 Found 131 hits with Last Name = 'wang' and Initial = 'bl'
Found 131 hits with Last Name = 'wang' and Initial = 'bl' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Ketol-acid reductoisomerase (NADP(+))
(Escherichia coli) | BDBM82145
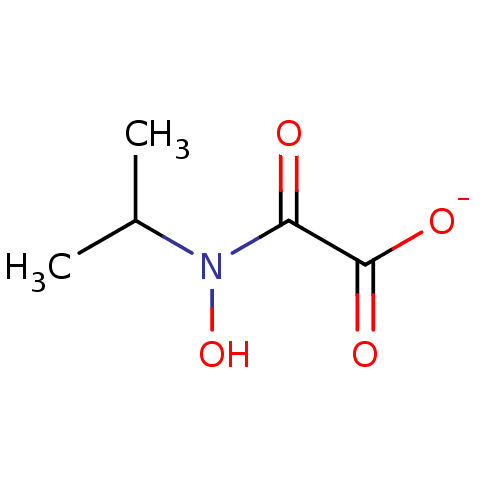
(N-hydroxy-N-isopropyloxamate, IpOHA)Show InChI InChI=1S/C5H9NO4/c1-3(2)6(10)4(7)5(8)9/h3,10H,1-2H3,(H,8,9)/p-1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| 2.75E+3 | -32.3 | n/a | n/a | n/a | n/a | n/a | 8.0 | 30 |
Nankai University
| Assay Description
Inhibition of E. coli KARI (ketol-acid reductoisomerase) is time dependent, and activity was measured by the decrease in A340 at 30 C in solutions co... |
Chem Biol Drug Des 75: 228-32 (2010)
Article DOI: 10.1111/j.1747-0285.2009.00924.x
BindingDB Entry DOI: 10.7270/Q2BV7F45 |
More data for this
Ligand-Target Pair | |
Ketol-acid reductoisomerase (NADP(+))
(Escherichia coli) | BDBM82144
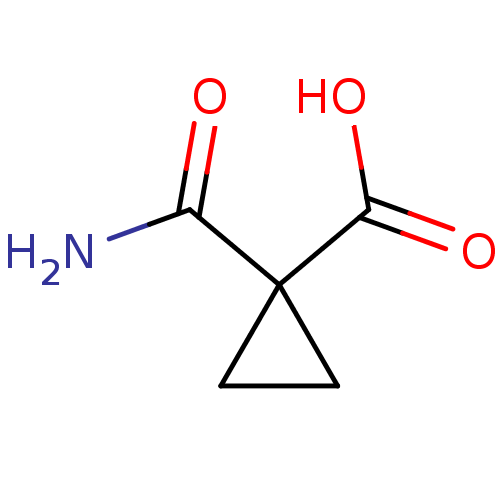
(Cyclopropane, 5)Show InChI InChI=1S/C5H7NO3/c6-3(7)5(1-2-5)4(8)9/h1-2H2,(H2,6,7)(H,8,9) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| 3.12E+4 | -26.2 | n/a | n/a | n/a | n/a | n/a | 8.0 | 30 |
Nankai University
| Assay Description
Inhibition of E. coli KARI (ketol-acid reductoisomerase) is time dependent, and activity was measured by the decrease in A340 at 30 C in solutions co... |
Chem Biol Drug Des 75: 228-32 (2010)
Article DOI: 10.1111/j.1747-0285.2009.00924.x
BindingDB Entry DOI: 10.7270/Q2BV7F45 |
More data for this
Ligand-Target Pair | |
Ketol-acid reductoisomerase (NADP(+))
(Escherichia coli) | BDBM82142
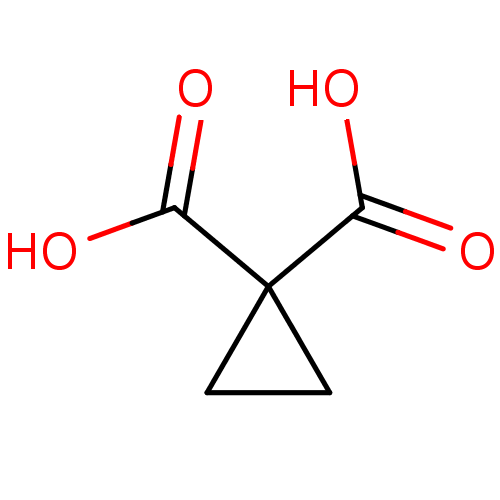
(Cyclopropane, 3)Show InChI InChI=1S/C5H6O4/c6-3(7)5(1-2-5)4(8)9/h1-2H2,(H,6,7)(H,8,9) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
Patents
| Article
PubMed
| 7.66E+4 | -23.9 | n/a | n/a | n/a | n/a | n/a | 8.0 | 30 |
Nankai University
| Assay Description
Inhibition of E. coli KARI (ketol-acid reductoisomerase) is time dependent, and activity was measured by the decrease in A340 at 30 C in solutions co... |
Chem Biol Drug Des 75: 228-32 (2010)
Article DOI: 10.1111/j.1747-0285.2009.00924.x
BindingDB Entry DOI: 10.7270/Q2BV7F45 |
More data for this
Ligand-Target Pair | |
Ketol-acid reductoisomerase (NADP(+))
(Escherichia coli) | BDBM82143

(Cyclopropane, 4)Show InChI InChI=1S/C5H5NO2/c6-3-5(1-2-5)4(7)8/h1-2H2,(H,7,8) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| 9.53E+4 | -23.3 | n/a | n/a | n/a | n/a | n/a | 8.0 | 30 |
Nankai University
| Assay Description
Inhibition of E. coli KARI (ketol-acid reductoisomerase) is time dependent, and activity was measured by the decrease in A340 at 30 C in solutions co... |
Chem Biol Drug Des 75: 228-32 (2010)
Article DOI: 10.1111/j.1747-0285.2009.00924.x
BindingDB Entry DOI: 10.7270/Q2BV7F45 |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, inducible
(Homo sapiens (Human)) | BDBM50226527

