 Found 121 hits with Last Name = 'vik' and Initial = 'a'
Found 121 hits with Last Name = 'vik' and Initial = 'a' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50552011
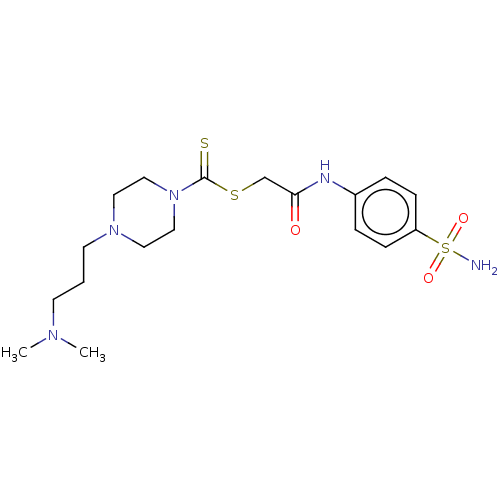
(CHEMBL4792992)Show SMILES CN(C)CCCN1CCN(CC1)C(=S)SCC(=O)Nc1ccc(cc1)S(N)(=O)=O | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human erythrocyte CA2 using 4-nitrophenyl acetate as substrate by Lineweaver-Burk plot analysis |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.112392
BindingDB Entry DOI: 10.7270/Q2C2512X |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50552018

(CHEMBL4764100)Show SMILES CN1CCN(CC1)C(=S)SCC(=O)Nc1ccc(cc1)S(N)(=O)=O | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 21 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human erythrocyte CA2 using 4-nitrophenyl acetate as substrate by Lineweaver-Burk plot analysis |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.112392
BindingDB Entry DOI: 10.7270/Q2C2512X |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50552012
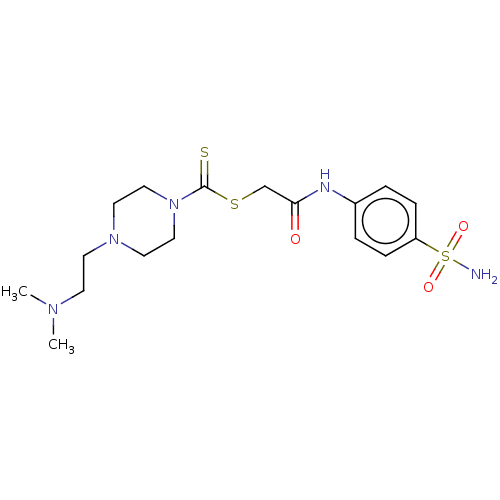
(CHEMBL4786423)Show SMILES CN(C)CCN1CCN(CC1)C(=S)SCC(=O)Nc1ccc(cc1)S(N)(=O)=O | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 26 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human erythrocyte CA2 using 4-nitrophenyl acetate as substrate by Lineweaver-Burk plot analysis |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.112392
BindingDB Entry DOI: 10.7270/Q2C2512X |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50552009
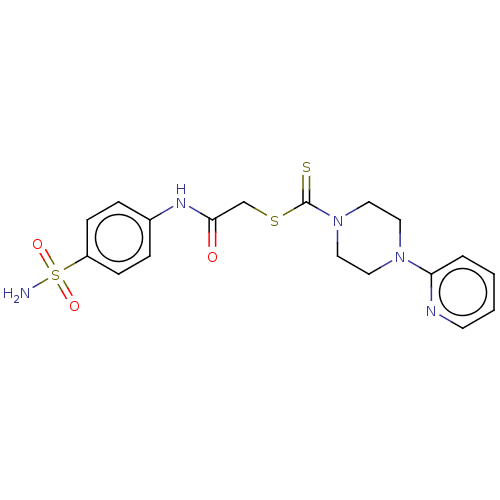
(CHEMBL4747356)Show SMILES NS(=O)(=O)c1ccc(NC(=O)CSC(=S)N2CCN(CC2)c2ccccn2)cc1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 28 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human erythrocyte CA2 using 4-nitrophenyl acetate as substrate by Lineweaver-Burk plot analysis |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.112392
BindingDB Entry DOI: 10.7270/Q2C2512X |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 1
(Homo sapiens (Human)) | BDBM50552011

