 Found 432 hits with Last Name = 'warszycki' and Initial = 'd'
Found 432 hits with Last Name = 'warszycki' and Initial = 'd' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Sodium-dependent serotonin transporter
(Homo sapiens (Human)) | BDBM22416

((3S,4R)-3-[(1,3-benzodioxol-5-yloxy)methyl]-4-(4-f...)Show SMILES Fc1ccc(cc1)[C@@H]1CCNC[C@H]1COc1ccc2OCOc2c1 Show InChI InChI=1S/C19H20FNO3/c20-15-3-1-13(2-4-15)17-7-8-21-10-14(17)11-22-16-5-6-18-19(9-16)24-12-23-18/h1-6,9,14,17,21H,7-8,10-12H2/t14-,17-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| 0.0800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [125I]RTI55 binding from human wild type SERT |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113533
BindingDB Entry DOI: 10.7270/Q24T6P6C |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50232666

(CHEMBL4083059)Show SMILES CN(C)Cc1cc2cn(c3cccc(o1)c23)S(=O)(=O)c1cccc2ccccc12 Show InChI InChI=1S/C23H20N2O3S/c1-24(2)15-18-13-17-14-25(20-10-6-11-21(28-18)23(17)20)29(26,27)22-12-5-8-16-7-3-4-9-19(16)22/h3-14H,15H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Polish Academy of Sciences
Curated by ChEMBL
| Assay Description
Displacement of [3H]-LSD from 5-HT6 receptor (unknown origin) |
ACS Med Chem Lett 8: 390-394 (2017)
Article DOI: 10.1021/acsmedchemlett.6b00482
BindingDB Entry DOI: 10.7270/Q2DN479B |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50562710

(CHEMBL4759730)Show SMILES CN1CCN(CC1)c1cccc2n(ccc12)S(=O)(=O)c1ccccc1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]-LSD from human 5-HT6 receptor incubated for 1 hr by microbeta counting method |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.112916
BindingDB Entry DOI: 10.7270/Q22R3WD3 |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Homo sapiens (Human)) | BDBM50578580
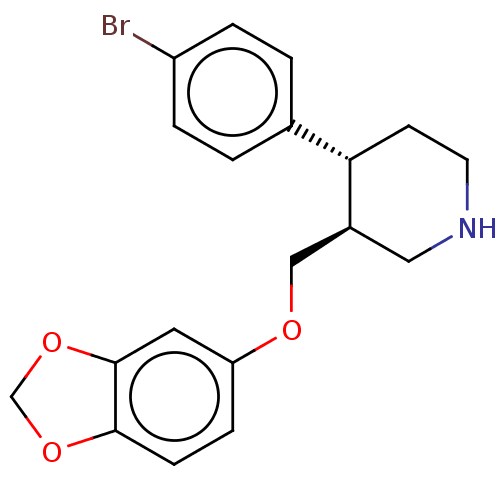
(CHEMBL4848081)Show SMILES Brc1ccc(cc1)[C@@H]1CCNC[C@H]1COc1ccc2OCOc2c1 |r| | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [125I]RTI55 binding from human wild type SERT |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113533
BindingDB Entry DOI: 10.7270/Q24T6P6C |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50504839

(CHEMBL4584504)Show SMILES Oc1ccc(cc1)-c1ccc(CNCCc2c[nH]c3cc(F)ccc23)o1 Show InChI InChI=1S/C21H19FN2O2/c22-16-3-7-19-15(12-24-20(19)11-16)9-10-23-13-18-6-8-21(26-18)14-1-4-17(25)5-2-14/h1-8,11-12,23-25H,9-10,13H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Polish Academy of Sciences
Curated by ChEMBL
| Assay Description
Displacement of [3H]-LSD from human 5-HT6R expressed in HEK293 cells after 1 hr by microbeta plate reader analysis |
Eur J Med Chem 185: (2020)
Article DOI: 10.1016/j.ejmech.2019.111857
BindingDB Entry DOI: 10.7270/Q2833W92 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50504838

(CHEMBL4437523)Show SMILES Oc1ccc(cc1)-c1ccc(CNCCc2c[nH]c3ccc(F)cc23)o1 Show InChI InChI=1S/C21H19FN2O2/c22-16-3-7-20-19(11-16)15(12-24-20)9-10-23-13-18-6-8-21(26-18)14-1-4-17(25)5-2-14/h1-8,11-12,23-25H,9-10,13H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Polish Academy of Sciences
Curated by ChEMBL
| Assay Description
Displacement of [3H]-LSD from human 5-HT6R expressed in HEK293 cells after 1 hr by microbeta plate reader analysis |
Eur J Med Chem 185: (2020)
Article DOI: 10.1016/j.ejmech.2019.111857
BindingDB Entry DOI: 10.7270/Q2833W92 |
More data for this
Ligand-Target Pair | |
Cathepsin S
(Homo sapiens (Human)) | BDBM50463221
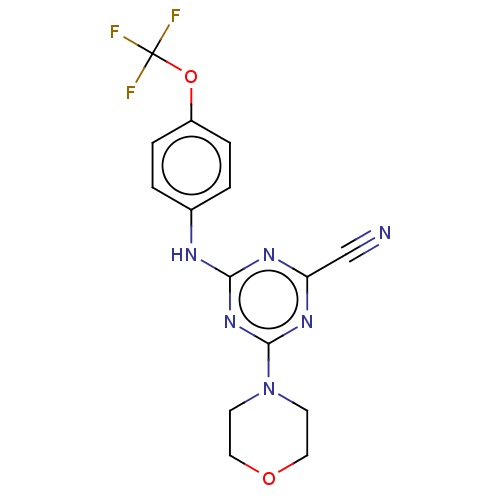
(CHEMBL4251253)Show SMILES FC(F)(F)Oc1ccc(Nc2nc(nc(n2)N2CCOCC2)C#N)cc1 Show InChI InChI=1S/C15H13F3N6O2/c16-15(17,18)26-11-3-1-10(2-4-11)20-13-21-12(9-19)22-14(23-13)24-5-7-25-8-6-24/h1-4H,5-8H2,(H,20,21,22,23) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ d'Orl£ans
Curated by ChEMBL
| Assay Description
Inhibition of human cathepsin S using Z-LR-AMC as substrate after 30 mins by Cheng-Prusoff equation analysis |
Bioorg Med Chem 26: 4310-4319 (2018)
Article DOI: 10.1016/j.bmc.2018.07.032
BindingDB Entry DOI: 10.7270/Q2P271RF |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Homo sapiens (Human)) | BDBM50048803

