
BindingDB Entry DOI: 10.7270/Q2G73C6R |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M4
(RAT) | BDBM86231

(ATR | ATROPINE | Atropine,(-) | CAS_51-55-8 | CHEM...)Show SMILES CN1C2CCC1CC(C2)OC(=O)C(CO)c1ccccc1 |THB:9:7:1:3.4| Show InChI InChI=1S/C17H23NO3/c1-18-13-7-8-14(18)10-15(9-13)21-17(20)16(11-19)12-5-3-2-4-6-12/h2-6,13-16,19H,7-11H2,1H3 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Mayo Clinic Jacksonville
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 257: 1121-9 (1991)
BindingDB Entry DOI: 10.7270/Q2G73C6R |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M4
(RAT) | BDBM50176065
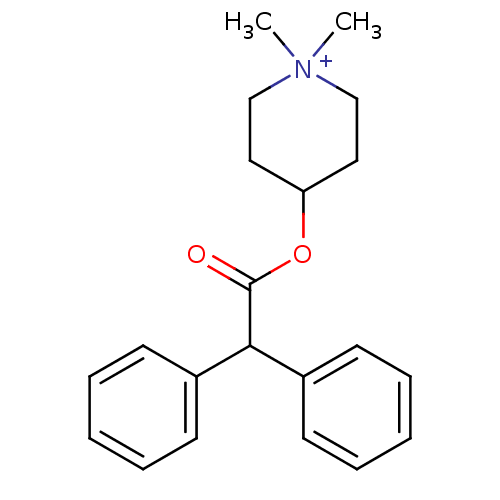
(4-DAMP | 4-Diphenylacetoxy-1,1-dimethyl-piperidini...)Show InChI InChI=1S/C21H26NO2/c1-22(2)15-13-19(14-16-22)24-21(23)20(17-9-5-3-6-10-17)18-11-7-4-8-12-18/h3-12,19-20H,13-16H2,1-2H3/q+1 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| PubMed
| 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Mayo Clinic Jacksonville
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 257: 1121-9 (1991)
BindingDB Entry DOI: 10.7270/Q2G73C6R |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1
(RAT) | BDBM81800
(CAS_57558-44-8 | NSC_42470 | Secoverine)Show InChI InChI=1S/C22H35NO2/c1-4-23(16-8-11-22(24)20-9-6-5-7-10-20)18(2)17-19-12-14-21(25-3)15-13-19/h12-15,18,20H,4-11,16-17H2,1-3H3 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
| PubMed
| 5.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Mayo Clinic Jacksonville
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 257: 1121-9 (1991)
BindingDB Entry DOI: 10.7270/Q2G73C6R |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M4
(RAT) | BDBM81800

(CAS_57558-44-8 | NSC_42470 | Secoverine)Show InChI InChI=1S/C22H35NO2/c1-4-23(16-8-11-22(24)20-9-6-5-7-10-20)18(2)17-19-12-14-21(25-3)15-13-19/h12-15,18,20H,4-11,16-17H2,1-3H3 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
| PubMed
| 7.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Mayo Clinic Jacksonville
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 257: 1121-9 (1991)
BindingDB Entry DOI: 10.7270/Q2G73C6R |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1
(RAT) | BDBM39341