(CHEMBL4792992)Show SMILES CN(C)CCCN1CCN(CC1)C(=S)SCC(=O)Nc1ccc(cc1)S(N)(=O)=O | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| 32 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human erythrocyte CA1 b using 4-nitrophenyl acetate as substrate by Lineweaver-Burk plot analysis |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.112392
BindingDB Entry DOI: 10.7270/Q2C2512X |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 1
(Homo sapiens (Human)) | BDBM50552008
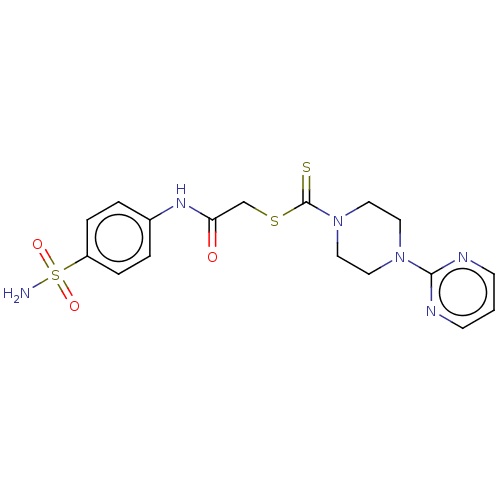
(CHEMBL4750069)Show SMILES NS(=O)(=O)c1ccc(NC(=O)CSC(=S)N2CCN(CC2)c2ncccn2)cc1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 41 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human erythrocyte CA1 b using 4-nitrophenyl acetate as substrate by Lineweaver-Burk plot analysis |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.112392
BindingDB Entry DOI: 10.7270/Q2C2512X |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 1
(Homo sapiens (Human)) | BDBM50552012

(CHEMBL4786423)Show SMILES CN(C)CCN1CCN(CC1)C(=S)SCC(=O)Nc1ccc(cc1)S(N)(=O)=O | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 49 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human erythrocyte CA1 b using 4-nitrophenyl acetate as substrate by Lineweaver-Burk plot analysis |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.112392
BindingDB Entry DOI: 10.7270/Q2C2512X |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50552014
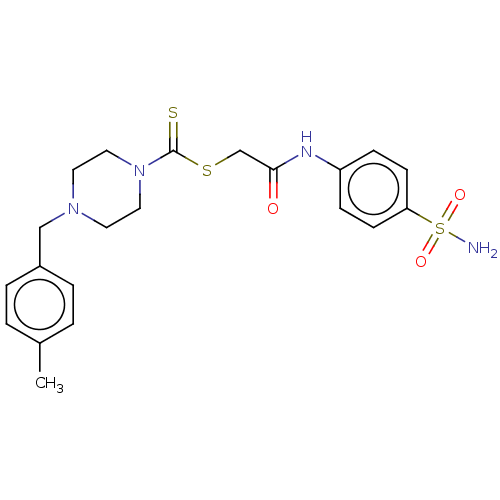
(CHEMBL4763412)Show SMILES Cc1ccc(CN2CCN(CC2)C(=S)SCC(=O)Nc2ccc(cc2)S(N)(=O)=O)cc1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 87 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human erythrocyte CA2 using 4-nitrophenyl acetate as substrate by Lineweaver-Burk plot analysis |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.112392
BindingDB Entry DOI: 10.7270/Q2C2512X |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 1
(Homo sapiens (Human)) | BDBM50552018

(CHEMBL4764100)Show SMILES CN1CCN(CC1)C(=S)SCC(=O)Nc1ccc(cc1)S(N)(=O)=O | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 98 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human erythrocyte CA1 b using 4-nitrophenyl acetate as substrate by Lineweaver-Burk plot analysis |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.112392
BindingDB Entry DOI: 10.7270/Q2C2512X |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50552008

(CHEMBL4750069)Show SMILES NS(=O)(=O)c1ccc(NC(=O)CSC(=S)N2CCN(CC2)c2ncccn2)cc1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 107 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human erythrocyte CA2 using 4-nitrophenyl acetate as substrate by Lineweaver-Burk plot analysis |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.112392
BindingDB Entry DOI: 10.7270/Q2C2512X |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM10880

(AZA | AZA2 | AZM acetazolamide | Acerazolamide, AA...)Show InChI InChI=1S/C4H6N4O3S2/c1-2(9)6-3-7-8-4(12-3)13(5,10)11/h1H3,(H2,5,10,11)(H,6,7,9) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
MMDB
PDB
Article
PubMed
| 135 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human erythrocyte CA2 using 4-nitrophenyl acetate as substrate by Lineweaver-Burk plot analysis |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.112392
BindingDB Entry DOI: 10.7270/Q2C2512X |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Carbonic anhydrase 1
(Homo sapiens (Human)) | BDBM50552009

(CHEMBL4747356)Show SMILES NS(=O)(=O)c1ccc(NC(=O)CSC(=S)N2CCN(CC2)c2ccccn2)cc1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 164 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human erythrocyte CA1 b using 4-nitrophenyl acetate as substrate by Lineweaver-Burk plot analysis |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.112392
BindingDB Entry DOI: 10.7270/Q2C2512X |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50552015
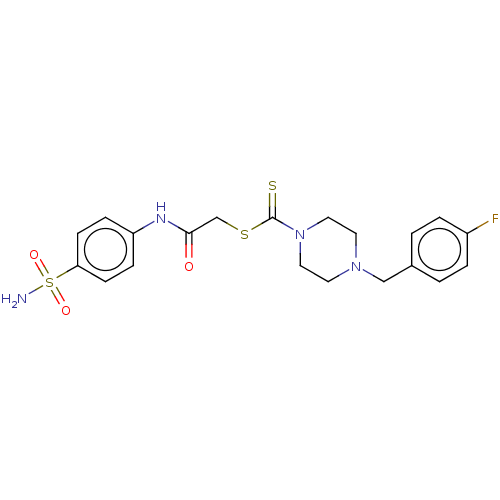
(CHEMBL4783836)Show SMILES NS(=O)(=O)c1ccc(NC(=O)CSC(=S)N2CCN(Cc3ccc(F)cc3)CC2)cc1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human erythrocyte CA2 using 4-nitrophenyl acetate as substrate by Lineweaver-Burk plot analysis |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.112392
BindingDB Entry DOI: 10.7270/Q2C2512X |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 1
(Homo sapiens (Human)) | BDBM50552014