(CHEMBL107251 | N-(3-(AMINOMETHYL)BENZYL)ACETAMIDIN...)Show InChI InChI=1S/C10H15N3/c1-8(12)13-7-10-4-2-3-9(5-10)6-11/h2-5H,6-7,11H2,1H3,(H2,12,13) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MMDB
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 8.40 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human iNOS assessed as reduction in L-[3H]-citrulline level using L-[3H]-arginine as substrate incubated for 1 hr |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00578
BindingDB Entry DOI: 10.7270/Q2H9993V |
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase EZH2 [Y641F]
(Homo sapiens (Human)) | BDBM441970
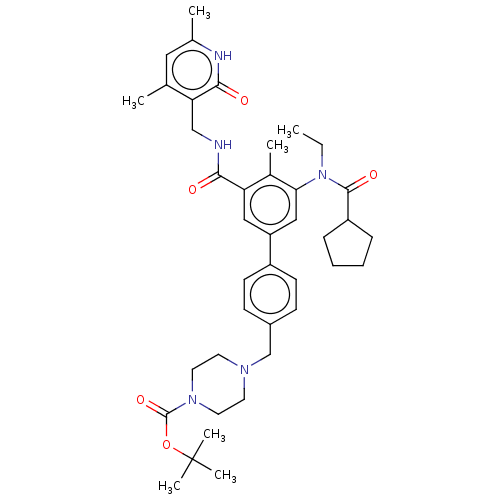
(US10647700, Compound 24)Show SMILES CCN(C(=O)C1CCCC1)c1cc(cc(C(=O)NCc2c(C)cc(C)[nH]c2=O)c1C)-c1ccc(CN2CCN(CC2)C(=O)OC(C)(C)C)cc1 Show InChI InChI=1S/C40H53N5O5/c1-8-45(38(48)31-11-9-10-12-31)35-23-32(22-33(28(35)4)36(46)41-24-34-26(2)21-27(3)42-37(34)47)30-15-13-29(14-16-30)25-43-17-19-44(20-18-43)39(49)50-40(5,6)7/h13-16,21-23,31H,8-12,17-20,24-25H2,1-7H3,(H,41,46)(H,42,47) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
TARAPEUTICS SCIENCE INC.
US Patent
| Assay Description
Enzymatic activity assay was conducted using EZH2(Y641F) TR-FRET assay KIT from Cisbio company on compounds that were shown to be active in primary s... |
US Patent US10647700 (2020)
BindingDB Entry DOI: 10.7270/Q2C82D9J |
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase EZH2 [Y641F]
(Homo sapiens (Human)) | BDBM441969
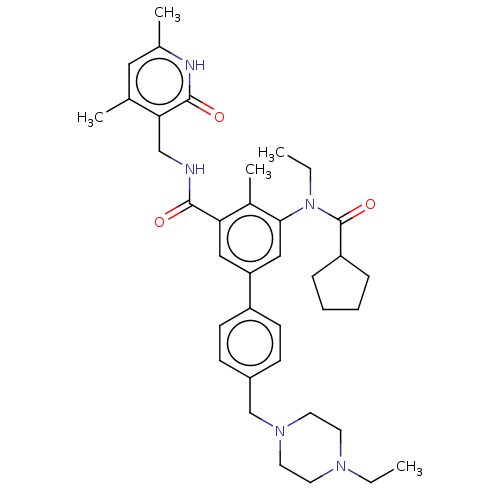
(US10647700, Compound 23)Show SMILES CCN(C(=O)C1CCCC1)c1cc(cc(C(=O)NCc2c(C)cc(C)[nH]c2=O)c1C)-c1ccc(CN2CCN(CC)CC2)cc1 Show InChI InChI=1S/C37H49N5O3/c1-6-40-16-18-41(19-17-40)24-28-12-14-29(15-13-28)31-21-32(35(43)38-23-33-25(3)20-26(4)39-36(33)44)27(5)34(22-31)42(7-2)37(45)30-10-8-9-11-30/h12-15,20-22,30H,6-11,16-19,23-24H2,1-5H3,(H,38,43)(H,39,44) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
TARAPEUTICS SCIENCE INC.
US Patent
| Assay Description
Enzymatic activity assay was conducted using EZH2(Y641F) TR-FRET assay KIT from Cisbio company on compounds that were shown to be active in primary s... |
US Patent US10647700 (2020)
BindingDB Entry DOI: 10.7270/Q2C82D9J |
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase EZH2 [Y641F]
(Homo sapiens (Human)) | BDBM441971
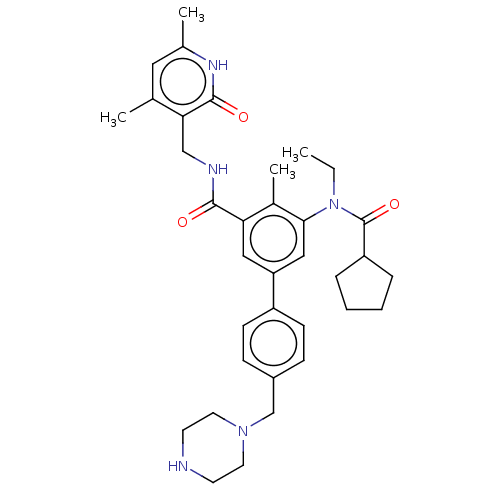
(US10647700, Compound 25)Show SMILES CCN(C(=O)C1CCCC1)c1cc(cc(C(=O)NCc2c(C)cc(C)[nH]c2=O)c1C)-c1ccc(CN2CCNCC2)cc1 Show InChI InChI=1S/C35H45N5O3/c1-5-40(35(43)28-8-6-7-9-28)32-20-29(27-12-10-26(11-13-27)22-39-16-14-36-15-17-39)19-30(25(32)4)33(41)37-21-31-23(2)18-24(3)38-34(31)42/h10-13,18-20,28,36H,5-9,14-17,21-22H2,1-4H3,(H,37,41)(H,38,42) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 23 | n/a | n/a | n/a | n/a | n/a | n/a |
TARAPEUTICS SCIENCE INC.
US Patent
| Assay Description
Enzymatic activity assay was conducted using EZH2(Y641F) TR-FRET assay KIT from Cisbio company on compounds that were shown to be active in primary s... |
US Patent US10647700 (2020)
BindingDB Entry DOI: 10.7270/Q2C82D9J |
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase EZH2 [Y641F]
(Homo sapiens (Human)) | BDBM441968
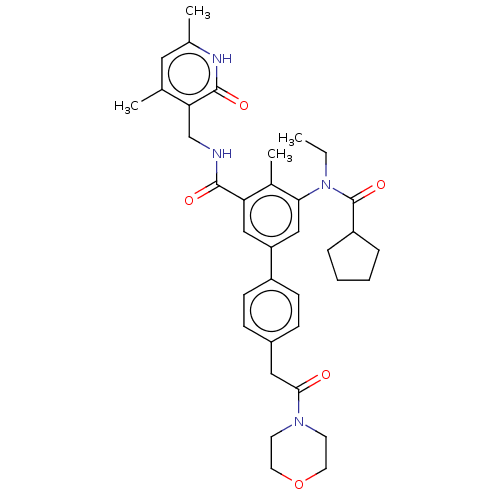
(US10647700, Compound 22)Show SMILES CCN(C(=O)C1CCCC1)c1cc(cc(C(=O)NCc2c(C)cc(C)[nH]c2=O)c1C)-c1ccc(CC(=O)N2CCOCC2)cc1 Show InChI InChI=1S/C36H44N4O5/c1-5-40(36(44)28-8-6-7-9-28)32-21-29(27-12-10-26(11-13-27)19-33(41)39-14-16-45-17-15-39)20-30(25(32)4)34(42)37-22-31-23(2)18-24(3)38-35(31)43/h10-13,18,20-21,28H,5-9,14-17,19,22H2,1-4H3,(H,37,42)(H,38,43) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 29 | n/a | n/a | n/a | n/a | n/a | n/a |
TARAPEUTICS SCIENCE INC.
US Patent
| Assay Description
Enzymatic activity assay was conducted using EZH2(Y641F) TR-FRET assay KIT from Cisbio company on compounds that were shown to be active in primary s... |
US Patent US10647700 (2020)
BindingDB Entry DOI: 10.7270/Q2C82D9J |
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase EZH2 [Y641F]
(Homo sapiens (Human)) | BDBM441962
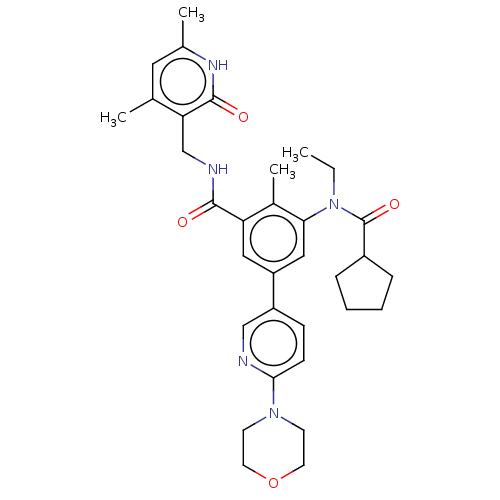
(US10647700, Compound 16)Show SMILES CCN(C(=O)C1CCCC1)c1cc(cc(C(=O)NCc2c(C)cc(C)[nH]c2=O)c1C)-c1ccc(nc1)N1CCOCC1 Show InChI InChI=1S/C33H41N5O4/c1-5-38(33(41)24-8-6-7-9-24)29-18-26(25-10-11-30(34-19-25)37-12-14-42-15-13-37)17-27(23(29)4)31(39)35-20-28-21(2)16-22(3)36-32(28)40/h10-11,16-19,24H,5-9,12-15,20H2,1-4H3,(H,35,39)(H,36,40) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 58.6 | n/a | n/a | n/a | n/a | n/a | n/a |
TARAPEUTICS SCIENCE INC.
US Patent
| Assay Description
Enzymatic activity assay was conducted using EZH2(Y641F) TR-FRET assay KIT from Cisbio company on compounds that were shown to be active in primary s... |
US Patent US10647700 (2020)
BindingDB Entry DOI: 10.7270/Q2C82D9J |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, inducible
(Homo sapiens (Human)) | BDBM50585157