(11-[(4-methylpiperazin-1-yl)acetyl]-5,11-dihydro-6...)Show InChI InChI=1S/C19H21N5O2/c1-22-9-11-23(12-10-22)13-17(25)24-16-7-3-2-5-14(16)19(26)21-15-6-4-8-20-18(15)24/h2-8H,9-13H2,1H3,(H,21,26) | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
UniChem
Similars
| PubMed
| 9 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Mayo Clinic Jacksonville
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 257: 1121-9 (1991)
BindingDB Entry DOI: 10.7270/Q2G73C6R |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M2
(RAT) | BDBM50005854
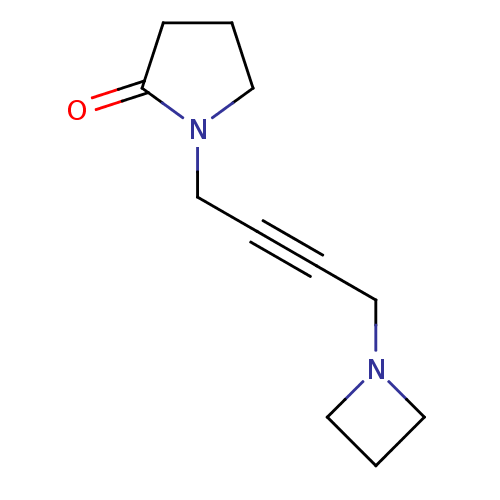
(1-(4-Azetidin-1-yl-but-2-ynyl)-pyrrolidin-2-one | ...)Show InChI InChI=1S/C11H16N2O/c14-11-5-3-10-13(11)9-2-1-6-12-7-4-8-12/h3-10H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 27 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Compound was evaluated for its binding affinity towards muscarinic acetylcholine receptor M2 in rat brainstem |
J Med Chem 35: 1550-7 (1992)
BindingDB Entry DOI: 10.7270/Q2MK6BT7 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M4
(RAT) | BDBM81799
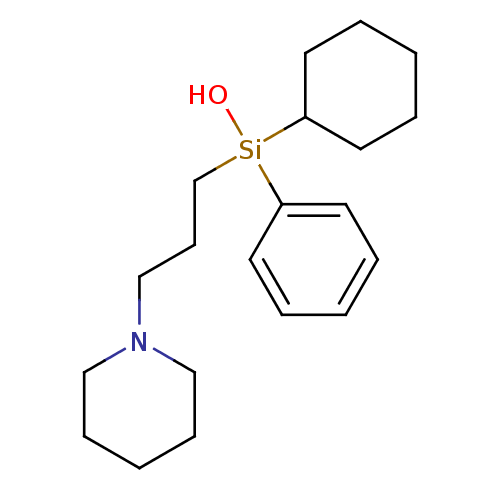
(CAS_98299-40-2 | HHSiD | NSC_3602 | hexahydrosilad...)Show InChI InChI=1S/C20H33NOSi/c22-23(19-11-4-1-5-12-19,20-13-6-2-7-14-20)18-10-17-21-15-8-3-9-16-21/h1,4-5,11-12,20,22H,2-3,6-10,13-18H2 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 47 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Mayo Clinic Jacksonville
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 257: 1121-9 (1991)
BindingDB Entry DOI: 10.7270/Q2G73C6R |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1
(RAT) | BDBM81799

(CAS_98299-40-2 | HHSiD | NSC_3602 | hexahydrosilad...)Show InChI InChI=1S/C20H33NOSi/c22-23(19-11-4-1-5-12-19,20-13-6-2-7-14-20)18-10-17-21-15-8-3-9-16-21/h1,4-5,11-12,20,22H,2-3,6-10,13-18H2 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Mayo Clinic Jacksonville
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 257: 1121-9 (1991)
BindingDB Entry DOI: 10.7270/Q2G73C6R |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M2
(RAT) | BDBM50004665

((oxotremorine)1-(4-Pyrrolidin-1-yl-but-2-ynyl)-pyr...)Show InChI InChI=1S/C12H18N2O/c15-12-6-5-11-14(12)10-4-3-9-13-7-1-2-8-13/h1-2,5-11H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 84 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Compound was evaluated for its binding affinity towards muscarinic acetylcholine receptor M2 in rat brainstem |
J Med Chem 35: 1550-7 (1992)
BindingDB Entry DOI: 10.7270/Q2MK6BT7 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1
(RAT) | BDBM50004665

((oxotremorine)1-(4-Pyrrolidin-1-yl-but-2-ynyl)-pyr...)Show InChI InChI=1S/C12H18N2O/c15-12-6-5-11-14(12)10-4-3-9-13-7-1-2-8-13/h1-2,5-11H2 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 196 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Compound was evaluated for its binding affinity towards muscarinic acetylcholine receptor in rat cortex |
J Med Chem 35: 1550-7 (1992)
BindingDB Entry DOI: 10.7270/Q2MK6BT7 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M4
(RAT) | BDBM39341