(CHEMBL4763412)Show SMILES Cc1ccc(CN2CCN(CC2)C(=S)SCC(=O)Nc2ccc(cc2)S(N)(=O)=O)cc1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 280 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human erythrocyte CA1 b using 4-nitrophenyl acetate as substrate by Lineweaver-Burk plot analysis |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.112392
BindingDB Entry DOI: 10.7270/Q2C2512X |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 1
(Homo sapiens (Human)) | BDBM10880

(AZA | AZA2 | AZM acetazolamide | Acerazolamide, AA...)Show InChI InChI=1S/C4H6N4O3S2/c1-2(9)6-3-7-8-4(12-3)13(5,10)11/h1H3,(H2,5,10,11)(H,6,7,9) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| 289 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human erythrocyte CA1 b using 4-nitrophenyl acetate as substrate by Lineweaver-Burk plot analysis |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.112392
BindingDB Entry DOI: 10.7270/Q2C2512X |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Carbonic anhydrase 1
(Homo sapiens (Human)) | BDBM50552015

(CHEMBL4783836)Show SMILES NS(=O)(=O)c1ccc(NC(=O)CSC(=S)N2CCN(Cc3ccc(F)cc3)CC2)cc1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 299 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human erythrocyte CA1 b using 4-nitrophenyl acetate as substrate by Lineweaver-Burk plot analysis |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.112392
BindingDB Entry DOI: 10.7270/Q2C2512X |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50552017
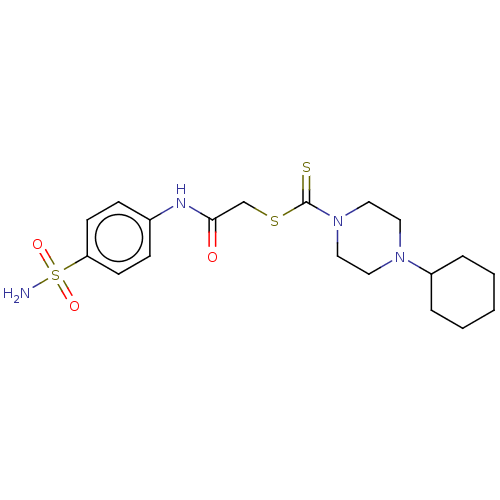
(CHEMBL4741640)Show SMILES NS(=O)(=O)c1ccc(NC(=O)CSC(=S)N2CCN(CC2)C2CCCCC2)cc1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 360 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human erythrocyte CA2 using 4-nitrophenyl acetate as substrate by Lineweaver-Burk plot analysis |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.112392
BindingDB Entry DOI: 10.7270/Q2C2512X |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50552010
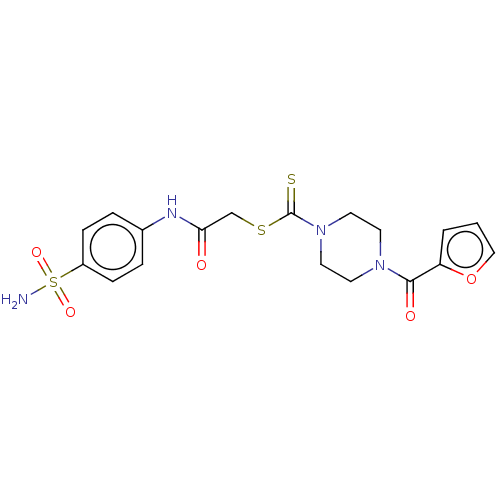
(CHEMBL4759737)Show SMILES NS(=O)(=O)c1ccc(NC(=O)CSC(=S)N2CCN(CC2)C(=O)c2ccco2)cc1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 597 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human erythrocyte CA2 using 4-nitrophenyl acetate as substrate by Lineweaver-Burk plot analysis |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.112392
BindingDB Entry DOI: 10.7270/Q2C2512X |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 1
(Homo sapiens (Human)) | BDBM50552016
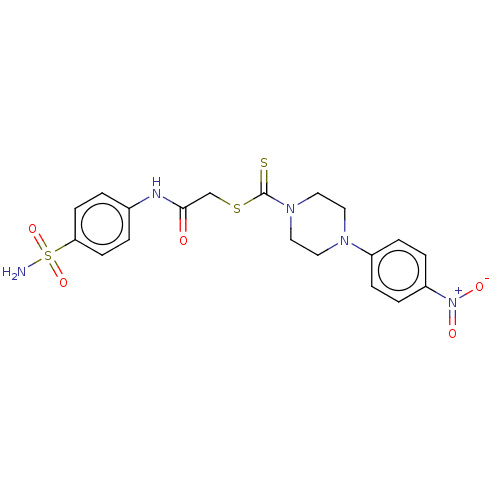
(CHEMBL4746908)Show SMILES NS(=O)(=O)c1ccc(NC(=O)CSC(=S)N2CCN(CC2)c2ccc(cc2)[N+]([O-])=O)cc1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.89E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human erythrocyte CA1 b using 4-nitrophenyl acetate as substrate by Lineweaver-Burk plot analysis |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.112392
BindingDB Entry DOI: 10.7270/Q2C2512X |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50552016