(CHEMBL5087665)Show SMILES COC(=O)[C@H](Cc1ccc(F)cc1)NC(=O)\C=C\c1ccc(cc1)C(F)(F)F |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 74 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human iNOS assessed as reduction in L-[3H]-citrulline level using L-[3H]-arginine as substrate incubated for 1 hr |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00578
BindingDB Entry DOI: 10.7270/Q2H9993V |
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase EZH2 [Y641F]
(Homo sapiens (Human)) | BDBM441960
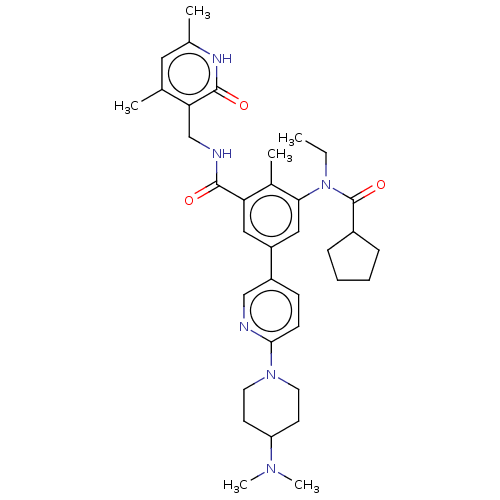
(US10647700, Compound 14)Show SMILES CCN(C(=O)C1CCCC1)c1cc(cc(C(=O)NCc2c(C)cc(C)[nH]c2=O)c1C)-c1ccc(nc1)N1CCC(CC1)N(C)C Show InChI InChI=1S/C36H48N6O3/c1-7-42(36(45)26-10-8-9-11-26)32-20-28(27-12-13-33(37-21-27)41-16-14-29(15-17-41)40(5)6)19-30(25(32)4)34(43)38-22-31-23(2)18-24(3)39-35(31)44/h12-13,18-21,26,29H,7-11,14-17,22H2,1-6H3,(H,38,43)(H,39,44) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 75 | n/a | n/a | n/a | n/a | n/a | n/a |
TARAPEUTICS SCIENCE INC.
US Patent
| Assay Description
Enzymatic activity assay was conducted using EZH2(Y641F) TR-FRET assay KIT from Cisbio company on compounds that were shown to be active in primary s... |
US Patent US10647700 (2020)
BindingDB Entry DOI: 10.7270/Q2C82D9J |
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase EZH2 [Y641F]
(Homo sapiens (Human)) | BDBM441961
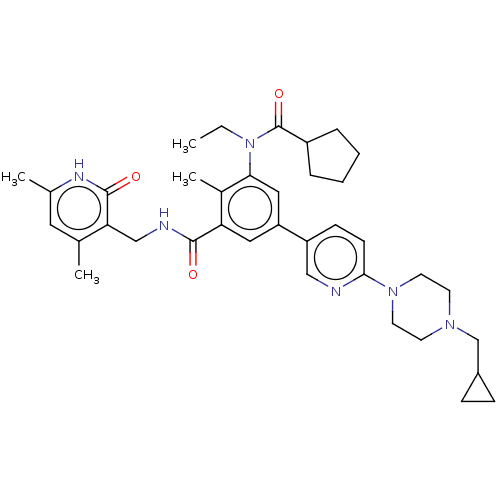
(US10647700, Compound 15)Show SMILES CCN(C(=O)C1CCCC1)c1cc(cc(C(=O)NCc2c(C)cc(C)[nH]c2=O)c1C)-c1ccc(nc1)N1CCN(CC2CC2)CC1 Show InChI InChI=1S/C37H48N6O3/c1-5-43(37(46)28-8-6-7-9-28)33-20-30(19-31(26(33)4)35(44)39-22-32-24(2)18-25(3)40-36(32)45)29-12-13-34(38-21-29)42-16-14-41(15-17-42)23-27-10-11-27/h12-13,18-21,27-28H,5-11,14-17,22-23H2,1-4H3,(H,39,44)(H,40,45) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 80.6 | n/a | n/a | n/a | n/a | n/a | n/a |
TARAPEUTICS SCIENCE INC.
US Patent
| Assay Description
Enzymatic activity assay was conducted using EZH2(Y641F) TR-FRET assay KIT from Cisbio company on compounds that were shown to be active in primary s... |
US Patent US10647700 (2020)
BindingDB Entry DOI: 10.7270/Q2C82D9J |
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase EZH2 [Y641F]
(Homo sapiens (Human)) | BDBM441967
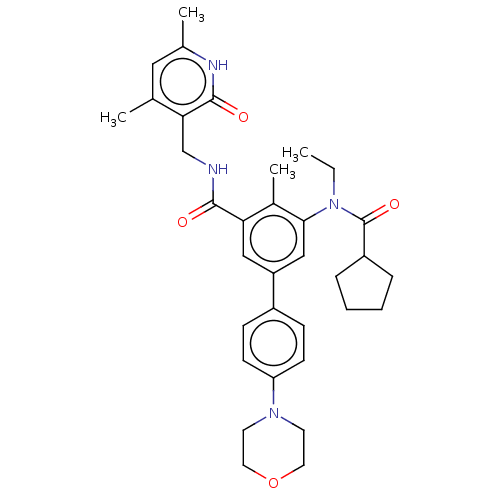
(US10647700, Compound 21)Show SMILES CCN(C(=O)C1CCCC1)c1cc(cc(C(=O)NCc2c(C)cc(C)[nH]c2=O)c1C)-c1ccc(cc1)N1CCOCC1 Show InChI InChI=1S/C34H42N4O4/c1-5-38(34(41)26-8-6-7-9-26)31-20-27(25-10-12-28(13-11-25)37-14-16-42-17-15-37)19-29(24(31)4)32(39)35-21-30-22(2)18-23(3)36-33(30)40/h10-13,18-20,26H,5-9,14-17,21H2,1-4H3,(H,35,39)(H,36,40) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 103 | n/a | n/a | n/a | n/a | n/a | n/a |
TARAPEUTICS SCIENCE INC.
US Patent
| Assay Description
Enzymatic activity assay was conducted using EZH2(Y641F) TR-FRET assay KIT from Cisbio company on compounds that were shown to be active in primary s... |
US Patent US10647700 (2020)
BindingDB Entry DOI: 10.7270/Q2C82D9J |
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase EZH2 [Y641F]
(Homo sapiens (Human)) | BDBM441966