(11-[(4-methylpiperazin-1-yl)acetyl]-5,11-dihydro-6...)Show InChI InChI=1S/C19H21N5O2/c1-22-9-11-23(12-10-22)13-17(25)24-16-7-3-2-5-14(16)19(26)21-15-6-4-8-20-18(15)24/h2-8H,9-13H2,1H3,(H,21,26) | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
UniChem
Similars
| PubMed
| 214 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Mayo Clinic Jacksonville
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 257: 1121-9 (1991)
BindingDB Entry DOI: 10.7270/Q2G73C6R |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M4
(RAT) | BDBM50064176
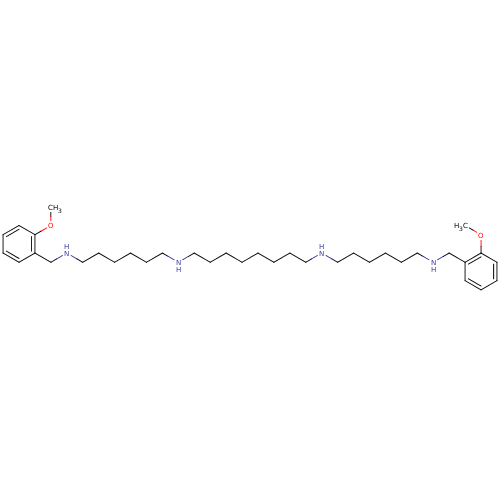
(CHEMBL27673 | CHEMBL500996 | METHOCTRAMINE | N,N''...)Show SMILES COc1ccccc1CNCCCCCCNCCCCCCCCNCCCCCCNCc1ccccc1OC Show InChI InChI=1S/C36H62N4O2/c1-41-35-23-13-11-21-33(35)31-39-29-19-9-7-17-27-37-25-15-5-3-4-6-16-26-38-28-18-8-10-20-30-40-32-34-22-12-14-24-36(34)42-2/h11-14,21-24,37-40H,3-10,15-20,25-32H2,1-2H3 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 410 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Mayo Clinic Jacksonville
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 257: 1121-9 (1991)
BindingDB Entry DOI: 10.7270/Q2G73C6R |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1
(RAT) | BDBM50064176

(CHEMBL27673 | CHEMBL500996 | METHOCTRAMINE | N,N''...)Show SMILES COc1ccccc1CNCCCCCCNCCCCCCCCNCCCCCCNCc1ccccc1OC Show InChI InChI=1S/C36H62N4O2/c1-41-35-23-13-11-21-33(35)31-39-29-19-9-7-17-27-37-25-15-5-3-4-6-16-26-38-28-18-8-10-20-30-40-32-34-22-12-14-24-36(34)42-2/h11-14,21-24,37-40H,3-10,15-20,25-32H2,1-2H3 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Mayo Clinic Jacksonville
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 257: 1121-9 (1991)
BindingDB Entry DOI: 10.7270/Q2G73C6R |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M4
(RAT) | BDBM50018056
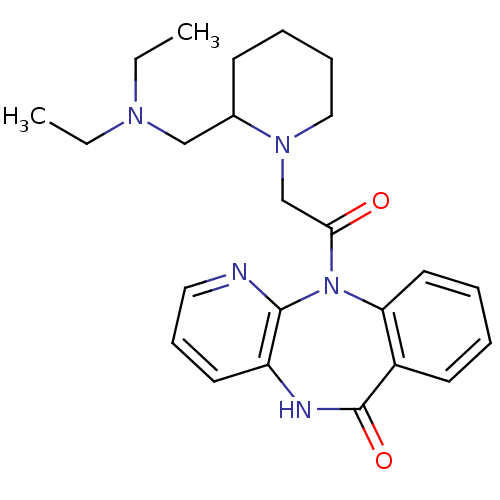
((AF-DX 116) 11-[2-(2-Diethylaminomethyl-piperidin-...)Show SMILES CCN(CC)CC1CCCCN1CC(=O)N1c2ccccc2C(=O)Nc2cccnc12 Show InChI InChI=1S/C24H31N5O2/c1-3-27(4-2)16-18-10-7-8-15-28(18)17-22(30)29-21-13-6-5-11-19(21)24(31)26-20-12-9-14-25-23(20)29/h5-6,9,11-14,18H,3-4,7-8,10,15-17H2,1-2H3,(H,26,31) | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 614 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Mayo Clinic Jacksonville
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 257: 1121-9 (1991)
BindingDB Entry DOI: 10.7270/Q2G73C6R |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M2
(RAT) | BDBM50455987