(CHEMBL4746908)Show SMILES NS(=O)(=O)c1ccc(NC(=O)CSC(=S)N2CCN(CC2)c2ccc(cc2)[N+]([O-])=O)cc1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 2.03E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human erythrocyte CA2 using 4-nitrophenyl acetate as substrate by Lineweaver-Burk plot analysis |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.112392
BindingDB Entry DOI: 10.7270/Q2C2512X |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 1
(Homo sapiens (Human)) | BDBM50552010

(CHEMBL4759737)Show SMILES NS(=O)(=O)c1ccc(NC(=O)CSC(=S)N2CCN(CC2)C(=O)c2ccco2)cc1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 7.71E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human erythrocyte CA1 b using 4-nitrophenyl acetate as substrate by Lineweaver-Burk plot analysis |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.112392
BindingDB Entry DOI: 10.7270/Q2C2512X |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 1
(Homo sapiens (Human)) | BDBM50552017

(CHEMBL4741640)Show SMILES NS(=O)(=O)c1ccc(NC(=O)CSC(=S)N2CCN(CC2)C2CCCCC2)cc1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.16E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human erythrocyte CA1 b using 4-nitrophenyl acetate as substrate by Lineweaver-Burk plot analysis |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.112392
BindingDB Entry DOI: 10.7270/Q2C2512X |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 1
(Homo sapiens (Human)) | BDBM50552013
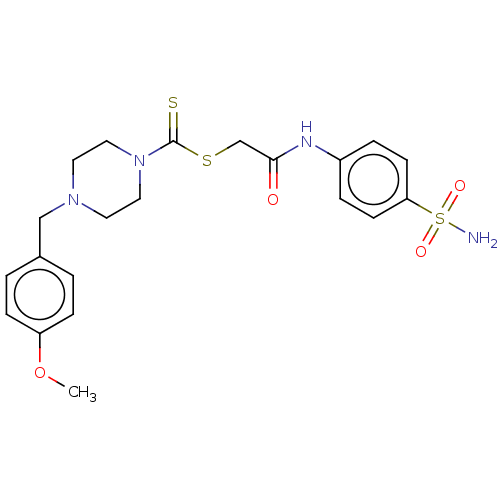
(CHEMBL4761403)Show SMILES COc1ccc(CN2CCN(CC2)C(=S)SCC(=O)Nc2ccc(cc2)S(N)(=O)=O)cc1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 6.48E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human erythrocyte CA1 b using 4-nitrophenyl acetate as substrate by Lineweaver-Burk plot analysis |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.112392
BindingDB Entry DOI: 10.7270/Q2C2512X |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50552013

(CHEMBL4761403)Show SMILES COc1ccc(CN2CCN(CC2)C(=S)SCC(=O)Nc2ccc(cc2)S(N)(=O)=O)cc1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 6.77E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human erythrocyte CA2 using 4-nitrophenyl acetate as substrate by Lineweaver-Burk plot analysis |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.112392
BindingDB Entry DOI: 10.7270/Q2C2512X |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor delta
(Homo sapiens (Human)) | BDBM50415440
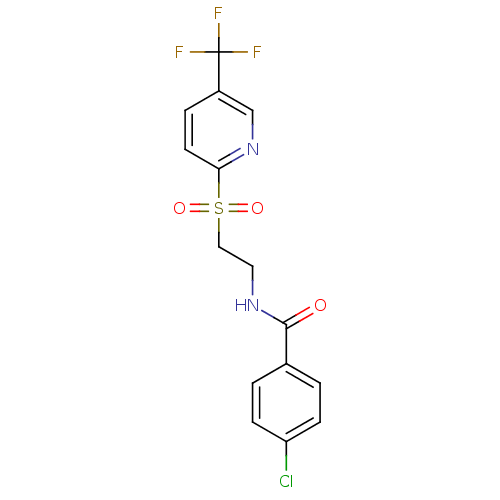
(CHEMBL598608 | GSK-3787)Show SMILES FC(F)(F)c1ccc(nc1)S(=O)(=O)CCNC(=O)c1ccc(Cl)cc1 Show InChI InChI=1S/C15H12ClF3N2O3S/c16-12-4-1-10(2-5-12)14(22)20-7-8-25(23,24)13-6-3-11(9-21-13)15(17,18)19/h1-6,9H,7-8H2,(H,20,22) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Oslo
Curated by ChEMBL
| Assay Description
Binding affinity to GST-PPAR-beta/delta LBP (unknown origin) after 24 hrs by TR-FRET assay |
Bioorg Med Chem 24: 247-60 (2016)
Article DOI: 10.1016/j.bmc.2015.12.012
BindingDB Entry DOI: 10.7270/Q2Z89F70 |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50552011