(US10647700, Compound 20)Show SMILES CCN(C(=O)C1CCCC1)c1cc(cc(C(=O)NCc2c(C)cc(C)[nH]c2=O)c1C)-c1ccc(cc1)C(=O)N1CCOCC1 Show InChI InChI=1S/C35H42N4O5/c1-5-39(35(43)26-8-6-7-9-26)31-20-28(25-10-12-27(13-11-25)34(42)38-14-16-44-17-15-38)19-29(24(31)4)32(40)36-21-30-22(2)18-23(3)37-33(30)41/h10-13,18-20,26H,5-9,14-17,21H2,1-4H3,(H,36,40)(H,37,41) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 103 | n/a | n/a | n/a | n/a | n/a | n/a |
TARAPEUTICS SCIENCE INC.
US Patent
| Assay Description
Enzymatic activity assay was conducted using EZH2(Y641F) TR-FRET assay KIT from Cisbio company on compounds that were shown to be active in primary s... |
US Patent US10647700 (2020)
BindingDB Entry DOI: 10.7270/Q2C82D9J |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, inducible
(Homo sapiens (Human)) | BDBM50585156
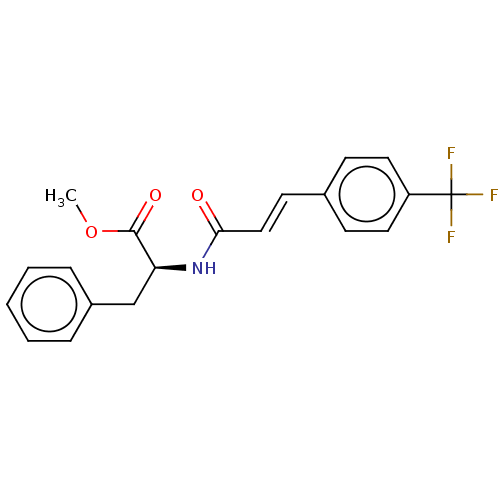
(CHEMBL5090551)Show SMILES COC(=O)[C@H](Cc1ccccc1)NC(=O)\C=C\c1ccc(cc1)C(F)(F)F |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 110 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human iNOS assessed as reduction in L-[3H]-citrulline level using L-[3H]-arginine as substrate incubated for 1 hr |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00578
BindingDB Entry DOI: 10.7270/Q2H9993V |
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase EZH2 [Y641F]
(Homo sapiens (Human)) | BDBM50017293