(CHEMBL2115360)Show InChI InChI=1S/C13H20N2O/c16-13-8-5-11-15(13)10-4-2-7-12-6-1-3-9-14-12/h12,14H,1,3,5-11H2/t12-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 636 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Compound was evaluated for its binding affinity towards M2 receptor in rat brainstem |
J Med Chem 35: 1550-7 (1992)
BindingDB Entry DOI: 10.7270/Q2MK6BT7 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M2
(RAT) | BDBM50005851
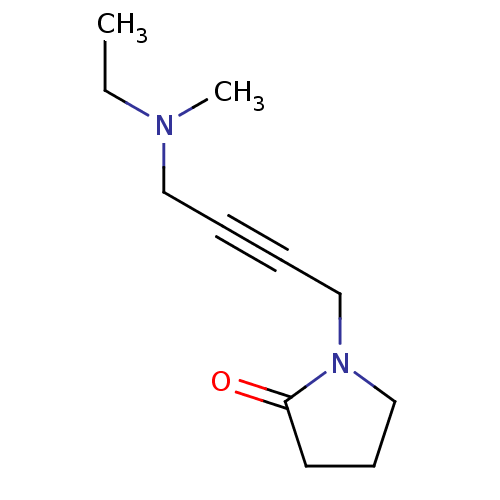
(1-[4-(Ethyl-methyl-amino)-but-2-ynyl]-pyrrolidin-2...)Show InChI InChI=1S/C11H18N2O/c1-3-12(2)8-4-5-9-13-10-6-7-11(13)14/h3,6-10H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 813 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Compound was evaluated for its binding affinity towards muscarinic acetylcholine receptor M2 in rat brainstem |
J Med Chem 35: 1550-7 (1992)
BindingDB Entry DOI: 10.7270/Q2MK6BT7 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M2
(RAT) | BDBM50005856
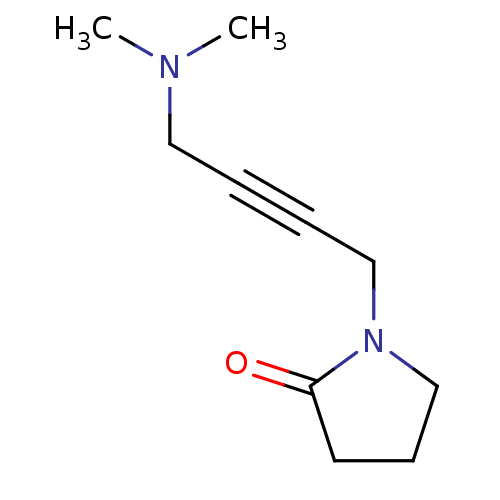
(1-(4-Dimethylamino-but-2-ynyl)-pyrrolidin-2-one | ...)Show InChI InChI=1S/C10H16N2O/c1-11(2)7-3-4-8-12-9-5-6-10(12)13/h5-9H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 926 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Compound was evaluated for its binding affinity towards Muscarinic acetylcholine receptor M2 in rat brainstem |
J Med Chem 35: 1550-7 (1992)
BindingDB Entry DOI: 10.7270/Q2MK6BT7 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1
(RAT) | BDBM50018056