(5-(2-(4-(benzo[d]isothiazol-3-yl)piperazin-1-yl)et...)Show SMILES Clc1cc2NC(=O)Cc2cc1CCN1CCN(CC1)c1nsc2ccccc12 Show InChI InChI=1S/C21H21ClN4OS/c22-17-13-18-15(12-20(27)23-18)11-14(17)5-6-25-7-9-26(10-8-25)21-16-3-1-2-4-19(16)28-24-21/h1-4,11,13H,5-10,12H2,(H,23,27) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| 2.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Polish Academy of Sciences
Curated by ChEMBL
| Assay Description
Displacement of [3H]-raclopride from human D2R expressed in HEK293 cells after 1 hr by microbeta plate reader analysis |
Eur J Med Chem 185: (2020)
Article DOI: 10.1016/j.ejmech.2019.111857
BindingDB Entry DOI: 10.7270/Q2833W92 |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Homo sapiens (Human)) | BDBM50048803

(5-(2-(4-(benzo[d]isothiazol-3-yl)piperazin-1-yl)et...)Show SMILES Clc1cc2NC(=O)Cc2cc1CCN1CCN(CC1)c1nsc2ccccc12 Show InChI InChI=1S/C21H21ClN4OS/c22-17-13-18-15(12-20(27)23-18)11-14(17)5-6-25-7-9-26(10-8-25)21-16-3-1-2-4-19(16)28-24-21/h1-4,11,13H,5-10,12H2,(H,23,27) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| 2.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Polish Academy of Sciences
Curated by ChEMBL
| Assay Description
Displacement of [3H]-Raclopride from D2L receptor (unknown origin) |
ACS Med Chem Lett 8: 390-394 (2017)
Article DOI: 10.1021/acsmedchemlett.6b00482
BindingDB Entry DOI: 10.7270/Q2DN479B |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Homo sapiens (Human)) | BDBM50463238