(CHEMBL3287735 | US10647700, Compound GSK126)Show SMILES CC[C@H](C)n1cc(C)c2c(cc(cc12)-c1ccc(nc1)N1CCNCC1)C(=O)NCc1c(C)cc(C)[nH]c1=O |r| Show InChI InChI=1S/C31H38N6O2/c1-6-22(5)37-18-20(3)29-25(30(38)34-17-26-19(2)13-21(4)35-31(26)39)14-24(15-27(29)37)23-7-8-28(33-16-23)36-11-9-32-10-12-36/h7-8,13-16,18,22,32H,6,9-12,17H2,1-5H3,(H,34,38)(H,35,39)/t22-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
US Patent
| n/a | n/a | 121 | n/a | n/a | n/a | n/a | n/a | n/a |
TARAPEUTICS SCIENCE INC.
US Patent
| Assay Description
Enzymatic activity assay was conducted using EZH2(Y641F) TR-FRET assay KIT from Cisbio company on compounds that were shown to be active in primary s... |
US Patent US10647700 (2020)
BindingDB Entry DOI: 10.7270/Q2C82D9J |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Nitric oxide synthase, inducible
(Homo sapiens (Human)) | BDBM50585159
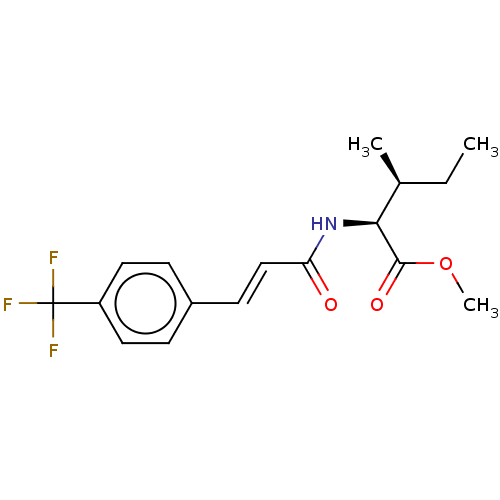
(CHEMBL5081853)Show SMILES CC[C@H](C)[C@H](NC(=O)\C=C\c1ccc(cc1)C(F)(F)F)C(=O)OC |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 178 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human iNOS assessed as reduction in L-[3H]-citrulline level using L-[3H]-arginine as substrate incubated for 1 hr |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00578
BindingDB Entry DOI: 10.7270/Q2H9993V |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, inducible
(Homo sapiens (Human)) | BDBM50585153
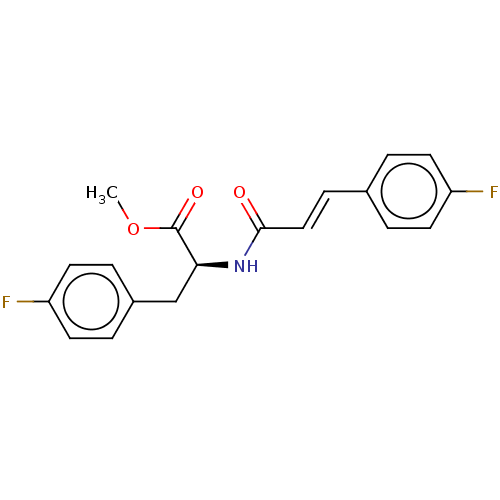
(CHEMBL5085929)Show SMILES COC(=O)[C@H](Cc1ccc(F)cc1)NC(=O)\C=C\c1ccc(F)cc1 |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 188 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human iNOS assessed as reduction in L-[3H]-citrulline level using L-[3H]-arginine as substrate incubated for 1 hr |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00578
BindingDB Entry DOI: 10.7270/Q2H9993V |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, inducible
(Homo sapiens (Human)) | BDBM50585158
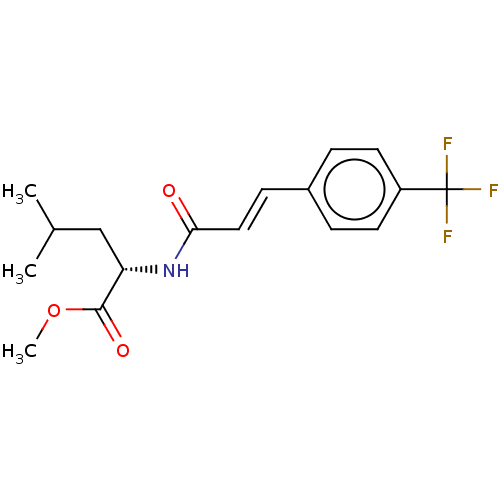
(CHEMBL5082466)Show SMILES COC(=O)[C@H](CC(C)C)NC(=O)\C=C\c1ccc(cc1)C(F)(F)F |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 195 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human iNOS assessed as reduction in L-[3H]-citrulline level using L-[3H]-arginine as substrate incubated for 1 hr |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00578
BindingDB Entry DOI: 10.7270/Q2H9993V |
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase EZH2 [Y641F]
(Homo sapiens (Human)) | BDBM172038
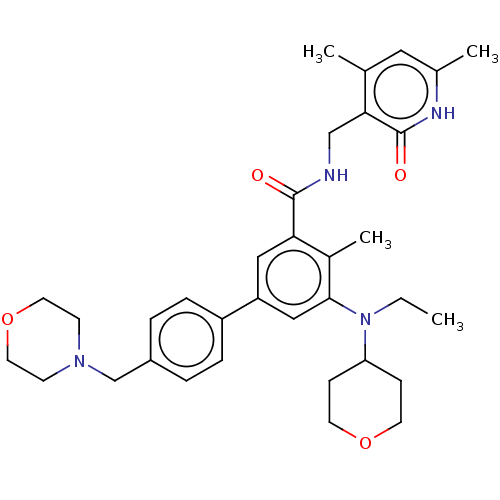
(US10155002, Compound 44 | US10647700, Compound EPZ...)Show SMILES CCN(C1CCOCC1)c1cc(cc(C(=O)NCc2c(C)cc(C)[nH]c2=O)c1C)-c1ccc(CN2CCOCC2)cc1 Show InChI InChI=1S/C34H44N4O4/c1-5-38(29-10-14-41-15-11-29)32-20-28(27-8-6-26(7-9-27)22-37-12-16-42-17-13-37)19-30(25(32)4)33(39)35-21-31-23(2)18-24(3)36-34(31)40/h6-9,18-20,29H,5,10-17,21-22H2,1-4H3,(H,35,39)(H,36,40) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 219 | n/a | n/a | n/a | n/a | n/a | n/a |
TARAPEUTICS SCIENCE INC.
US Patent
| Assay Description
Enzymatic activity assay was conducted using EZH2(Y641F) TR-FRET assay KIT from Cisbio company on compounds that were shown to be active in primary s... |
US Patent US10647700 (2020)
BindingDB Entry DOI: 10.7270/Q2C82D9J |
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase EZH2 [Y641F]
(Homo sapiens (Human)) | BDBM441965
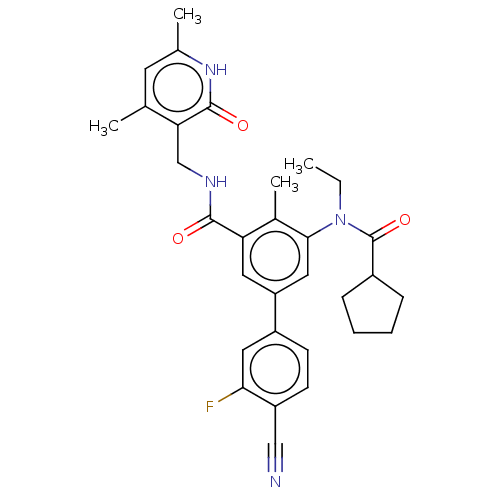
(US10647700, Compound 19)Show SMILES CCN(C(=O)C1CCCC1)c1cc(cc(C(=O)NCc2c(C)cc(C)[nH]c2=O)c1C)-c1ccc(C#N)c(F)c1 Show InChI InChI=1S/C31H33FN4O3/c1-5-36(31(39)21-8-6-7-9-21)28-15-24(22-10-11-23(16-33)27(32)14-22)13-25(20(28)4)29(37)34-17-26-18(2)12-19(3)35-30(26)38/h10-15,21H,5-9,17H2,1-4H3,(H,34,37)(H,35,38) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 220 | n/a | n/a | n/a | n/a | n/a | n/a |
TARAPEUTICS SCIENCE INC.
US Patent
| Assay Description
Enzymatic activity assay was conducted using EZH2(Y641F) TR-FRET assay KIT from Cisbio company on compounds that were shown to be active in primary s... |
US Patent US10647700 (2020)
BindingDB Entry DOI: 10.7270/Q2C82D9J |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, inducible
(Homo sapiens (Human)) | BDBM50585152
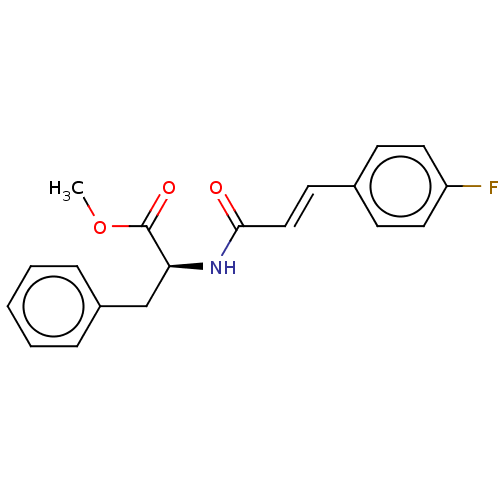
(CHEMBL5086634)Show SMILES COC(=O)[C@H](Cc1ccccc1)NC(=O)\C=C\c1ccc(F)cc1 |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 291 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human iNOS assessed as reduction in L-[3H]-citrulline level using L-[3H]-arginine as substrate incubated for 1 hr |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00578
BindingDB Entry DOI: 10.7270/Q2H9993V |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, inducible
(Homo sapiens (Human)) | BDBM50585155

(CHEMBL5093141)Show SMILES CC[C@H](C)[C@H](NC(=O)\C=C\c1ccc(F)cc1)C(=O)OC |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 421 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human iNOS assessed as reduction in L-[3H]-citrulline level using L-[3H]-arginine as substrate incubated for 1 hr |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00578
BindingDB Entry DOI: 10.7270/Q2H9993V |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, inducible
(Homo sapiens (Human)) | BDBM50585154
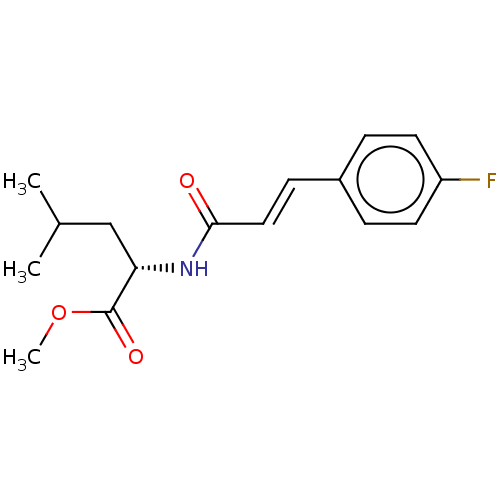
(CHEMBL5070156)Show SMILES COC(=O)[C@H](CC(C)C)NC(=O)\C=C\c1ccc(F)cc1 |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 434 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human iNOS assessed as reduction in L-[3H]-citrulline level using L-[3H]-arginine as substrate incubated for 1 hr |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00578
BindingDB Entry DOI: 10.7270/Q2H9993V |
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase EZH2 [Y641F]
(Homo sapiens (Human)) | BDBM441963
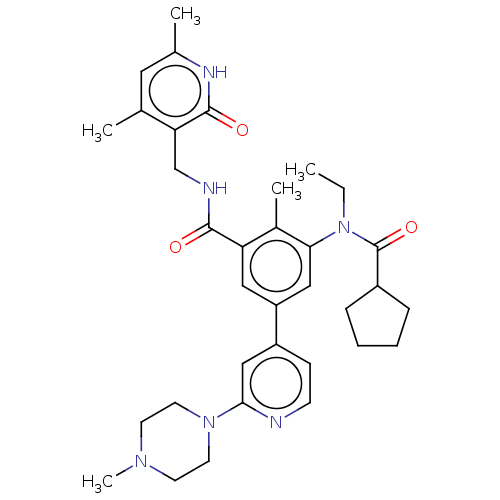
(US10647700, Compound 17)Show SMILES CCN(C(=O)C1CCCC1)c1cc(cc(C(=O)NCc2c(C)cc(C)[nH]c2=O)c1C)-c1ccnc(c1)N1CCN(C)CC1 Show InChI InChI=1S/C34H44N6O3/c1-6-40(34(43)25-9-7-8-10-25)30-19-27(26-11-12-35-31(20-26)39-15-13-38(5)14-16-39)18-28(24(30)4)32(41)36-21-29-22(2)17-23(3)37-33(29)42/h11-12,17-20,25H,6-10,13-16,21H2,1-5H3,(H,36,41)(H,37,42) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 444 | n/a | n/a | n/a | n/a | n/a | n/a |
TARAPEUTICS SCIENCE INC.
US Patent
| Assay Description
Enzymatic activity assay was conducted using EZH2(Y641F) TR-FRET assay KIT from Cisbio company on compounds that were shown to be active in primary s... |
US Patent US10647700 (2020)
BindingDB Entry DOI: 10.7270/Q2C82D9J |
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase EZH2 [Y641F]
(Homo sapiens (Human)) | BDBM441964

