

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM912 ((3S,4S)-3-hydroxy-4-[(2S)-2-[(3S,4S)-3-hydroxy-6-m...) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 38 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Lyon-ENS Curated by ChEMBL | Assay Description Inhibition of HIV1 protease by Hanes method | Bioorg Med Chem 17: 3671-9 (2009) Article DOI: 10.1016/j.bmc.2009.03.059 BindingDB Entry DOI: 10.7270/Q2G163NB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM912 ((3S,4S)-3-hydroxy-4-[(2S)-2-[(3S,4S)-3-hydroxy-6-m...) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 38 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Lyon-ENS Curated by ChEMBL | Assay Description Inhibition of HIV1 protease by Lineweaver-Burke method | Bioorg Med Chem 17: 3671-9 (2009) Article DOI: 10.1016/j.bmc.2009.03.059 BindingDB Entry DOI: 10.7270/Q2G163NB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM912 ((3S,4S)-3-hydroxy-4-[(2S)-2-[(3S,4S)-3-hydroxy-6-m...) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 50 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Lyon-ENS Curated by ChEMBL | Assay Description Inhibition of HIV1 protease by Dixon method | Bioorg Med Chem 17: 3671-9 (2009) Article DOI: 10.1016/j.bmc.2009.03.059 BindingDB Entry DOI: 10.7270/Q2G163NB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50480048 (CHEMBL466684) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 2.62E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Lyon-ENS Curated by ChEMBL | Assay Description Inhibition of HIV1 protease by Lineweaver-Burke method | Bioorg Med Chem 17: 3671-9 (2009) Article DOI: 10.1016/j.bmc.2009.03.059 BindingDB Entry DOI: 10.7270/Q2G163NB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50480048 (CHEMBL466684) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 2.62E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Lyon-ENS Curated by ChEMBL | Assay Description Inhibition of HIV1 protease by Hanes method | Bioorg Med Chem 17: 3671-9 (2009) Article DOI: 10.1016/j.bmc.2009.03.059 BindingDB Entry DOI: 10.7270/Q2G163NB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50480043 (CHEMBL466688) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 3.43E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Lyon-ENS Curated by ChEMBL | Assay Description Inhibition of HIV1 protease by Lineweaver-Burke method | Bioorg Med Chem 17: 3671-9 (2009) Article DOI: 10.1016/j.bmc.2009.03.059 BindingDB Entry DOI: 10.7270/Q2G163NB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50480043 (CHEMBL466688) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 3.43E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Lyon-ENS Curated by ChEMBL | Assay Description Inhibition of HIV1 protease by Hanes method | Bioorg Med Chem 17: 3671-9 (2009) Article DOI: 10.1016/j.bmc.2009.03.059 BindingDB Entry DOI: 10.7270/Q2G163NB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50480048 (CHEMBL466684) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 3.48E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Lyon-ENS Curated by ChEMBL | Assay Description Inhibition of HIV1 protease by Dixon method | Bioorg Med Chem 17: 3671-9 (2009) Article DOI: 10.1016/j.bmc.2009.03.059 BindingDB Entry DOI: 