(CHEMBL4238973)Show SMILES CSCC[C@H](NC(=O)[C@@H]1CCCN1C(=O)[C@H](Cc1c[nH]cn1)NC(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)CNC(=O)CNC(C)=O)C(=O)NNC(=O)N[C@@H](C)C(=O)N1CCC[C@H]1C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@H](C)C(=O)N[C@H](CCCNC(N)=N)C(N)=O |r| Show InChI InChI=1S/C67H92N22O16S/c1-35(57(96)81-45(56(68)95)16-9-22-72-66(69)70)78-58(97)46(19-20-55(93)94)82-60(99)49(27-39-30-75-44-15-8-6-13-42(39)44)84-63(102)51-17-10-23-88(51)64(103)36(2)79-67(105)87-86-61(100)47(21-25-106-4)83-62(101)52-18-11-24-89(52)65(104)50(28-40-31-71-34-77-40)85-59(98)48(26-38-29-74-43-14-7-5-12-41(38)43)80-54(92)33-76-53(91)32-73-37(3)90/h5-8,12-15,29-31,34-36,45-52,74-75H,9-11,16-28,32-33H2,1-4H3,(H2,68,95)(H,71,77)(H,73,90)(H,76,91)(H,78,97)(H,80,92)(H,81,96)(H,82,99)(H,83,101)(H,84,102)(H,85,98)(H,86,100)(H,93,94)(H4,69,70,72)(H2,79,87,105)/t35-,36+,45-,46+,47+,48+,49+,50+,51+,52+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ d'Orl£ans
Curated by ChEMBL
| Assay Description
Inhibition of human cathepsin K using Z-Phe-Arg-AMC as substrate after 30 mins by Cheng-Prusoff equation analysis |
Bioorg Med Chem 26: 4310-4319 (2018)
Article DOI: 10.1016/j.bmc.2018.07.032
BindingDB Entry DOI: 10.7270/Q2P271RF |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50504842
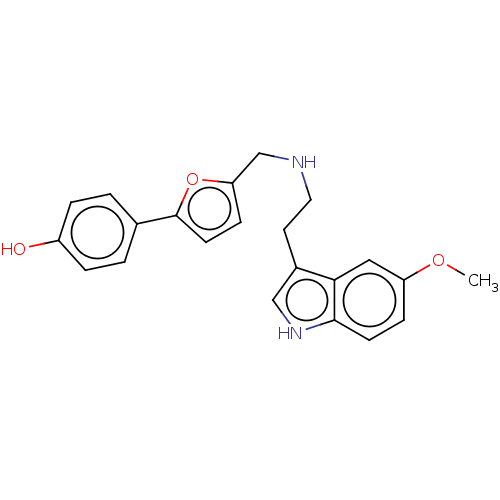
(CHEMBL4583082)Show SMILES COc1ccc2[nH]cc(CCNCc3ccc(o3)-c3ccc(O)cc3)c2c1 Show InChI InChI=1S/C22H22N2O3/c1-26-18-6-8-21-20(12-18)16(13-24-21)10-11-23-14-19-7-9-22(27-19)15-2-4-17(25)5-3-15/h2-9,12-13,23-25H,10-11,14H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Polish Academy of Sciences
Curated by ChEMBL
| Assay Description
Displacement of [3H]-LSD from human 5-HT6R expressed in HEK293 cells after 1 hr by microbeta plate reader analysis |
Eur J Med Chem 185: (2020)
Article DOI: 10.1016/j.ejmech.2019.111857
BindingDB Entry DOI: 10.7270/Q2833W92 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50019754
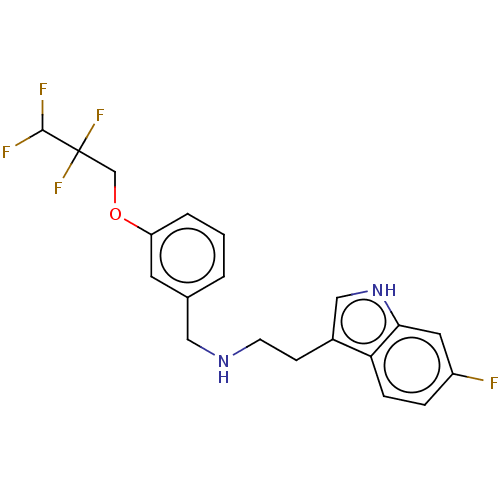
(IDALOPIRDINE | LU-AE58054)Show SMILES FC(F)C(F)(F)COc1cccc(CNCCc2c[nH]c3cc(F)ccc23)c1 Show InChI InChI=1S/C20H19F5N2O/c21-15-4-5-17-14(11-27-18(17)9-15)6-7-26-10-13-2-1-3-16(8-13)28-12-20(24,25)19(22)23/h1-5,8-9,11,19,26-27H,6-7,10,12H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Polish Academy of Sciences
Curated by ChEMBL
| Assay Description
Displacement of [3H]-LSD from human 5-HT6R expressed in HEK293 cells after 1 hr by microbeta plate reader analysis |
Eur J Med Chem 185: (2020)
Article DOI: 10.1016/j.ejmech.2019.111857
BindingDB Entry DOI: 10.7270/Q2833W92 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50334252
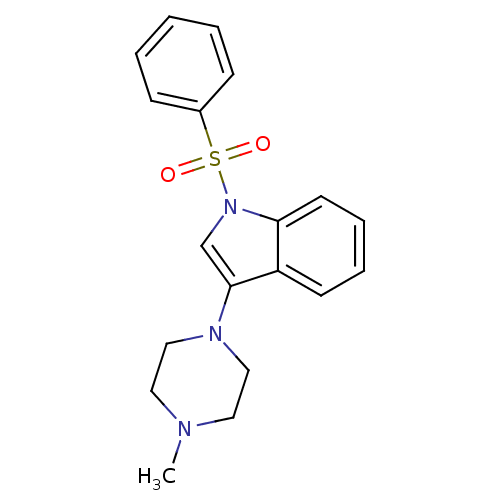
(3-(4-methylpiperazin-1-yl)-1-(phenylsulfonyl)-1H-i...)Show SMILES CN1CCN(CC1)c1cn(c2ccccc12)S(=O)(=O)c1ccccc1 Show InChI InChI=1S/C19H21N3O2S/c1-20-11-13-21(14-12-20)19-15-22(18-10-6-5-9-17(18)19)25(23,24)16-7-3-2-4-8-16/h2-10,15H,11-14H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]-LSD from human 5-HT6 receptor incubated for 1 hr by microbeta counting method |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.112916
BindingDB Entry DOI: 10.7270/Q22R3WD3 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50232674
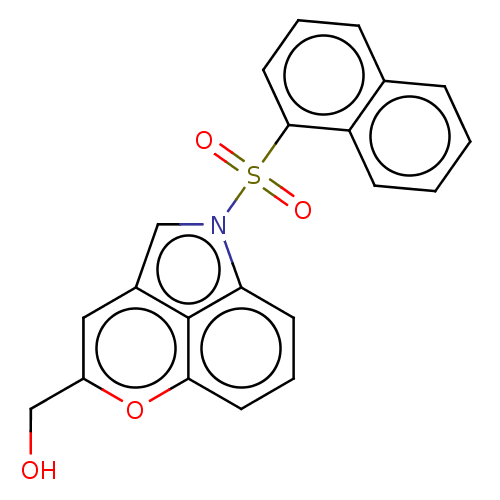
(CHEMBL4068850)Show SMILES OCc1cc2cn(c3cccc(o1)c23)S(=O)(=O)c1cccc2ccccc12 Show InChI InChI=1S/C21H15NO4S/c23-13-16-11-15-12-22(18-8-4-9-19(26-16)21(15)18)27(24,25)20-10-3-6-14-5-1-2-7-17(14)20/h1-12,23H,13H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Polish Academy of Sciences
Curated by ChEMBL
| Assay Description
Displacement of [3H]-LSD from 5-HT6 receptor (unknown origin) |
ACS Med Chem Lett 8: 390-394 (2017)
Article DOI: 10.1021/acsmedchemlett.6b00482
BindingDB Entry DOI: 10.7270/Q2DN479B |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50232673
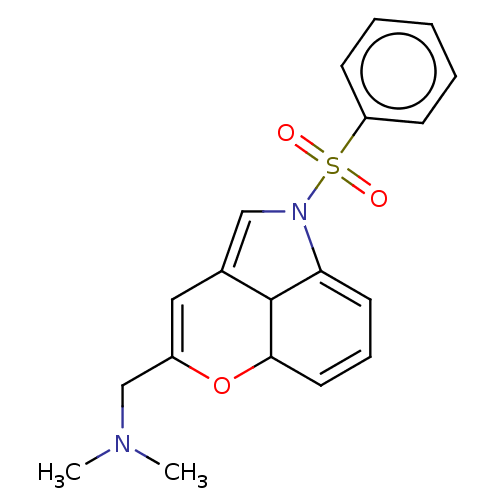
(CHEMBL4061189)Show SMILES CN(C)CC1=CC2=CN(C3=CC=CC(O1)C23)S(=O)(=O)c1ccccc1 |c:11,t:4,6,9| Show InChI InChI=1S/C19H20N2O3S/c1-20(2)13-15-11-14-12-21(17-9-6-10-18(24-15)19(14)17)25(22,23)16-7-4-3-5-8-16/h3-12,18-19H,13H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Polish Academy of Sciences
Curated by ChEMBL
| Assay Description
Displacement of [3H]-LSD from 5-HT6 receptor (unknown origin) |
ACS Med Chem Lett 8: 390-394 (2017)
Article DOI: 10.1021/acsmedchemlett.6b00482
BindingDB Entry DOI: 10.7270/Q2DN479B |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50504843

(CHEMBL4438434)Show SMILES Cc1ccc2[nH]cc(CCNCc3ccc(o3)-c3ccc(O)cc3)c2c1 Show InChI InChI=1S/C22H22N2O2/c1-15-2-8-21-20(12-15)17(13-24-21)10-11-23-14-19-7-9-22(26-19)16-3-5-18(25)6-4-16/h2-9,12-13,23-25H,10-11,14H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Polish Academy of Sciences
Curated by ChEMBL
| Assay Description
Displacement of [3H]-LSD from human 5-HT6R expressed in HEK293 cells after 1 hr by microbeta plate reader analysis |
Eur J Med Chem 185: (2020)
Article DOI: 10.1016/j.ejmech.2019.111857
BindingDB Entry DOI: 10.7270/Q2833W92 |
More data for this
Ligand-Target Pair | |
Procathepsin L
(Homo sapiens (Human)) | BDBM50463238