(US10647700, Compound 18)Show SMILES CCN(C(=O)C1CCCC1)c1cc(cc(C(=O)NCc2c(C)cc(C)[nH]c2=O)c1C)-c1ccc(nc1)N1CCN(CC1)C(C)C Show InChI InChI=1S/C36H48N6O3/c1-7-42(36(45)27-10-8-9-11-27)32-20-29(28-12-13-33(37-21-28)41-16-14-40(15-17-41)23(2)3)19-30(26(32)6)34(43)38-22-31-24(4)18-25(5)39-35(31)44/h12-13,18-21,23,27H,7-11,14-17,22H2,1-6H3,(H,38,43)(H,39,44) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 515 | n/a | n/a | n/a | n/a | n/a | n/a |
TARAPEUTICS SCIENCE INC.
US Patent
| Assay Description
Enzymatic activity assay was conducted using EZH2(Y641F) TR-FRET assay KIT from Cisbio company on compounds that were shown to be active in primary s... |
US Patent US10647700 (2020)
BindingDB Entry DOI: 10.7270/Q2C82D9J |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, inducible
(Homo sapiens (Human)) | BDBM50585142
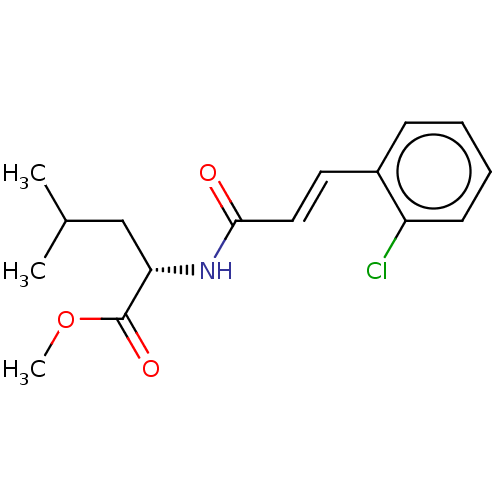
(CHEMBL5091916)Show SMILES COC(=O)[C@H](CC(C)C)NC(=O)\C=C\c1ccccc1Cl |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 594 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human iNOS assessed as reduction in L-[3H]-citrulline level using L-[3H]-arginine as substrate incubated for 1 hr |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00578
BindingDB Entry DOI: 10.7270/Q2H9993V |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, inducible
(Homo sapiens (Human)) | BDBM50585140
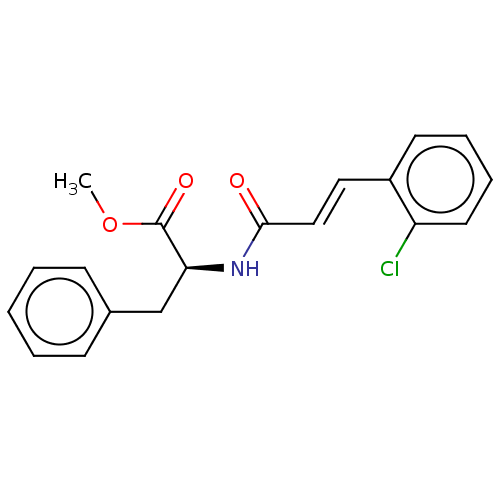
(CHEMBL5083240)Show SMILES COC(=O)[C@H](Cc1ccccc1)NC(=O)\C=C\c1ccccc1Cl |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 630 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human iNOS assessed as reduction in L-[3H]-citrulline level using L-[3H]-arginine as substrate incubated for 1 hr |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00578
BindingDB Entry DOI: 10.7270/Q2H9993V |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, inducible
(Homo sapiens (Human)) | BDBM50585151

(CHEMBL5094665)Show SMILES CC[C@H](C)[C@H](NC(=O)\C=C\c1ccc(Cl)c(Cl)c1)C(=O)OC |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 640 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human iNOS assessed as reduction in L-[3H]-citrulline level using L-[3H]-arginine as substrate incubated for 1 hr |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00578
BindingDB Entry DOI: 10.7270/Q2H9993V |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, inducible
(Homo sapiens (Human)) | BDBM50585143
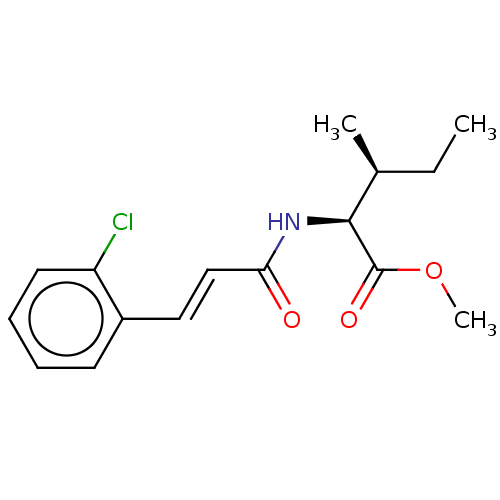
(CHEMBL5082696)Show SMILES CC[C@H](C)[C@H](NC(=O)\C=C\c1ccccc1Cl)C(=O)OC |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 645 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human iNOS assessed as reduction in L-[3H]-citrulline level using L-[3H]-arginine as substrate incubated for 1 hr |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00578
BindingDB Entry DOI: 10.7270/Q2H9993V |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, inducible
(Homo sapiens (Human)) | BDBM50585144
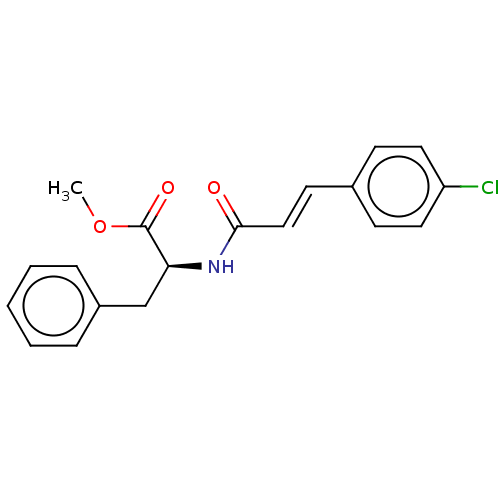
(CHEMBL2289691)Show SMILES COC(=O)[C@H](Cc1ccccc1)NC(=O)\C=C\c1ccc(Cl)cc1 |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 731 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human iNOS assessed as reduction in L-[3H]-citrulline level using L-[3H]-arginine as substrate incubated for 1 hr |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00578
BindingDB Entry DOI: 10.7270/Q2H9993V |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, inducible
(Homo sapiens (Human)) | BDBM50585150