(CHEMBL4792992)Show SMILES CN(C)CCCN1CCN(CC1)C(=S)SCC(=O)Nc1ccc(cc1)S(N)(=O)=O | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 24 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human erythrocyte CA2 using 4-nitrophenyl acetate as substrate by spectrophotometry |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.112392
BindingDB Entry DOI: 10.7270/Q2C2512X |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50552018

(CHEMBL4764100)Show SMILES CN1CCN(CC1)C(=S)SCC(=O)Nc1ccc(cc1)S(N)(=O)=O | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 28 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human erythrocyte CA2 using 4-nitrophenyl acetate as substrate by spectrophotometry |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.112392
BindingDB Entry DOI: 10.7270/Q2C2512X |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor delta
(Homo sapiens (Human)) | BDBM50383371
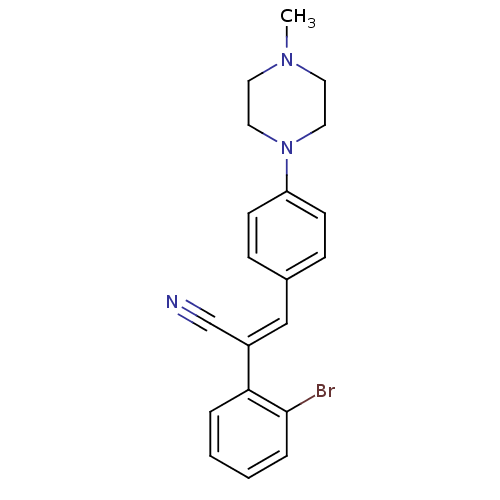
(CHEMBL2030550)Show InChI InChI=1S/C20H20BrN3/c1-23-10-12-24(13-11-23)18-8-6-16(7-9-18)14-17(15-22)19-4-2-3-5-20(19)21/h2-9,14H,10-13H2,1H3/b17-14+ | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 29 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Oslo
Curated by ChEMBL
| Assay Description
Binding affinity to GST-PPAR-beta/delta LBP (unknown origin) after 40 mins by TR-FRET assay |
Bioorg Med Chem 24: 247-60 (2016)
Article DOI: 10.1016/j.bmc.2015.12.012
BindingDB Entry DOI: 10.7270/Q2Z89F70 |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor delta
(Homo sapiens (Human)) | BDBM50383371

(CHEMBL2030550)Show InChI InChI=1S/C20H20BrN3/c1-23-10-12-24(13-11-23)18-8-6-16(7-9-18)14-17(15-22)19-4-2-3-5-20(19)21/h2-9,14H,10-13H2,1H3/b17-14+ | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Oslo
Curated by ChEMBL
| Assay Description
Binding affinity to GST-PPAR-beta/delta LBP (unknown origin) after 40 mins by TR-FRET assay |
Bioorg Med Chem 24: 247-60 (2016)
Article DOI: 10.1016/j.bmc.2015.12.012
BindingDB Entry DOI: 10.7270/Q2Z89F70 |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50552012

(CHEMBL4786423)Show SMILES CN(C)CCN1CCN(CC1)C(=S)SCC(=O)Nc1ccc(cc1)S(N)(=O)=O | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 33 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human erythrocyte CA2 using 4-nitrophenyl acetate as substrate by spectrophotometry |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.112392
BindingDB Entry DOI: 10.7270/Q2C2512X |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50552009

(CHEMBL4747356)Show SMILES NS(=O)(=O)c1ccc(NC(=O)CSC(=S)N2CCN(CC2)c2ccccn2)cc1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 39 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human erythrocyte CA2 using 4-nitrophenyl acetate as substrate by spectrophotometry |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.112392
BindingDB Entry DOI: 10.7270/Q2C2512X |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 1
(Homo sapiens (Human)) | BDBM50552011

(CHEMBL4792992)Show SMILES CN(C)CCCN1CCN(CC1)C(=S)SCC(=O)Nc1ccc(cc1)S(N)(=O)=O | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 47 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human erythrocyte CA1 using 4-nitrophenyl acetate as substrate by spectrophotometry |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.112392
BindingDB Entry DOI: 10.7270/Q2C2512X |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 1
(Homo sapiens (Human)) | BDBM50552008

(CHEMBL4750069)Show SMILES NS(=O)(=O)c1ccc(NC(=O)CSC(=S)N2CCN(CC2)c2ncccn2)cc1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 65 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human erythrocyte CA1 using 4-nitrophenyl acetate as substrate by spectrophotometry |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.112392
BindingDB Entry DOI: 10.7270/Q2C2512X |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 1
(Homo sapiens (Human)) | BDBM50552012