10.7270/Q2G163NB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50480043 (CHEMBL466688) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 4.39E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Lyon-ENS Curated by ChEMBL | Assay Description Inhibition of HIV1 protease by Dixon method | Bioorg Med Chem 17: 3671-9 (2009) Article DOI: 10.1016/j.bmc.2009.03.059 BindingDB Entry DOI: 10.7270/Q2G163NB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50480047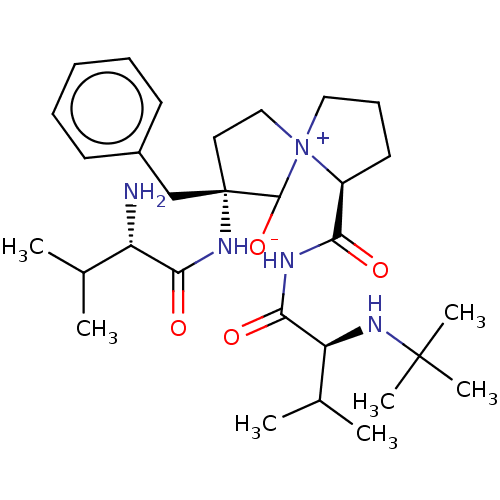 (CHEMBL466502) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 9.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Lyon-ENS Curated by ChEMBL | Assay Description Inhibition of HIV1 protease by Dixon method | Bioorg Med Chem 17: 3671-9 (2009) Article DOI: 10.1016/j.bmc.2009.03.059 BindingDB Entry DOI: 10.7270/Q2G163NB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50480047 (CHEMBL466502) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 9.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Lyon-ENS Curated by ChEMBL | Assay Description Inhibition of HIV1 protease by Hanes method | Bioorg Med Chem 17: 3671-9 (2009) Article DOI: 10.1016/j.bmc.2009.03.059 BindingDB Entry DOI: 10.7270/Q2G163NB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50480047 (CHEMBL466502) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 9.90E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Lyon-ENS Curated by ChEMBL | Assay Description Inhibition of HIV1 protease by Lineweaver-Burke method | Bioorg Med Chem 17: 3671-9 (2009) Article DOI: 10.1016/j.bmc.2009.03.059 BindingDB Entry DOI: 10.7270/Q2G163NB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50480044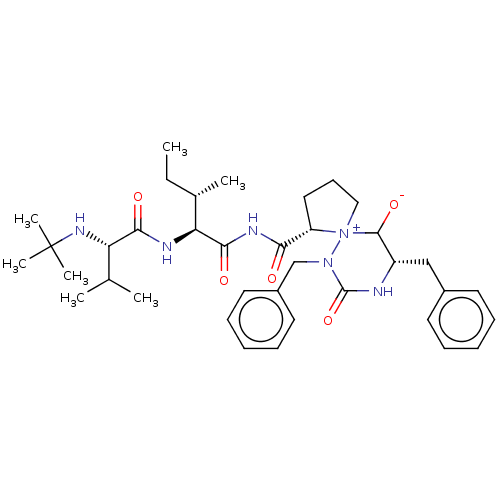 (CHEMBL471433) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.38E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Lyon-ENS Curated by ChEMBL | Assay Description Inhibition of HIV1 protease by Dixon method | Bioorg Med Chem 17: 3671-9 (2009) Article DOI: 10.1016/j.bmc.2009.03.059 BindingDB Entry DOI: 10.7270/Q2G163NB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50480044 (CHEMBL471433) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.49E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Lyon-ENS Curated by ChEMBL | Assay Description Inhibition of HIV1 protease by Hanes method | Bioorg Med Chem 17: 3671-9 (2009) Article DOI: 10.1016/j.bmc.2009.03.059 BindingDB Entry DOI: 10.7270/Q2G163NB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50480044 (CHEMBL471433) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.49E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Lyon-ENS Curated by ChEMBL | Assay Description Inhibition of HIV1 protease by Lineweaver-Burke method | Bioorg Med Chem 17: 3671-9 (2009) Article DOI: 10.1016/j.bmc.2009.03.059 BindingDB Entry DOI: 10.7270/Q2G163NB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50480049 (CHEMBL512599) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 2.86E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Lyon-ENS Curated by ChEMBL | Assay Description Inhibition of HIV1 protease by Dixon method | Bioorg Med Chem 17: 3671-9 (2009) Article DOI: 10.1016/j.bmc.2009.03.059 BindingDB Entry DOI: 10.7270/Q2G163NB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50480049 (CHEMBL512599) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 2.92E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Lyon-ENS Curated by ChEMBL | Assay Description Inhibition of HIV1 protease by Hanes method | Bioorg Med Chem 17: 3671-9 (2009) Article DOI: 10.1016/j.bmc.2009.03.059 BindingDB Entry DOI: 10.7270/Q2G163NB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50480049 (CHEMBL512599) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 2.92E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Lyon-ENS Curated by ChEMBL | Assay Description Inhibition of HIV1 protease by Lineweaver-Burke method | Bioorg Med Chem 17: 3671-9 (2009) Article DOI: 10.1016/j.bmc.2009.03.059 BindingDB Entry DOI: 10.7270/Q2G163NB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50480042 (CHEMBL466687) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 4.54E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Lyon-ENS Curated by ChEMBL | Assay Description Inhibition of HIV1 protease by Dixon method | Bioorg Med Chem 17: 3671-9 (2009) Article DOI: 10.1016/j.bmc.2009.03.059 BindingDB Entry DOI: 10.7270/Q2G163NB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50480042 (CHEMBL466687) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 4.61E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Lyon-ENS Curated by ChEMBL | Assay Description Inhibition of HIV1 protease by Hanes method | Bioorg Med Chem 17: 3671-9 (2009) Article DOI: 10.1016/j.bmc.2009.03.059 BindingDB Entry DOI: 10.7270/Q2G163NB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50480042 (CHEMBL466687) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 4.61E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Lyon-ENS Curated by ChEMBL | Assay Description Inhibition of HIV1 protease by Lineweaver-Burke method | Bioorg Med Chem 17: 3671-9 (2009) Article DOI: 10.1016/j.bmc.2009.03.059 BindingDB Entry DOI: 10.7270/Q2G163NB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50480046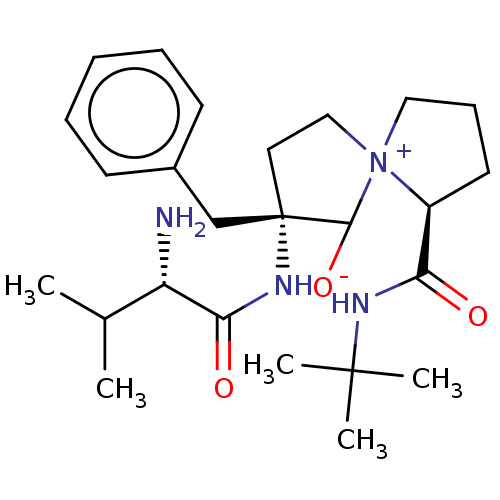 (CHEMBL511210) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 5.74E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Lyon-ENS Curated by ChEMBL | Assay Description Inhibition of HIV1 protease by Lineweaver-Burke method | Bioorg Med Chem 17: 3671-9 (2009) Article DOI: 10.1016/j.bmc.2009.03.059 BindingDB Entry DOI: 10.7270/Q2G163NB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50480046 (CHEMBL511210) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 5.77E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Lyon-ENS Curated by ChEMBL | Assay Description Inhibition of HIV1 protease by Dixon method | Bioorg Med Chem 17: 3671-9 (2009) Article DOI: 10.1016/j.bmc.2009.03.059 BindingDB Entry DOI: 10.7270/Q2G163NB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50480046 (CHEMBL511210) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 6.18E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Lyon-ENS Curated by ChEMBL | Assay Description Inhibition of HIV1 protease by Hanes method | Bioorg Med Chem 17: 3671-9 (2009) Article DOI: 10.1016/j.bmc.2009.03.059 BindingDB Entry DOI: 10.7270/Q2G163NB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM912 ((3S,4S)-3-hydroxy-4-[(2S)-2-[(3S,4S)-3-hydroxy-6-m...) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 41 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Lyon-ENS Curated by ChEMBL | Assay Description Inhibition of HIV1 protease by FRET assay | Bioorg Med Chem 17: 3671-9 (2009) Article DOI: 10.1016/j.bmc.2009.03.059 BindingDB Entry DOI: 10.7270/Q2G163NB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50480048 (CHEMBL466684) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.72E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Lyon-ENS Curated by ChEMBL | Assay Description Inhibition of HIV1 protease by FRET assay | Bioorg Med Chem 17: 3671-9 (2009) Article DOI: 10.1016/j.bmc.2009.03.059 BindingDB Entry DOI: 10.7270/Q2G163NB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50480043 (CHEMBL466688) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4.45E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Lyon-ENS Curated by ChEMBL | Assay Description Inhibition of HIV1 protease by FRET assay | Bioorg Med Chem 17: 3671-9 (2009) Article DOI: 10.1016/j.bmc.2009.03.059 BindingDB Entry DOI: 10.7270/Q2G163NB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50480044 (CHEMBL471433) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.69E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Lyon-ENS Curated by ChEMBL | Assay Description Inhibition of HIV1 protease by FRET assay | Bioorg Med Chem 17: 3671-9 (2009) Article DOI: 10.1016/j.bmc.2009.03.059 BindingDB Entry DOI: 10.7270/Q2G163NB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50480047 (CHEMBL466502) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.04E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Lyon-ENS Curated by ChEMBL | Assay Description Inhibition of HIV1 protease by FRET assay | Bioorg Med Chem 17: 3671-9 (2009) Article DOI: 10.1016/j.bmc.2009.03.059 BindingDB Entry DOI: 10.7270/Q2G163NB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50480049 (CHEMBL512599) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.41E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Lyon-ENS Curated by ChEMBL | Assay Description Inhibition of HIV1 protease by FRET assay | Bioorg Med Chem 17: 3671-9 (2009) Article DOI: 10.1016/j.bmc.2009.03.059 BindingDB Entry DOI: 10.7270/Q2G163NB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50480042 (CHEMBL466687) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 5.43E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Lyon-ENS Curated by ChEMBL | Assay Description Inhibition of HIV1 protease by FRET assay | Bioorg Med Chem 17: 3671-9 (2009) Article DOI: 10.1016/j.bmc.2009.03.059 BindingDB Entry DOI: 10.7270/Q2G163NB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50480046 (CHEMBL511210) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 5.45E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Lyon-ENS Curated by ChEMBL | Assay Description Inhibition of HIV1 protease by FRET assay | Bioorg Med Chem 17: 3671-9 (2009) Article DOI: 10.1016/j.bmc.2009.03.059 BindingDB Entry DOI: 10.7270/Q2G163NB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50480045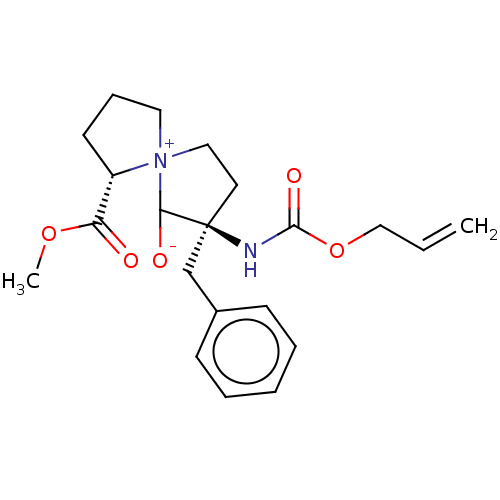 (CHEMBL466085) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >1.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Lyon-ENS Curated by ChEMBL | Assay Description Inhibition of HIV1 protease by FRET assay | Bioorg Med Chem 17: 3671-9 (2009) Article DOI: 10.1016/j.bmc.2009.03.059 BindingDB Entry DOI: 10.7270/Q2G163NB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM50297513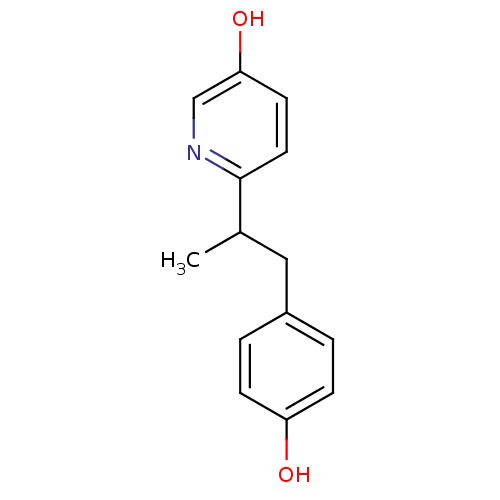 (6-[2-(4-Hydroxy-phenyl)-1-methyl-ethyl]-pyridin-3-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 330 | n/a | n/a | n/a | n/a |