(CHEMBL4238973)Show SMILES CSCC[C@H](NC(=O)[C@@H]1CCCN1C(=O)[C@H](Cc1c[nH]cn1)NC(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)CNC(=O)CNC(C)=O)C(=O)NNC(=O)N[C@@H](C)C(=O)N1CCC[C@H]1C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@H](C)C(=O)N[C@H](CCCNC(N)=N)C(N)=O |r| Show InChI InChI=1S/C67H92N22O16S/c1-35(57(96)81-45(56(68)95)16-9-22-72-66(69)70)78-58(97)46(19-20-55(93)94)82-60(99)49(27-39-30-75-44-15-8-6-13-42(39)44)84-63(102)51-17-10-23-88(51)64(103)36(2)79-67(105)87-86-61(100)47(21-25-106-4)83-62(101)52-18-11-24-89(52)65(104)50(28-40-31-71-34-77-40)85-59(98)48(26-38-29-74-43-14-7-5-12-41(38)43)80-54(92)33-76-53(91)32-73-37(3)90/h5-8,12-15,29-31,34-36,45-52,74-75H,9-11,16-28,32-33H2,1-4H3,(H2,68,95)(H,71,77)(H,73,90)(H,76,91)(H,78,97)(H,80,92)(H,81,96)(H,82,99)(H,83,101)(H,84,102)(H,85,98)(H,86,100)(H,93,94)(H4,69,70,72)(H2,79,87,105)/t35-,36+,45-,46+,47+,48+,49+,50+,51+,52+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ d'Orl£ans
Curated by ChEMBL
| Assay Description
Inhibition of human cathepsin L using Z-Phe-Arg-AMC as substrate after 30 mins by Cheng-Prusoff equation analysis |
Bioorg Med Chem 26: 4310-4319 (2018)
Article DOI: 10.1016/j.bmc.2018.07.032
BindingDB Entry DOI: 10.7270/Q2P271RF |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Homo sapiens (Human)) | BDBM50578573

(CHEMBL4869180) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]citalopram from human SERT in HEK293 cells by Topcount scintillation analysis |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113533
BindingDB Entry DOI: 10.7270/Q24T6P6C |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Homo sapiens (Human)) | BDBM35254

(2-methyl-4-(4-methylpiperazin-1-yl)-10H-thieno[2,3...)Show InChI InChI=1S/C17H20N4S/c1-12-11-13-16(21-9-7-20(2)8-10-21)18-14-5-3-4-6-15(14)19-17(13)22-12/h3-6,11,19H,7-10H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 6.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Polish Academy of Sciences
Curated by ChEMBL
| Assay Description
Binding affinity to human 5-HT2AR expressed in HEK293 cells by competitive binding assay |
Eur J Med Chem 185: (2020)
Article DOI: 10.1016/j.ejmech.2019.111857
BindingDB Entry DOI: 10.7270/Q2833W92 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Homo sapiens (Human)) | BDBM35254

(2-methyl-4-(4-methylpiperazin-1-yl)-10H-thieno[2,3...)Show InChI InChI=1S/C17H20N4S/c1-12-11-13-16(21-9-7-20(2)8-10-21)18-14-5-3-4-6-15(14)19-17(13)22-12/h3-6,11,19H,7-10H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 6.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Polish Academy of Sciences
Curated by ChEMBL
| Assay Description
Binding affinity to 5HT2A receptor (unknown origin) |
ACS Med Chem Lett 8: 390-394 (2017)
Article DOI: 10.1021/acsmedchemlett.6b00482
BindingDB Entry DOI: 10.7270/Q2DN479B |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50504847

(CHEMBL4516220)Show InChI InChI=1S/C18H25N7/c1-23-8-10-25(11-9-23)18-21-16(15-13-20-24(2)17(15)22-18)19-12-14-6-4-3-5-7-14/h3-7,20H,8-13H2,1-2H3,(H,19,21,22) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Polish Academy of Sciences
Curated by ChEMBL
| Assay Description
Binding affinity to 5-HT6R (unknown origin) assessed as inhibitory constant |
Eur J Med Chem 185: (2020)
Article DOI: 10.1016/j.ejmech.2019.111857
BindingDB Entry DOI: 10.7270/Q2833W92 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50504814

(CHEMBL4465414)Show InChI InChI=1S/C21H20N2O2/c24-17-7-5-15(6-8-17)21-10-9-18(25-21)14-22-12-11-16-13-23-20-4-2-1-3-19(16)20/h1-10,13,22-24H,11-12,14H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Polish Academy of Sciences
Curated by ChEMBL
| Assay Description
Displacement of [3H]-LSD from human 5-HT6R expressed in HEK293 cells after 1 hr by microbeta plate reader analysis |
Eur J Med Chem 185: (2020)
Article DOI: 10.1016/j.ejmech.2019.111857
BindingDB Entry DOI: 10.7270/Q2833W92 |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Homo sapiens (Human)) | BDBM50578564
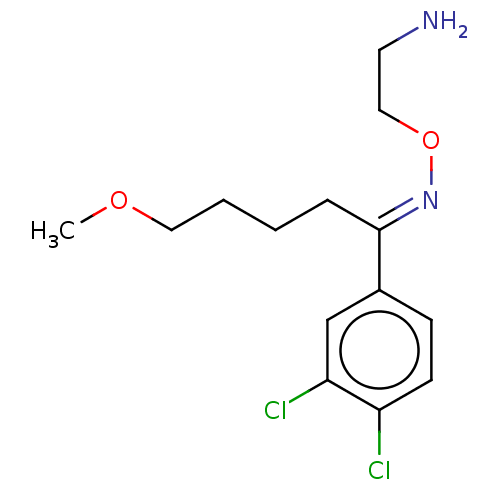
(CHEMBL4853402) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 9 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]citalopram from human SERT in HEK293 cells by Topcount scintillation analysis |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113533
BindingDB Entry DOI: 10.7270/Q24T6P6C |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Homo sapiens (Human)) | BDBM35254