(CHEMBL4786423)Show SMILES CN(C)CCN1CCN(CC1)C(=S)SCC(=O)Nc1ccc(cc1)S(N)(=O)=O | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 81 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human erythrocyte CA1 using 4-nitrophenyl acetate as substrate by spectrophotometry |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.112392
BindingDB Entry DOI: 10.7270/Q2C2512X |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50552014

(CHEMBL4763412)Show SMILES Cc1ccc(CN2CCN(CC2)C(=S)SCC(=O)Nc2ccc(cc2)S(N)(=O)=O)cc1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 131 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human erythrocyte CA2 using 4-nitrophenyl acetate as substrate by spectrophotometry |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.112392
BindingDB Entry DOI: 10.7270/Q2C2512X |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM11639

(4-[5-(4-methylphenyl)-3-(trifluoromethyl)-1H-pyraz...)Show SMILES Cc1ccc(cc1)-c1cc(nn1-c1ccc(cc1)S(N)(=O)=O)C(F)(F)F Show InChI InChI=1S/C17H14F3N3O2S/c1-11-2-4-12(5-3-11)15-10-16(17(18,19)20)22-23(15)13-6-8-14(9-7-13)26(21,24)25/h2-10H,1H3,(H2,21,24,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| n/a | n/a | 132 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of COX-2 (unknown origin) using arachidonic acid as substrate measured after 10 mins by fluorometric based multimode microplate reader |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.112918
BindingDB Entry DOI: 10.7270/Q2BC438V |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50562258
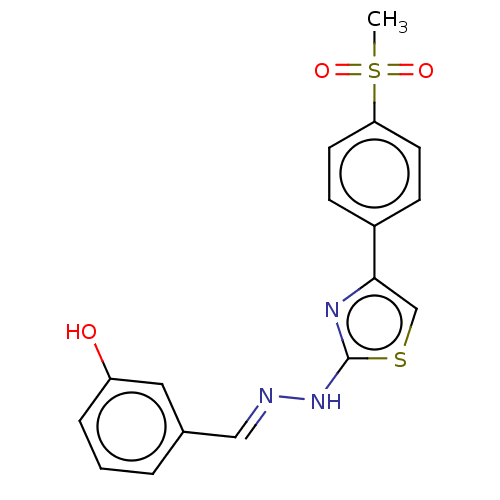
(CHEMBL4754406)Show SMILES CS(=O)(=O)c1ccc(cc1)-c1csc(N\N=C\c2cccc(O)c2)n1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 140 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of COX-2 (unknown origin) using arachidonic acid as substrate measured after 10 mins by fluorometric based multimode microplate reader |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.112918
BindingDB Entry DOI: 10.7270/Q2BC438V |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 1
(Homo sapiens (Human)) | BDBM50552018

(CHEMBL4764100)Show SMILES CN1CCN(CC1)C(=S)SCC(=O)Nc1ccc(cc1)S(N)(=O)=O | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 152 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human erythrocyte CA1 using 4-nitrophenyl acetate as substrate by spectrophotometry |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.112392
BindingDB Entry DOI: 10.7270/Q2C2512X |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM10880

(AZA | AZA2 | AZM acetazolamide | Acerazolamide, AA...)Show InChI InChI=1S/C4H6N4O3S2/c1-2(9)6-3-7-8-4(12-3)13(5,10)11/h1H3,(H2,5,10,11)(H,6,7,9) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
MMDB
PDB
Article
PubMed
| n/a | n/a | 175 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human erythrocyte CA2 using 4-nitrophenyl acetate as substrate by spectrophotometry |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.112392
BindingDB Entry DOI: 10.7270/Q2C2512X |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50562263
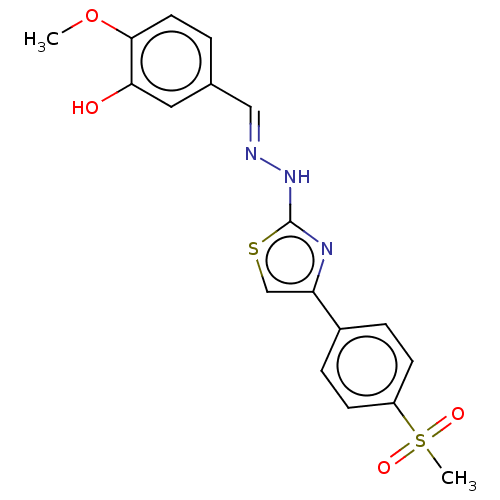
(CHEMBL4763059)Show SMILES COc1ccc(\C=N\Nc2nc(cs2)-c2ccc(cc2)S(C)(=O)=O)cc1O | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 175 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of COX-2 (unknown origin) using arachidonic acid as substrate measured after 10 mins by fluorometric based multimode microplate reader |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.112918
BindingDB Entry DOI: 10.7270/Q2BC438V |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50562264
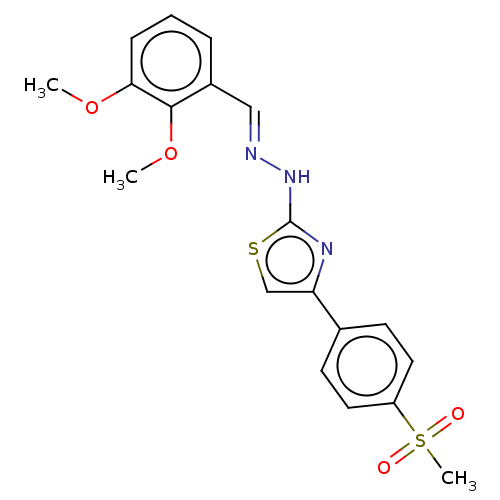
(CHEMBL4790676)Show SMILES COc1cccc(\C=N\Nc2nc(cs2)-c2ccc(cc2)S(C)(=O)=O)c1OC | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 184 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of COX-2 (unknown origin) using arachidonic acid as substrate measured after 10 mins by fluorometric based multimode microplate reader |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.112918
BindingDB Entry DOI: 10.7270/Q2BC438V |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50552008