University of Illinois Curated by ChEMBL | Assay Description Activity at human ERalpha expressed in HEC1 cells cotransfected with 2ERE-pS2-Luc assessed as relative transcriptional potency by luciferase reporter... | Eur J Med Chem 44: 3560-70 (2009) Article DOI: 10.1016/j.ejmech.2009.03.013 BindingDB Entry DOI: 10.7270/Q21Z44G1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM50297514 (6-[1-(4-Hydroxy-benzyl)-propyl]-pyridin-3-ol | CHE...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 65 | n/a | n/a | n/a | n/a |
University of Illinois Curated by ChEMBL | Assay Description Activity at human ERalpha expressed in HEC1 cells cotransfected with 2ERE-pS2-Luc assessed as relative transcriptional potency by luciferase reporter... | Eur J Med Chem 44: 3560-70 (2009) Article DOI: 10.1016/j.ejmech.2009.03.013 BindingDB Entry DOI: 10.7270/Q21Z44G1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM50297515 (6-[2-(4-Hydroxy-phenyl)-propyl]-pyridin-3-ol | CHE...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 440 | n/a | n/a | n/a | n/a |
University of Illinois Curated by ChEMBL | Assay Description Activity at human ERalpha expressed in HEC1 cells cotransfected with 2ERE-pS2-Luc assessed as relative transcriptional potency by luciferase reporter... | Eur J Med Chem 44: 3560-70 (2009) Article DOI: 10.1016/j.ejmech.2009.03.013 BindingDB Entry DOI: 10.7270/Q21Z44G1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM50297516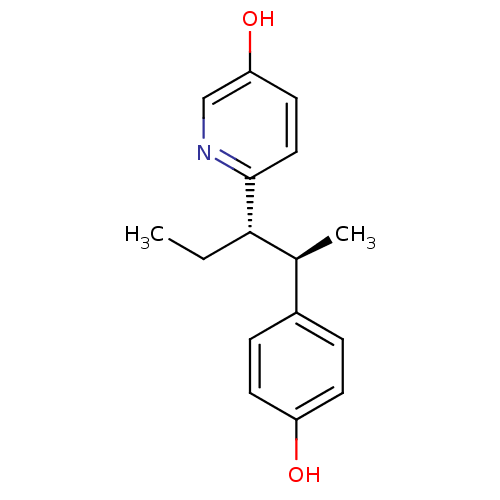 (CHEMBL538148 | trans-6-[1-ethyl-2-(4-hydroxy-pheny...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 0.110 | n/a | n/a | n/a | n/a |
University of Illinois Curated by ChEMBL | Assay Description Activity at human ERalpha expressed in HEC1 cells cotransfected with 2ERE-pS2-Luc assessed as relative transcriptional potency by luciferase reporter... | Eur J Med Chem 44: 3560-70 (2009) Article DOI: 10.1016/j.ejmech.2009.03.013 BindingDB Entry DOI: 10.7270/Q21Z44G1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM50297517 (CHEMBL552019 | cis-6-[1-ethyl-2-(4-hydroxy-phenyl)...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 16 | n/a | n/a | n/a | n/a |
University of Illinois Curated by ChEMBL | Assay Description Activity at human ERalpha expressed in HEC1 cells cotransfected with 2ERE-pS2-Luc assessed as relative transcriptional potency by luciferase reporter... | Eur J Med Chem 44: 3560-70 (2009) Article DOI: 10.1016/j.ejmech.2009.03.013 BindingDB Entry DOI: 10.7270/Q21Z44G1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM50297518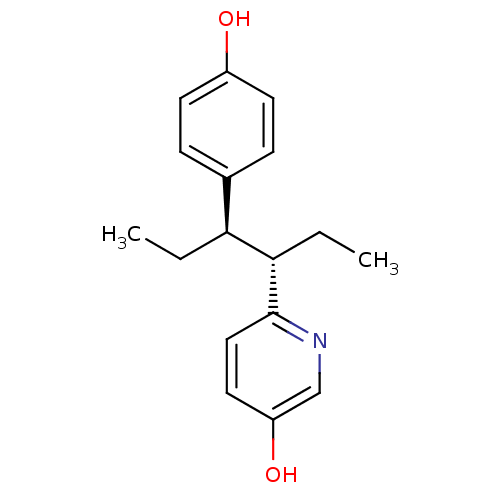 (CHEMBL556650 | trans-6-[1-ethyl-2-(4-hydroxy-pheny...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 0.110 | n/a | n/a | n/a | n/a |