(2-methyl-4-(4-methylpiperazin-1-yl)-10H-thieno[2,3...)Show InChI InChI=1S/C17H20N4S/c1-12-11-13-16(21-9-7-20(2)8-10-21)18-14-5-3-4-6-15(14)19-17(13)22-12/h3-6,11,19H,7-10H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| 9 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Jagiellonian University Medical College
Curated by ChEMBL
| Assay Description
Displacement of [3H]-Raclopride from human dopamine D2L receptor expressed in HEK293 cells |
Eur J Med Chem 112: 258-69 (2016)
Article DOI: 10.1016/j.ejmech.2016.02.024
BindingDB Entry DOI: 10.7270/Q2KH0Q50 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50562696
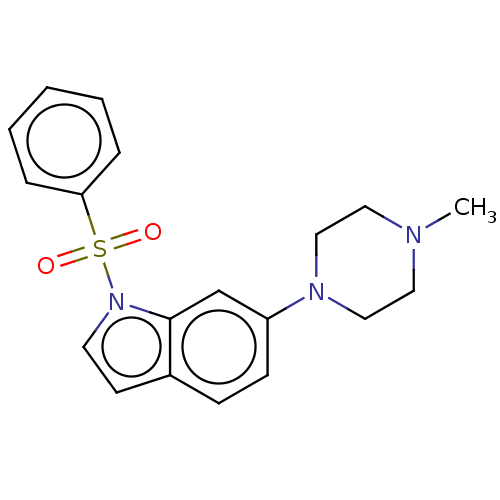
(CHEMBL4759208)Show SMILES CN1CCN(CC1)c1ccc2ccn(c2c1)S(=O)(=O)c1ccccc1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 9 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]-LSD from human 5-HT6 receptor incubated for 1 hr by microbeta counting method |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.112916
BindingDB Entry DOI: 10.7270/Q22R3WD3 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 7
(Homo sapiens (Human)) | BDBM50151315
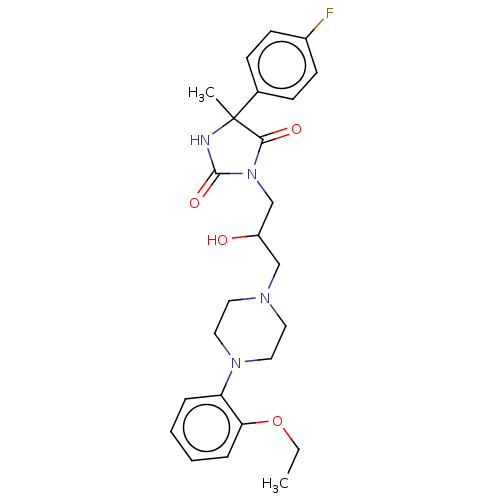
(CHEMBL3769534)Show SMILES CCOc1ccccc1N1CCN(CC(O)CN2C(=O)NC(C)(C2=O)c2ccc(F)cc2)CC1 Show InChI InChI=1S/C25H31FN4O4/c1-3-34-22-7-5-4-6-21(22)29-14-12-28(13-15-29)16-20(31)17-30-23(32)25(2,27-24(30)33)18-8-10-19(26)11-9-18/h4-11,20,31H,3,12-17H2,1-2H3,(H,27,33) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Jagiellonian University Medical College
Curated by ChEMBL
| Assay Description
Displacement of [3H]-5-CT from human 5-HT7 receptor expressed in HEK293 cells |
Eur J Med Chem 112: 258-69 (2016)
Article DOI: 10.1016/j.ejmech.2016.02.024
BindingDB Entry DOI: 10.7270/Q2KH0Q50 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Homo sapiens (Human)) | BDBM50504838

(CHEMBL4437523)Show SMILES Oc1ccc(cc1)-c1ccc(CNCCc2c[nH]c3ccc(F)cc23)o1 Show InChI InChI=1S/C21H19FN2O2/c22-16-3-7-20-19(11-16)15(12-24-20)9-10-23-13-18-6-8-21(26-18)14-1-4-17(25)5-2-14/h1-8,11-12,23-25H,9-10,13H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Polish Academy of Sciences
Curated by ChEMBL
| Assay Description
Binding affinity to human 5-HT2AR expressed in HEK293 cells by competitive binding assay |
Eur J Med Chem 185: (2020)
Article DOI: 10.1016/j.ejmech.2019.111857
BindingDB Entry DOI: 10.7270/Q2833W92 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM35254

(2-methyl-4-(4-methylpiperazin-1-yl)-10H-thieno[2,3...)Show InChI InChI=1S/C17H20N4S/c1-12-11-13-16(21-9-7-20(2)8-10-21)18-14-5-3-4-6-15(14)19-17(13)22-12/h3-6,11,19H,7-10H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Polish Academy of Sciences
Curated by ChEMBL
| Assay Description
Displacement of [3H]-LSD from 5-HT6 receptor (unknown origin) |
ACS Med Chem Lett 8: 390-394 (2017)
Article DOI: 10.1021/acsmedchemlett.6b00482
BindingDB Entry DOI: 10.7270/Q2DN479B |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM35254

(2-methyl-4-(4-methylpiperazin-1-yl)-10H-thieno[2,3...)Show InChI InChI=1S/C17H20N4S/c1-12-11-13-16(21-9-7-20(2)8-10-21)18-14-5-3-4-6-15(14)19-17(13)22-12/h3-6,11,19H,7-10H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Polish Academy of Sciences
Curated by ChEMBL
| Assay Description
Displacement of [3H]-LSD from human 5-HT6R expressed in HEK293 cells after 1 hr by microbeta plate reader analysis |
Eur J Med Chem 185: (2020)
Article DOI: 10.1016/j.ejmech.2019.111857
BindingDB Entry DOI: 10.7270/Q2833W92 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50504816