((AF-DX 116) 11-[2-(2-Diethylaminomethyl-piperidin-...)Show SMILES CCN(CC)CC1CCCCN1CC(=O)N1c2ccccc2C(=O)Nc2cccnc12 Show InChI InChI=1S/C24H31N5O2/c1-3-27(4-2)16-18-10-7-8-15-28(18)17-22(30)29-21-13-6-5-11-19(21)24(31)26-20-12-9-14-25-23(20)29/h5-6,9,11-14,18H,3-4,7-8,10,15-17H2,1-2H3,(H,26,31) | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 1.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Mayo Clinic Jacksonville
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 257: 1121-9 (1991)
BindingDB Entry DOI: 10.7270/Q2G73C6R |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1
(RAT) | BDBM50455986

(CHEMBL2115361)Show InChI InChI=1S/C14H22N2O/c1-15-10-4-2-7-13(15)8-3-5-11-16-12-6-9-14(16)17/h13H,2,4,6-12H2,1H3/t13-/m1/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 1.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Compound was evaluated for its binding affinity towards M1 receptor in rat cortex |
J Med Chem 35: 1550-7 (1992)
BindingDB Entry DOI: 10.7270/Q2MK6BT7 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1
(RAT) | BDBM50005854

(1-(4-Azetidin-1-yl-but-2-ynyl)-pyrrolidin-2-one | ...)Show InChI InChI=1S/C11H16N2O/c14-11-5-3-10-13(11)9-2-1-6-12-7-4-8-12/h3-10H2 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 2.25E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Compound was evaluated for its binding affinity towards muscarinic acetylcholine receptor in rat cortex |
J Med Chem 35: 1550-7 (1992)
BindingDB Entry DOI: 10.7270/Q2MK6BT7 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M2
(RAT) | BDBM50455988
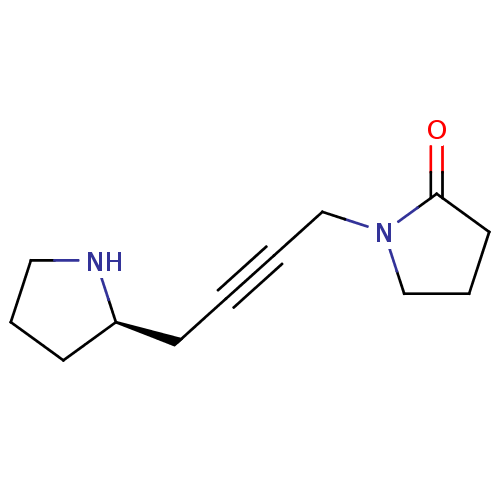
(CHEMBL2115359)Show InChI InChI=1S/C12H18N2O/c15-12-7-4-10-14(12)9-2-1-5-11-6-3-8-13-11/h11,13H,3-10H2/t11-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 2.46E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Compound was evaluated for its binding affinity towards M2 receptor in rat brainstem |
J Med Chem 35: 1550-7 (1992)
BindingDB Entry DOI: 10.7270/Q2MK6BT7 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1
(RAT) | BDBM50005851

(1-[4-(Ethyl-methyl-amino)-but-2-ynyl]-pyrrolidin-2...)Show InChI InChI=1S/C11H18N2O/c1-3-12(2)8-4-5-9-13-10-6-7-11(13)14/h3,6-10H2,1-2H3 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 2.69E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Compound was evaluated for its binding affinity towards muscarinic acetylcholine receptor in rat cortex |
J Med Chem 35: 1550-7 (1992)
BindingDB Entry DOI: 10.7270/Q2MK6BT7 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1
(RAT) | BDBM50455987

(CHEMBL2115360)Show InChI InChI=1S/C13H20N2O/c16-13-8-5-11-15(13)10-4-2-7-12-6-1-3-9-14-12/h12,14H,1,3,5-11H2/t12-/m1/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 3.03E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Compound was evaluated for its binding affinity towards M1 receptor in rat cortex |
J Med Chem 35: 1550-7 (1992)
BindingDB Entry DOI: 10.7270/Q2MK6BT7 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1
(RAT) | BDBM50455988