University of Illinois Curated by ChEMBL | Assay Description Activity at human ERalpha expressed in HEC1 cells cotransfected with 2ERE-pS2-Luc assessed as relative transcriptional potency by luciferase reporter... | Eur J Med Chem 44: 3560-70 (2009) Article DOI: 10.1016/j.ejmech.2009.03.013 BindingDB Entry DOI: 10.7270/Q21Z44G1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM50297519 ((+/-)-1,2-Bis-(4-hydroxy-phenyl)-propane | 4,4'-(p...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 6.5 | n/a | n/a | n/a | n/a |
University of Illinois Curated by ChEMBL | Assay Description Activity at human ERalpha expressed in HEC1 cells cotransfected with 2ERE-pS2-Luc assessed as relative transcriptional potency by luciferase reporter... | Eur J Med Chem 44: 3560-70 (2009) Article DOI: 10.1016/j.ejmech.2009.03.013 BindingDB Entry DOI: 10.7270/Q21Z44G1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor beta (Homo sapiens (Human)) | BDBM50297513 (6-[2-(4-Hydroxy-phenyl)-1-methyl-ethyl]-pyridin-3-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 60 | n/a | n/a | n/a | n/a |
University of Illinois Curated by ChEMBL | Assay Description Activity at human ERbeta expressed in HEC1 cells cotransfected with 2ERE-pS2-Luc assessed as relative transcriptional potency by luciferase reporter ... | Eur J Med Chem 44: 3560-70 (2009) Article DOI: 10.1016/j.ejmech.2009.03.013 BindingDB Entry DOI: 10.7270/Q21Z44G1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor beta (Homo sapiens (Human)) | BDBM50297514 (6-[1-(4-Hydroxy-benzyl)-propyl]-pyridin-3-ol | CHE...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 17 | n/a | n/a | n/a | n/a |
University of Illinois Curated by ChEMBL | Assay Description Activity at human ERbeta expressed in HEC1 cells cotransfected with 2ERE-pS2-Luc assessed as relative transcriptional potency by luciferase reporter ... | Eur J Med Chem 44: 3560-70 (2009) Article DOI: 10.1016/j.ejmech.2009.03.013 BindingDB Entry DOI: 10.7270/Q21Z44G1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor beta (Homo sapiens (Human)) | BDBM50297515 (6-[2-(4-Hydroxy-phenyl)-propyl]-pyridin-3-ol | CHE...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 170 | n/a | n/a | n/a | n/a |
University of Illinois Curated by ChEMBL | Assay Description Activity at human ERbeta expressed in HEC1 cells cotransfected with 2ERE-pS2-Luc assessed as relative transcriptional potency by luciferase reporter ... | Eur J Med Chem 44: 3560-70 (2009) Article DOI: 10.1016/j.ejmech.2009.03.013 BindingDB Entry DOI: 10.7270/Q21Z44G1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor beta (Homo sapiens (Human)) | BDBM50297516 (CHEMBL538148 | trans-6-[1-ethyl-2-(4-hydroxy-pheny...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 1.10 | n/a | n/a | n/a | n/a |
University of Illinois Curated by ChEMBL | Assay Description Activity at human ERbeta expressed in HEC1 cells cotransfected with 2ERE-pS2-Luc assessed as relative transcriptional potency by luciferase reporter ... | Eur J Med Chem 44: 3560-70 (2009) Article DOI: 10.1016/j.ejmech.2009.03.013 BindingDB Entry DOI: 10.7270/Q21Z44G1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor beta (Homo sapiens (Human)) | BDBM50297517 (CHEMBL552019 | cis-6-[1-ethyl-2-(4-hydroxy-phenyl)...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 26 | n/a | n/a | n/a | n/a |