(CHEMBL5093391)Show SMILES COC(=O)[C@H](CC(C)C)NC(=O)\C=C\c1ccc(Cl)c(Cl)c1 |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 765 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human iNOS assessed as reduction in L-[3H]-citrulline level using L-[3H]-arginine as substrate incubated for 1 hr |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00578
BindingDB Entry DOI: 10.7270/Q2H9993V |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, inducible
(Homo sapiens (Human)) | BDBM50585148
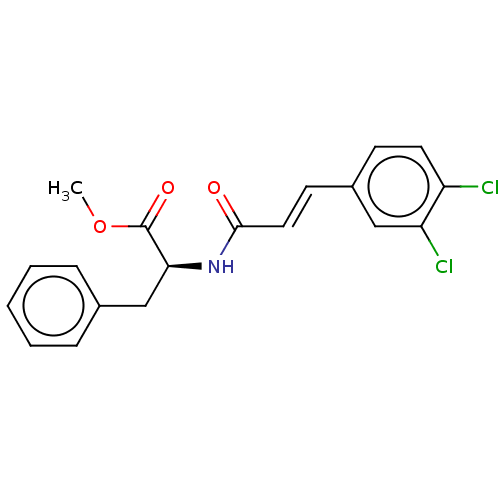
(CHEMBL5077818)Show SMILES COC(=O)[C@H](Cc1ccccc1)NC(=O)\C=C\c1ccc(Cl)c(Cl)c1 |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 766 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human iNOS assessed as reduction in L-[3H]-citrulline level using L-[3H]-arginine as substrate incubated for 1 hr |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00578
BindingDB Entry DOI: 10.7270/Q2H9993V |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, inducible
(Homo sapiens (Human)) | BDBM50585146

(CHEMBL2289685)Show SMILES COC(=O)[C@H](CC(C)C)NC(=O)\C=C\c1ccc(Cl)cc1 |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 782 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human iNOS assessed as reduction in L-[3H]-citrulline level using L-[3H]-arginine as substrate incubated for 1 hr |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00578
BindingDB Entry DOI: 10.7270/Q2H9993V |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, inducible
(Homo sapiens (Human)) | BDBM50585141
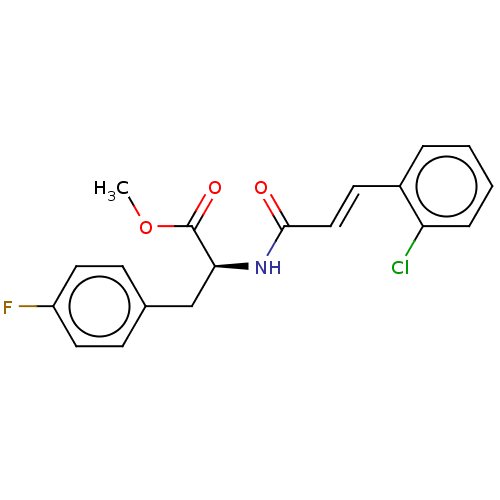
(CHEMBL5080023)Show SMILES COC(=O)[C@H](Cc1ccc(F)cc1)NC(=O)\C=C\c1ccccc1Cl |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 820 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human iNOS assessed as reduction in L-[3H]-citrulline level using L-[3H]-arginine as substrate incubated for 1 hr |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00578
BindingDB Entry DOI: 10.7270/Q2H9993V |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, inducible
(Homo sapiens (Human)) | BDBM50585160
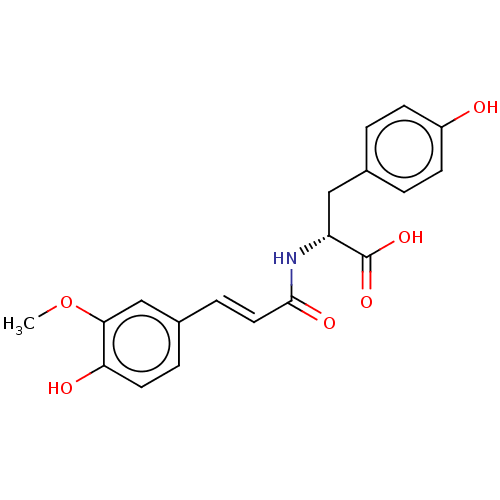
(CHEMBL5092908)Show SMILES COc1cc(\C=C\C(=O)N[C@H](Cc2ccc(O)cc2)C(O)=O)ccc1O |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 892 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human iNOS assessed as reduction in L-[3H]-citrulline level using L-[3H]-arginine as substrate incubated for 1 hr |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00578
BindingDB Entry DOI: 10.7270/Q2H9993V |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, inducible
(Homo sapiens (Human)) | BDBM50585147
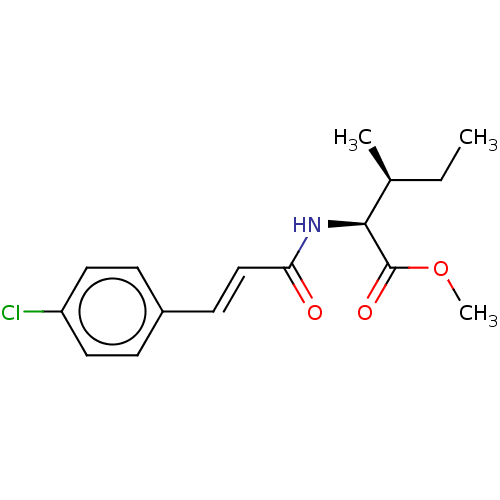
(CHEMBL2289689)Show SMILES CC[C@H](C)[C@H](NC(=O)\C=C\c1ccc(Cl)cc1)C(=O)OC |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 916 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human iNOS assessed as reduction in L-[3H]-citrulline level using L-[3H]-arginine as substrate incubated for 1 hr |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00578
BindingDB Entry DOI: 10.7270/Q2H9993V |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, inducible
(Homo sapiens (Human)) | BDBM50585145
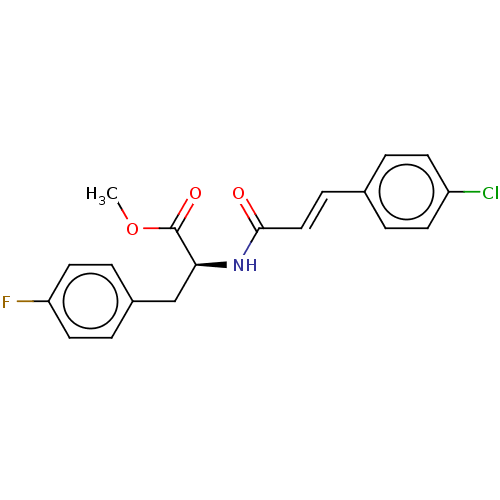
(CHEMBL5078616)Show SMILES COC(=O)[C@H](Cc1ccc(F)cc1)NC(=O)\C=C\c1ccc(Cl)cc1 |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 945 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human iNOS assessed as reduction in L-[3H]-citrulline level using L-[3H]-arginine as substrate incubated for 1 hr |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00578
BindingDB Entry DOI: 10.7270/Q2H9993V |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, inducible
(Homo sapiens (Human)) | BDBM50585149
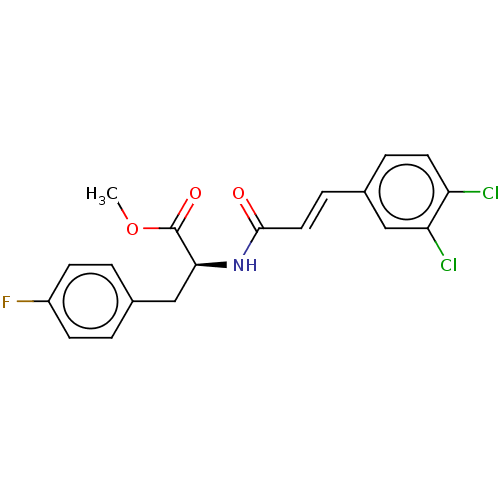
(CHEMBL5090181)Show SMILES COC(=O)[C@H](Cc1ccc(F)cc1)NC(=O)\C=C\c1ccc(Cl)c(Cl)c1 |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 993 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human iNOS assessed as reduction in L-[3H]-citrulline level using L-[3H]-arginine as substrate incubated for 1 hr |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00578
BindingDB Entry DOI: 10.7270/Q2H9993V |
More data for this
Ligand-Target Pair | |
Platelet-derived growth factor receptor alpha/beta
(Mus musculus (mouse)) | BDBM50607981