(CHEMBL2115359)Show InChI InChI=1S/C12H18N2O/c15-12-7-4-10-14(12)9-2-1-5-11-6-3-8-13-11/h11,13H,3-10H2/t11-/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 3.07E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Compound was evaluated for its binding affinity towards M1 receptor in rat cortex |
J Med Chem 35: 1550-7 (1992)
BindingDB Entry DOI: 10.7270/Q2MK6BT7 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M2
(RAT) | BDBM50455986

(CHEMBL2115361)Show InChI InChI=1S/C14H22N2O/c1-15-10-4-2-7-13(15)8-3-5-11-16-12-6-9-14(16)17/h13H,2,4,6-12H2,1H3/t13-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 3.66E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Compound was evaluated for its binding affinity towards M2 receptor in rat brainstem |
J Med Chem 35: 1550-7 (1992)
BindingDB Entry DOI: 10.7270/Q2MK6BT7 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1
(RAT) | BDBM50455982

(CHEMBL2114427)Show InChI InChI=1S/C14H22N2O/c1-15-10-4-2-7-13(15)8-3-5-11-16-12-6-9-14(16)17/h13H,2,4,6-12H2,1H3/t13-/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 5.38E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Compound was evaluated for its binding affinity towards M1 receptor in rat cortex |
J Med Chem 35: 1550-7 (1992)
BindingDB Entry DOI: 10.7270/Q2MK6BT7 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1
(RAT) | BDBM50455985

(CHEMBL2115362)Show InChI InChI=1S/C13H20N2O/c1-14-9-4-7-12(14)6-2-3-10-15-11-5-8-13(15)16/h12H,4-11H2,1H3/t12-/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 5.46E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Compound was evaluated for its binding affinity towards M1 receptor in rat cortex |
J Med Chem 35: 1550-7 (1992)
BindingDB Entry DOI: 10.7270/Q2MK6BT7 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M2
(RAT) | BDBM50455983

(CHEMBL2114425)Show InChI InChI=1S/C12H18N2O/c15-12-7-4-10-14(12)9-2-1-5-11-6-3-8-13-11/h11,13H,3-10H2/t11-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 5.64E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Compound was evaluated for its binding affinity towards M2 receptor in rat brainstem |
J Med Chem 35: 1550-7 (1992)
BindingDB Entry DOI: 10.7270/Q2MK6BT7 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M2
(RAT) | BDBM50455985

(CHEMBL2115362)Show InChI InChI=1S/C13H20N2O/c1-14-9-4-7-12(14)6-2-3-10-15-11-5-8-13(15)16/h12H,4-11H2,1H3/t12-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 5.86E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Compound was evaluated for its binding affinity towards M2 receptor in rat brainstem |
J Med Chem 35: 1550-7 (1992)
BindingDB Entry DOI: 10.7270/Q2MK6BT7 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M2
(RAT) | BDBM50455982

(CHEMBL2114427)Show InChI InChI=1S/C14H22N2O/c1-15-10-4-2-7-13(15)8-3-5-11-16-12-6-9-14(16)17/h13H,2,4,6-12H2,1H3/t13-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 7.06E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Compound was evaluated for its binding affinity towards M2 receptor in rat brainstem |
J Med Chem 35: 1550-7 (1992)
BindingDB Entry DOI: 10.7270/Q2MK6BT7 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1
(RAT) | BDBM50455984

(CHEMBL2114430)Show InChI InChI=1S/C13H20N2O/c1-14-9-4-7-12(14)6-2-3-10-15-11-5-8-13(15)16/h12H,4-11H2,1H3/t12-/m1/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 1.05E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Compound was evaluated for its binding affinity towards M1 receptor in rat cortex |
J Med Chem 35: 1550-7 (1992)
BindingDB Entry DOI: 10.7270/Q2MK6BT7 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M2
(RAT) | BDBM50455984