University of Illinois Curated by ChEMBL | Assay Description Activity at human ERbeta expressed in HEC1 cells cotransfected with 2ERE-pS2-Luc assessed as relative transcriptional potency by luciferase reporter ... | Eur J Med Chem 44: 3560-70 (2009) Article DOI: 10.1016/j.ejmech.2009.03.013 BindingDB Entry DOI: 10.7270/Q21Z44G1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor beta (Homo sapiens (Human)) | BDBM50297518 (CHEMBL556650 | trans-6-[1-ethyl-2-(4-hydroxy-pheny...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 0.610 | n/a | n/a | n/a | n/a |
University of Illinois Curated by ChEMBL | Assay Description Activity at human ERbeta expressed in HEC1 cells cotransfected with 2ERE-pS2-Luc assessed as relative transcriptional potency by luciferase reporter ... | Eur J Med Chem 44: 3560-70 (2009) Article DOI: 10.1016/j.ejmech.2009.03.013 BindingDB Entry DOI: 10.7270/Q21Z44G1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor beta (Homo sapiens (Human)) | BDBM50297519 ((+/-)-1,2-Bis-(4-hydroxy-phenyl)-propane | 4,4'-(p...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 1.20 | n/a | n/a | n/a | n/a |
University of Illinois Curated by ChEMBL | Assay Description Activity at human ERbeta expressed in HEC1 cells cotransfected with 2ERE-pS2-Luc assessed as relative transcriptional potency by luciferase reporter ... | Eur J Med Chem 44: 3560-70 (2009) Article DOI: 10.1016/j.ejmech.2009.03.013 BindingDB Entry DOI: 10.7270/Q21Z44G1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor beta (Homo sapiens (Human)) | BDBM50297519 ((+/-)-1,2-Bis-(4-hydroxy-phenyl)-propane | 4,4'-(p...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 1.20 | n/a | n/a | n/a | n/a |
University of Illinois Curated by ChEMBL | Assay Description Agonist activity at ERbeta expressed in human HEC1 cells assessed as transcriptional potency after 24 hrs by luciferase-beta galactosidase reporter g... | Eur J Med Chem 44: 3412-24 (2009) Article DOI: 10.1016/j.ejmech.2009.02.006 BindingDB Entry DOI: 10.7270/Q2NK3F37 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor beta (Homo sapiens (Human)) | BDBM50298235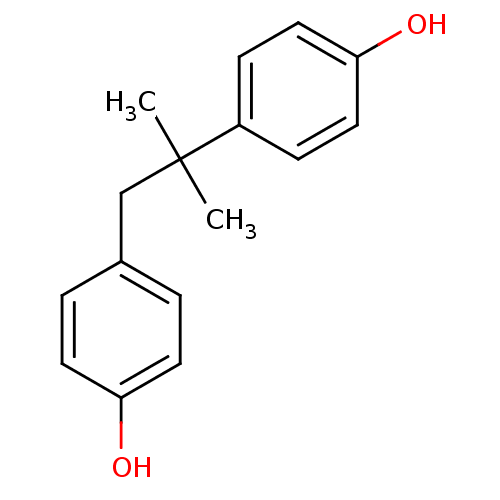 (1,2-Bis-(4-hydroxy-phenyl)-2-methyl-propane | CHEM...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 1.10 | n/a | n/a | n/a | n/a |
University of Illinois Curated by ChEMBL | Assay Description Agonist activity at ERbeta expressed in human HEC1 cells assessed as transcriptional potency after 24 hrs by luciferase-beta galactosidase reporter g... | Eur J Med Chem 44: 3412-24 (2009) Article DOI: 10.1016/j.ejmech.2009.02.006 BindingDB Entry DOI: 10.7270/Q2NK3F37 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor beta (Homo sapiens (Human)) | BDBM50106635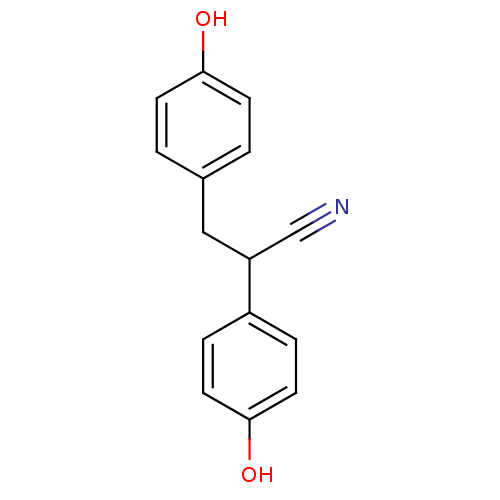 ((+/-)-2,3-bis(4-hydroxyphenyl)propanenitrile | 2,3...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | n/a | n/a | 0.850 | n/a | n/a | n/a | n/a |
University of Illinois Curated by ChEMBL | Assay Description Agonist activity at ERbeta expressed in human HEC1 cells assessed as transcriptional potency after 24 hrs by luciferase-beta galactosidase reporter g... | Eur J Med Chem 44: 3412-24 (2009) Article DOI: 10.1016/j.ejmech.2009.02.006 BindingDB Entry DOI: 10.7270/Q2NK3F37 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Displayed 1 to 50 (of 69 total ) | Next | Last >> |