(CHEMBL4750069)Show SMILES NS(=O)(=O)c1ccc(NC(=O)CSC(=S)N2CCN(CC2)c2ncccn2)cc1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 191 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human erythrocyte CA2 using 4-nitrophenyl acetate as substrate by spectrophotometry |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.112392
BindingDB Entry DOI: 10.7270/Q2C2512X |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50562267
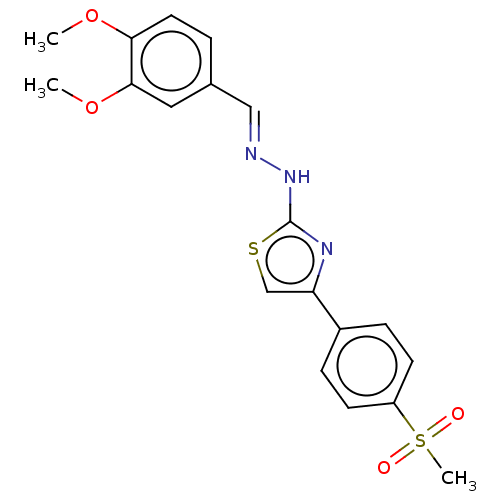
(CHEMBL4780951)Show SMILES COc1ccc(\C=N\Nc2nc(cs2)-c2ccc(cc2)S(C)(=O)=O)cc1OC | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 195 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of COX-2 (unknown origin) using arachidonic acid as substrate measured after 10 mins by fluorometric based multimode microplate reader |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.112918
BindingDB Entry DOI: 10.7270/Q2BC438V |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50562262
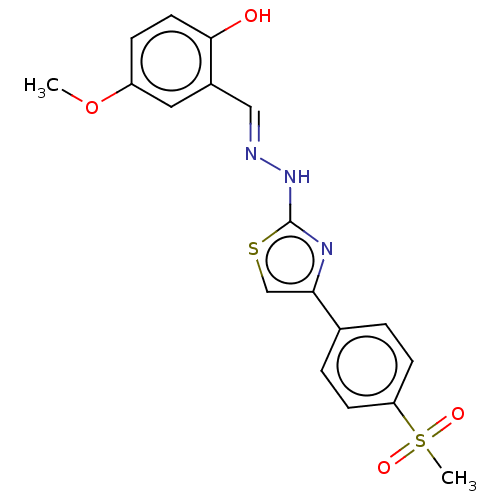
(CHEMBL4790377)Show SMILES COc1ccc(O)c(\C=N\Nc2nc(cs2)-c2ccc(cc2)S(C)(=O)=O)c1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 213 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of COX-2 (unknown origin) using arachidonic acid as substrate measured after 10 mins by fluorometric based multimode microplate reader |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.112918
BindingDB Entry DOI: 10.7270/Q2BC438V |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50562266
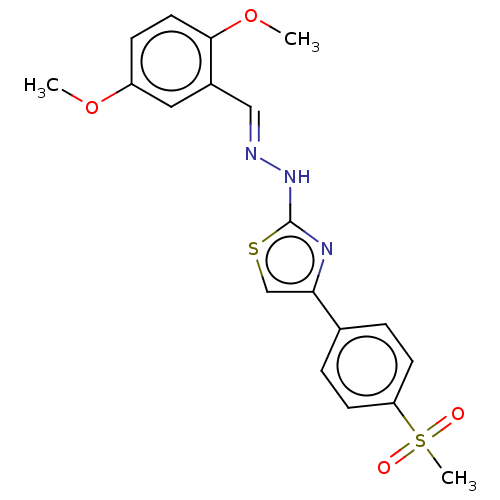
(CHEMBL4761194)Show SMILES COc1ccc(OC)c(\C=N\Nc2nc(cs2)-c2ccc(cc2)S(C)(=O)=O)c1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 239 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of COX-2 (unknown origin) using arachidonic acid as substrate measured after 10 mins by fluorometric based multimode microplate reader |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.112918
BindingDB Entry DOI: 10.7270/Q2BC438V |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor delta
(Homo sapiens (Human)) | BDBM50134238
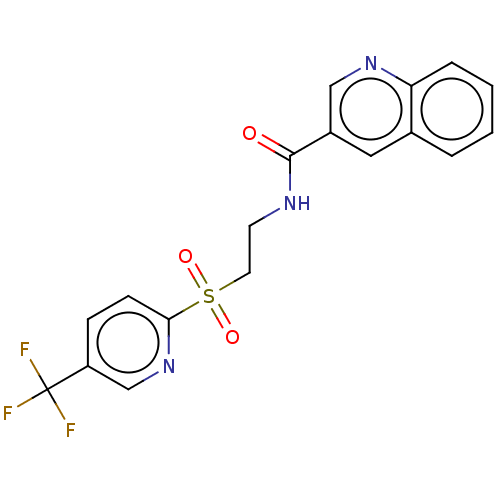
(CHEMBL3740002)Show SMILES FC(F)(F)c1ccc(nc1)S(=O)(=O)CCNC(=O)c1cnc2ccccc2c1 Show InChI InChI=1S/C18H14F3N3O3S/c19-18(20,21)14-5-6-16(24-11-14)28(26,27)8-7-22-17(25)13-9-12-3-1-2-4-15(12)23-10-13/h1-6,9-11H,7-8H2,(H,22,25) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 241 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Oslo
Curated by ChEMBL
| Assay Description
Binding affinity to GST-PPAR-beta/delta LBP (unknown origin) after 24 hrs by TR-FRET assay |
Bioorg Med Chem 24: 247-60 (2016)
Article DOI: 10.1016/j.bmc.2015.12.012
BindingDB Entry DOI: 10.7270/Q2Z89F70 |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50552015