(CHEMBL4474618)Show SMILES COc1cccc2[nH]cc(CCNCc3ccc(o3)-c3ccc(O)cc3)c12 Show InChI InChI=1S/C22H22N2O3/c1-26-21-4-2-3-19-22(21)16(13-24-19)11-12-23-14-18-9-10-20(27-18)15-5-7-17(25)8-6-15/h2-10,13,23-25H,11-12,14H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 14 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Polish Academy of Sciences
Curated by ChEMBL
| Assay Description
Displacement of [3H]-LSD from human 5-HT6R expressed in HEK293 cells after 1 hr by microbeta plate reader analysis |
Eur J Med Chem 185: (2020)
Article DOI: 10.1016/j.ejmech.2019.111857
BindingDB Entry DOI: 10.7270/Q2833W92 |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Homo sapiens (Human)) | BDBM50578556

(CHEMBL4868210) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 14 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]citalopram from human SERT in HEK293 cells by Topcount scintillation analysis |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113533
BindingDB Entry DOI: 10.7270/Q24T6P6C |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 7
(Homo sapiens (Human)) | BDBM50151314
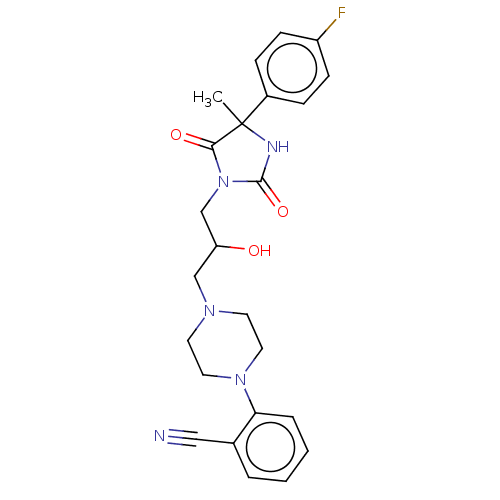
(CHEMBL3770639)Show SMILES CC1(NC(=O)N(CC(O)CN2CCN(CC2)c2ccccc2C#N)C1=O)c1ccc(F)cc1 Show InChI InChI=1S/C24H26FN5O3/c1-24(18-6-8-19(25)9-7-18)22(32)30(23(33)27-24)16-20(31)15-28-10-12-29(13-11-28)21-5-3-2-4-17(21)14-26/h2-9,20,31H,10-13,15-16H2,1H3,(H,27,33) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 14 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Jagiellonian University Medical College
Curated by ChEMBL
| Assay Description
Displacement of [3H]-5-CT from human 5-HT7 receptor expressed in HEK293 cells |
Eur J Med Chem 112: 258-69 (2016)
Article DOI: 10.1016/j.ejmech.2016.02.024
BindingDB Entry DOI: 10.7270/Q2KH0Q50 |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Homo sapiens (Human)) | BDBM50578561

(CHEMBL4850522) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 15 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]citalopram from human SERT in HEK293 cells by Topcount scintillation analysis |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113533
BindingDB Entry DOI: 10.7270/Q24T6P6C |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 7
(Homo sapiens (Human)) | BDBM50151313
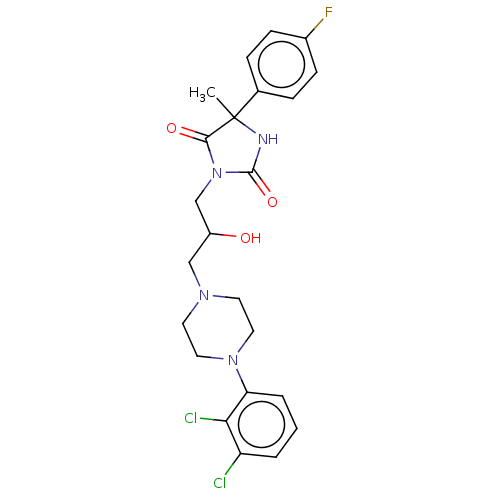
(CHEMBL3771309)Show SMILES CC1(NC(=O)N(CC(O)CN2CCN(CC2)c2cccc(Cl)c2Cl)C1=O)c1ccc(F)cc1 Show InChI InChI=1S/C23H25Cl2FN4O3/c1-23(15-5-7-16(26)8-6-15)21(32)30(22(33)27-23)14-17(31)13-28-9-11-29(12-10-28)19-4-2-3-18(24)20(19)25/h2-8,17,31H,9-14H2,1H3,(H,27,33) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 17 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Jagiellonian University Medical College
Curated by ChEMBL
| Assay Description
Displacement of [3H]-5-CT from human 5-HT7 receptor expressed in HEK293 cells |
Eur J Med Chem 112: 258-69 (2016)
Article DOI: 10.1016/j.ejmech.2016.02.024
BindingDB Entry DOI: 10.7270/Q2KH0Q50 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 7
(Homo sapiens (Human)) | BDBM50001884