(CHEMBL2114430)Show InChI InChI=1S/C13H20N2O/c1-14-9-4-7-12(14)6-2-3-10-15-11-5-8-13(15)16/h12H,4-11H2,1H3/t12-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 1.08E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Compound was evaluated for its binding affinity towards M2 receptor in rat brainstem |
J Med Chem 35: 1550-7 (1992)
BindingDB Entry DOI: 10.7270/Q2MK6BT7 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M2
(RAT) | BDBM50455981
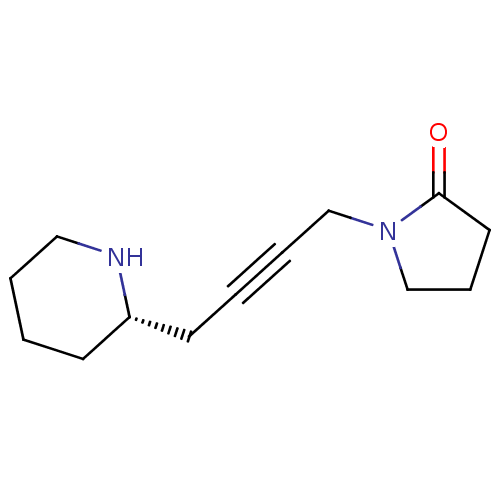
(CHEMBL2114426)Show InChI InChI=1S/C13H20N2O/c16-13-8-5-11-15(13)10-4-2-7-12-6-1-3-9-14-12/h12,14H,1,3,5-11H2/t12-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 1.58E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Compound was evaluated for its binding affinity towards M2 receptor in rat brainstem |
J Med Chem 35: 1550-7 (1992)
BindingDB Entry DOI: 10.7270/Q2MK6BT7 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1
(RAT) | BDBM50005856

(1-(4-Dimethylamino-but-2-ynyl)-pyrrolidin-2-one | ...)Show InChI InChI=1S/C10H16N2O/c1-11(2)7-3-4-8-12-9-5-6-10(12)13/h5-9H2,1-2H3 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 1.59E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Compound was evaluated for its binding affinity towards Muscarinic acetylcholine receptor M1 in rat cortex |
J Med Chem 35: 1550-7 (1992)
BindingDB Entry DOI: 10.7270/Q2MK6BT7 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1
(RAT) | BDBM50455981

(CHEMBL2114426)Show InChI InChI=1S/C13H20N2O/c16-13-8-5-11-15(13)10-4-2-7-12-6-1-3-9-14-12/h12,14H,1,3,5-11H2/t12-/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 2.76E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Compound was evaluated for its binding affinity towards M1 receptor in rat cortex |
J Med Chem 35: 1550-7 (1992)
BindingDB Entry DOI: 10.7270/Q2MK6BT7 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M2
(RAT) | BDBM50005855

(1-(4-Methylamino-but-2-ynyl)-pyrrolidin-2-one | CH...)Show InChI InChI=1S/C9H14N2O/c1-10-6-2-3-7-11-8-4-5-9(11)12/h10H,4-8H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 3.81E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Compound was evaluated for its binding affinity towards muscarinic acetylcholine receptor M2 in rat brainstem |
J Med Chem 35: 1550-7 (1992)
BindingDB Entry DOI: 10.7270/Q2MK6BT7 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1
(RAT) | BDBM50455983

(CHEMBL2114425)Show InChI InChI=1S/C12H18N2O/c15-12-7-4-10-14(12)9-2-1-5-11-6-3-8-13-11/h11,13H,3-10H2/t11-/m1/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 4.05E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Compound was evaluated for its binding affinity towards M1 receptor in rat cortex |
J Med Chem 35: 1550-7 (1992)
BindingDB Entry DOI: 10.7270/Q2MK6BT7 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1
(RAT) | BDBM50005855

(1-(4-Methylamino-but-2-ynyl)-pyrrolidin-2-one | CH...)Show InChI InChI=1S/C9H14N2O/c1-10-6-2-3-7-11-8-4-5-9(11)12/h10H,4-8H2,1H3 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 6.55E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Compound was evaluated for its binding affinity towards muscarinic acetylcholine receptor in rat cortex |
J Med Chem 35: 1550-7 (1992)
BindingDB Entry DOI: 10.7270/Q2MK6BT7 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data