(CHEMBL4783836)Show SMILES NS(=O)(=O)c1ccc(NC(=O)CSC(=S)N2CCN(Cc3ccc(F)cc3)CC2)cc1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 251 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human erythrocyte CA2 using 4-nitrophenyl acetate as substrate by spectrophotometry |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.112392
BindingDB Entry DOI: 10.7270/Q2C2512X |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 1
(Homo sapiens (Human)) | BDBM50552009

(CHEMBL4747356)Show SMILES NS(=O)(=O)c1ccc(NC(=O)CSC(=S)N2CCN(CC2)c2ccccn2)cc1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 281 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human erythrocyte CA1 using 4-nitrophenyl acetate as substrate by spectrophotometry |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.112392
BindingDB Entry DOI: 10.7270/Q2C2512X |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor delta
(Homo sapiens (Human)) | BDBM50415440

(CHEMBL598608 | GSK-3787)Show SMILES FC(F)(F)c1ccc(nc1)S(=O)(=O)CCNC(=O)c1ccc(Cl)cc1 Show InChI InChI=1S/C15H12ClF3N2O3S/c16-12-4-1-10(2-5-12)14(22)20-7-8-25(23,24)13-6-3-11(9-21-13)15(17,18)19/h1-6,9H,7-8H2,(H,20,22) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 284 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Oslo
Curated by ChEMBL
| Assay Description
Binding affinity to GST-PPAR-beta/delta LBP (unknown origin) after 2 hrs by TR-FRET assay |
Bioorg Med Chem 24: 247-60 (2016)
Article DOI: 10.1016/j.bmc.2015.12.012
BindingDB Entry DOI: 10.7270/Q2Z89F70 |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor delta
(Homo sapiens (Human)) | BDBM50079260
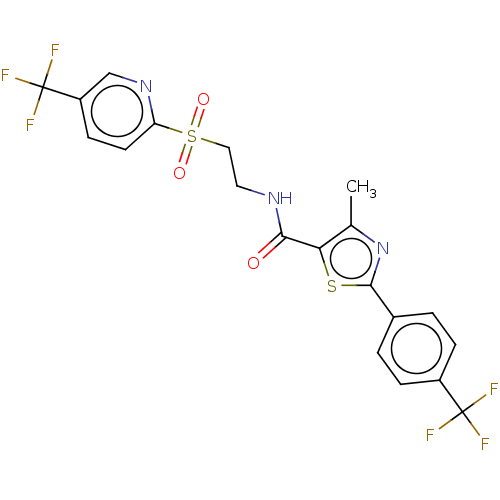
(CHEMBL3416764)Show SMILES Cc1nc(sc1C(=O)NCCS(=O)(=O)c1ccc(cn1)C(F)(F)F)-c1ccc(cc1)C(F)(F)F Show InChI InChI=1S/C20H15F6N3O3S2/c1-11-16(33-18(29-11)12-2-4-13(5-3-12)19(21,22)23)17(30)27-8-9-34(31,32)15-7-6-14(10-28-15)20(24,25)26/h2-7,10H,8-9H2,1H3,(H,27,30) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 284 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Oslo
Curated by ChEMBL
| Assay Description
Binding affinity to GST-PPAR-beta/delta LBP (unknown origin) after 3 hrs by TR-FRET assay |
Bioorg Med Chem 24: 247-60 (2016)
Article DOI: 10.1016/j.bmc.2015.12.012
BindingDB Entry DOI: 10.7270/Q2Z89F70 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data