(2-[4-(4-Methyl-benzyl)-piperazin-1-yl]-1-(2-methyl...)Show SMILES CN1CCN(CC1)C1=Nc2cc(Cl)ccc2Nc2ccccc12 |t:8| Show InChI InChI=1S/C18H19ClN4/c1-22-8-10-23(11-9-22)18-14-4-2-3-5-15(14)20-16-7-6-13(19)12-17(16)21-18/h2-7,12,20H,8-11H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| 18 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Jagiellonian University Medical College
Curated by ChEMBL
| Assay Description
Displacement of [3H]-5-CT from human 5-HT7 receptor expressed in HEK293 cells |
Eur J Med Chem 112: 258-69 (2016)
Article DOI: 10.1016/j.ejmech.2016.02.024
BindingDB Entry DOI: 10.7270/Q2KH0Q50 |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Homo sapiens (Human)) | BDBM50578552
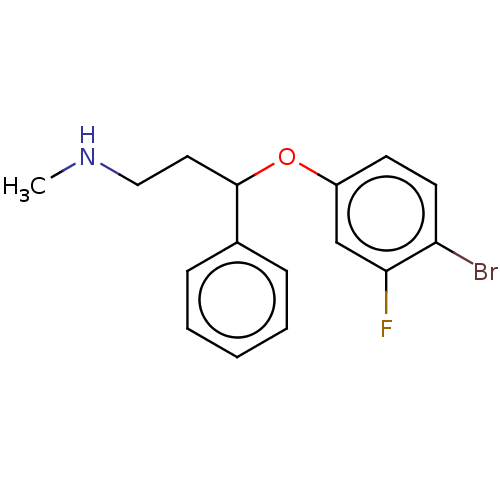
(CHEMBL4853473) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 19 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]citalopram from human SERT in HEK293 cells by Topcount scintillation analysis |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113533
BindingDB Entry DOI: 10.7270/Q24T6P6C |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1A
(Homo sapiens (Human)) | BDBM50001859
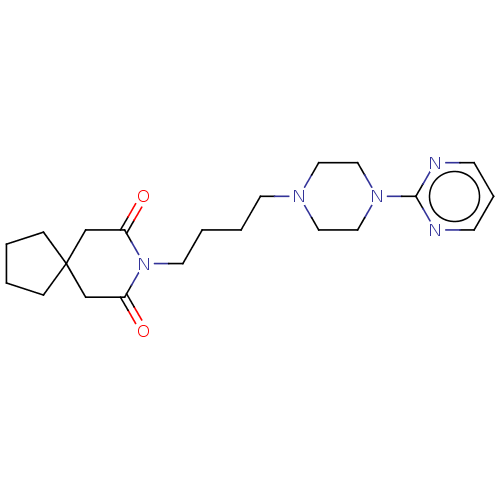
((buspirone) 8-[4-(4-Pyrimidin-2-yl-piperazin-1-yl)...)Show SMILES O=C1CC2(CCCC2)CC(=O)N1CCCCN1CCN(CC1)c1ncccn1 Show InChI InChI=1S/C21H31N5O2/c27-18-16-21(6-1-2-7-21)17-19(28)26(18)11-4-3-10-24-12-14-25(15-13-24)20-22-8-5-9-23-20/h5,8-9H,1-4,6-7,10-17H2 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Jagiellonian University Medical College
Curated by ChEMBL
| Assay Description
Displacement of [3H]-8-OH-DPAT from human 5-HT1A receptor expressed in HEK293 cells |
Eur J Med Chem 112: 258-69 (2016)
Article DOI: 10.1016/j.ejmech.2016.02.024
BindingDB Entry DOI: 10.7270/Q2KH0Q50 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50504849
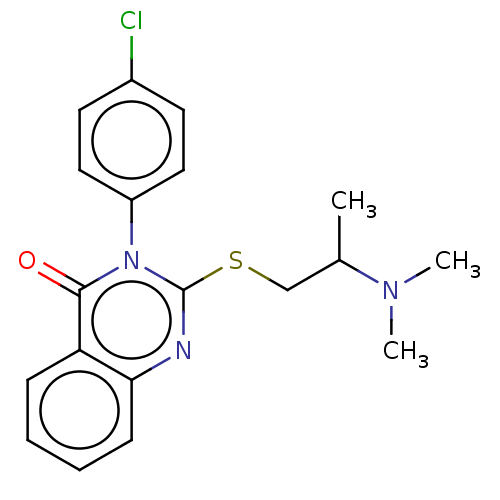
(CHEMBL4434670)Show InChI InChI=1S/C19H20ClN3OS/c1-13(22(2)3)12-25-19-21-17-7-5-4-6-16(17)18(24)23(19)15-10-8-14(20)9-11-15/h4-11,13H,12H2,1-3H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
| Article
PubMed
| 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Polish Academy of Sciences
Curated by ChEMBL
| Assay Description
Binding affinity to 5-HT6R (unknown origin) assessed as inhibitory constant |
Eur J Med Chem 185: (2020)
Article DOI: 10.1016/j.ejmech.2019.111857
BindingDB Entry DOI: 10.7270/Q2833W92 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50232672
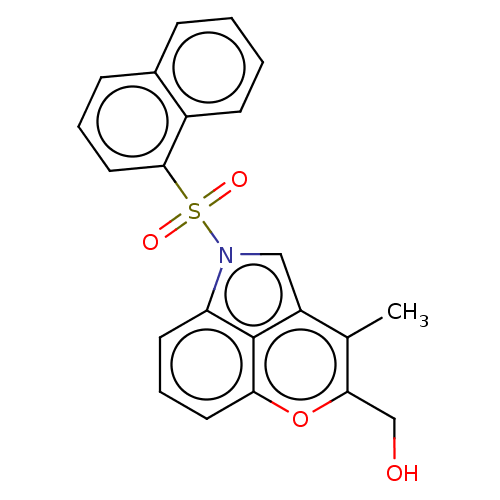
(CHEMBL4090862)Show SMILES Cc1c(CO)oc2cccc3n(cc1c23)S(=O)(=O)c1cccc2ccccc12 Show InChI InChI=1S/C22H17NO4S/c1-14-17-12-23(18-9-5-10-19(22(17)18)27-20(14)13-24)28(25,26)21-11-4-7-15-6-2-3-8-16(15)21/h2-12,24H,13H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Polish Academy of Sciences
Curated by ChEMBL
| Assay Description
Displacement of [3H]-LSD from 5-HT6 receptor (unknown origin) |
ACS Med Chem Lett 8: 390-394 (2017)
Article DOI: 10.1021/acsmedchemlett.6b00482
BindingDB Entry DOI: 10.7270/Q2DN479B |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Homo sapiens (Human)) | BDBM50578563
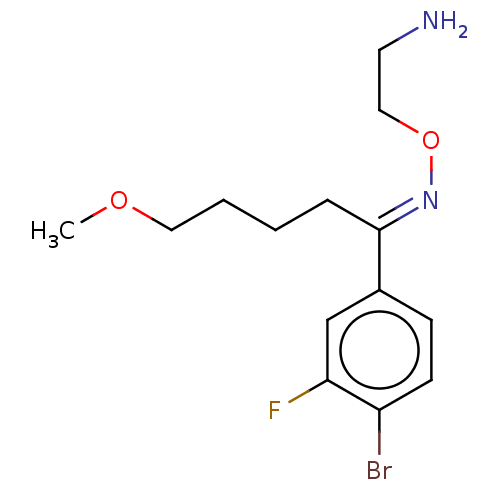
(CHEMBL4877693) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 21 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]citalopram from human SERT in HEK293 cells by Topcount scintillation analysis |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113533
BindingDB Entry DOI: 10.7270/Q24T6P6C |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50504851
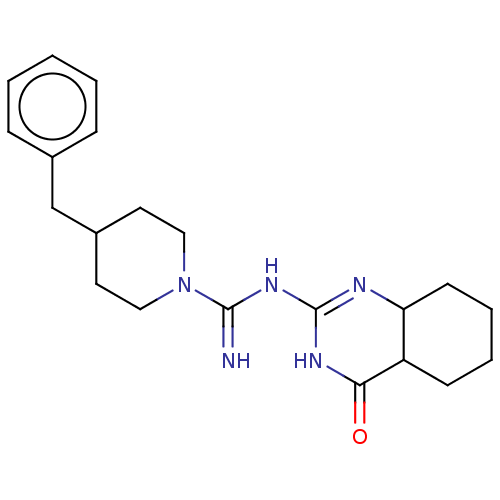
(CHEMBL4483706)Show SMILES N=C(NC1=NC2CCCCC2C(=O)N1)N1CCC(Cc2ccccc2)CC1 |t:3| Show InChI InChI=1S/C21H29N5O/c22-20(25-21-23-18-9-5-4-8-17(18)19(27)24-21)26-12-10-16(11-13-26)14-15-6-2-1-3-7-15/h1-3,6-7,16-18H,4-5,8-14H2,(H3,22,23,24,25,27) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 21 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Polish Academy of Sciences
Curated by ChEMBL
| Assay Description
Binding affinity to 5-HT6R (unknown origin) assessed as inhibitory constant |
Eur J Med Chem 185: (2020)
Article DOI: 10.1016/j.ejmech.2019.111857
BindingDB Entry DOI: 10.7270/Q2833W92 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50504812
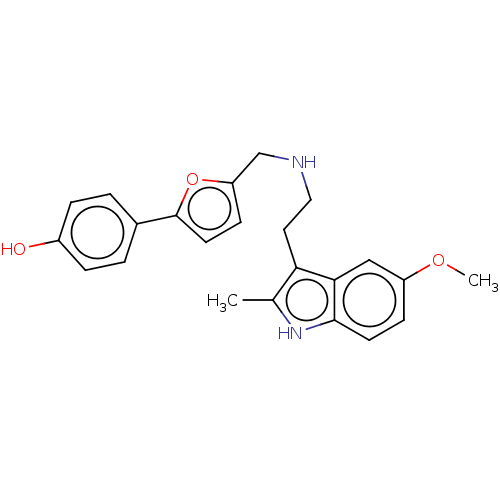
(CHEMBL4536315)Show SMILES COc1ccc2[nH]c(C)c(CCNCc3ccc(o3)-c3ccc(O)cc3)c2c1 Show InChI InChI=1S/C23H24N2O3/c1-15-20(21-13-18(27-2)7-9-22(21)25-15)11-12-24-14-19-8-10-23(28-19)16-3-5-17(26)6-4-16/h3-10,13,24-26H,11-12,14H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 21 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Polish Academy of Sciences
Curated by ChEMBL
| Assay Description
Displacement of [3H]-LSD from human 5-HT6R expressed in HEK293 cells after 1 hr by microbeta plate reader analysis |
Eur J Med Chem 185: (2020)
Article DOI: 10.1016/j.ejmech.2019.111857
BindingDB Entry DOI: 10.7270/Q2833W92 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Homo sapiens (Human)) | BDBM50504843