(CHEMBL4782984)Show SMILES [H][C@@]12C[C@@](C)(CC[C@]1(C)CC[C@]1(C)C3=CC=C4C(C)=C(O)C(=O)C=C4[C@]3(C)CC[C@@]21C)C(=O)OCC#C |r,c:24,t:14,16,19| | MMDB
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| UniChem
| | n/a | n/a | 1.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, brain
(Homo sapiens (Human)) | BDBM50226527

(CHEMBL107251 | N-(3-(AMINOMETHYL)BENZYL)ACETAMIDIN...)Show InChI InChI=1S/C10H15N3/c1-8(12)13-7-10-4-2-3-9(5-10)6-11/h2-5H,6-7,11H2,1H3,(H2,12,13) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MMDB
PC cid
PC sid
UniChem
Patents
Similars
| MMDB
Article
PubMed
| n/a | n/a | 1.98E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human nNOS assessed as reduction in L-[3H]-citrulline level using L-[3H]-arginine as substrate incubated for 1 hr |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00578
BindingDB Entry DOI: 10.7270/Q2H9993V |
More data for this
Ligand-Target Pair | |
Platelet-derived growth factor receptor alpha/beta
(Mus musculus (mouse)) | BDBM50607983

(CHEMBL1170915)Show SMILES [H][C@@]12C[C@@](C)(CC[C@]1(C)CC[C@]1(C)C3=CC=C4C(C)=C(O)C(=O)C=C4[C@]3(C)CC[C@@]21C)C(=O)OCc1ccccc1 |r,c:24,t:14,16,19| | MMDB
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| UniChem
| | n/a | n/a | 2.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Platelet-derived growth factor receptor alpha/beta
(Mus musculus (mouse)) | BDBM50607982

(CHEMBL4789502)Show SMILES [H][C@@]12C[C@@](C)(CC[C@]1(C)CC[C@]1(C)C3=CC=C4C(C)=C(O)C(=O)C=C4[C@]3(C)CC[C@@]21C)C(=O)OCC1CCCCC1 |r,c:24,t:14,16,19| | MMDB
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| UniChem
| | n/a | n/a | 2.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, endothelial
(Homo sapiens (Human)) | BDBM50585158

(CHEMBL5082466)Show SMILES COC(=O)[C@H](CC(C)C)NC(=O)\C=C\c1ccc(cc1)C(F)(F)F |r| | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 5.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human eNOS assessed as reduction in L-[3H]-citrulline level using L-[3H]-arginine as substrate incubated for 1 hr |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00578
BindingDB Entry DOI: 10.7270/Q2H9993V |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, brain
(Homo sapiens (Human)) | BDBM50585158

(CHEMBL5082466)Show SMILES COC(=O)[C@H](CC(C)C)NC(=O)\C=C\c1ccc(cc1)C(F)(F)F |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 6.12E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human nNOS assessed as reduction in L-[3H]-citrulline level using L-[3H]-arginine as substrate incubated for 1 hr |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00578
BindingDB Entry DOI: 10.7270/Q2H9993V |
More data for this
Ligand-Target Pair | |
Platelet-derived growth factor receptor alpha/beta
(Mus musculus (mouse)) | BDBM50607984

(CHEMBL4758598)Show SMILES [H][C@@]12C[C@@](C)(CC[C@]1(C)CC[C@]1(C)C3=CC=C4C(C)=C(O)C(=O)C=C4[C@]3(C)CC[C@@]21C)C(=O)OCc1cccc(C)c1 |r,c:24,t:14,16,19| | MMDB
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| UniChem
| | n/a | n/a | 6.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Platelet-derived growth factor receptor alpha/beta
(Mus musculus (mouse)) | BDBM50607980
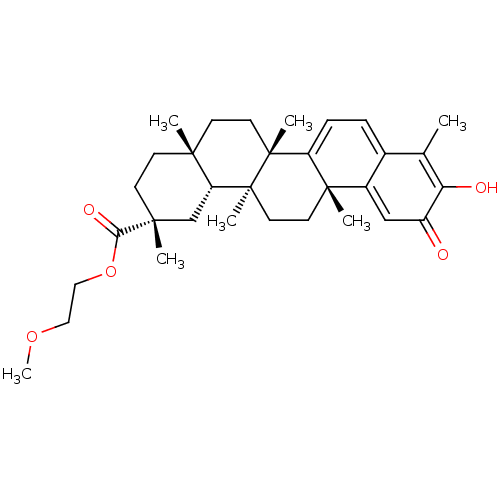
(CHEMBL4751648)Show SMILES [H][C@@]12C[C@@](C)(CC[C@]1(C)CC[C@]1(C)C3=CC=C4C(C)=C(O)C(=O)C=C4[C@]3(C)CC[C@@]21C)C(=O)OCCOC |r,c:24,t:14,16,19| | MMDB
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| UniChem
| | n/a | n/a | 6.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, brain
(Homo sapiens (Human)) | BDBM50585159

(CHEMBL5081853)Show SMILES CC[C@H](C)[C@H](NC(=O)\C=C\c1ccc(cc1)C(F)(F)F)C(=O)OC |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 8.36E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human nNOS assessed as reduction in L-[3H]-citrulline level using L-[3H]-arginine as substrate incubated for 1 hr |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00578
BindingDB Entry DOI: 10.7270/Q2H9993V |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, endothelial
(Homo sapiens (Human)) | BDBM50585159

(CHEMBL5081853)Show SMILES CC[C@H](C)[C@H](NC(=O)\C=C\c1ccc(cc1)C(F)(F)F)C(=O)OC |r| | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 9.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human eNOS assessed as reduction in L-[3H]-citrulline level using L-[3H]-arginine as substrate incubated for 1 hr |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00578
BindingDB Entry DOI: 10.7270/Q2H9993V |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data