(CHEMBL4438434)Show SMILES Cc1ccc2[nH]cc(CCNCc3ccc(o3)-c3ccc(O)cc3)c2c1 Show InChI InChI=1S/C22H22N2O2/c1-15-2-8-21-20(12-15)17(13-24-21)10-11-23-14-19-7-9-22(26-19)16-3-5-18(25)6-4-16/h2-9,12-13,23-25H,10-11,14H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 22 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Polish Academy of Sciences
Curated by ChEMBL
| Assay Description
Binding affinity to human 5-HT2AR expressed in HEK293 cells by competitive binding assay |
Eur J Med Chem 185: (2020)
Article DOI: 10.1016/j.ejmech.2019.111857
BindingDB Entry DOI: 10.7270/Q2833W92 |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Homo sapiens (Human)) | BDBM50578570

(CHEMBL4853132) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 23 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]citalopram from human SERT in HEK293 cells by Topcount scintillation analysis |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113533
BindingDB Entry DOI: 10.7270/Q24T6P6C |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Homo sapiens (Human)) | BDBM50578554
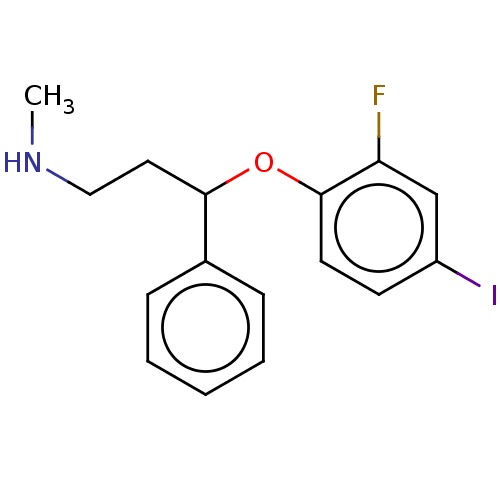
(CHEMBL4848149) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 23 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]citalopram from human SERT in HEK293 cells by Topcount scintillation analysis |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113533
BindingDB Entry DOI: 10.7270/Q24T6P6C |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Homo sapiens (Human)) | BDBM50578566
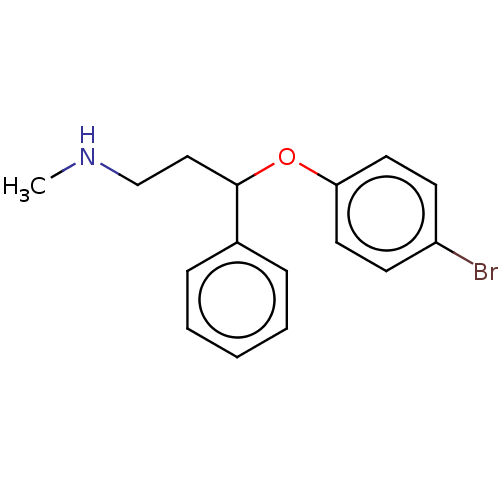
(CHEMBL4850285) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 24 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]citalopram from human SERT in HEK293 cells by Topcount scintillation analysis |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113533
BindingDB Entry DOI: 10.7270/Q24T6P6C |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50504810
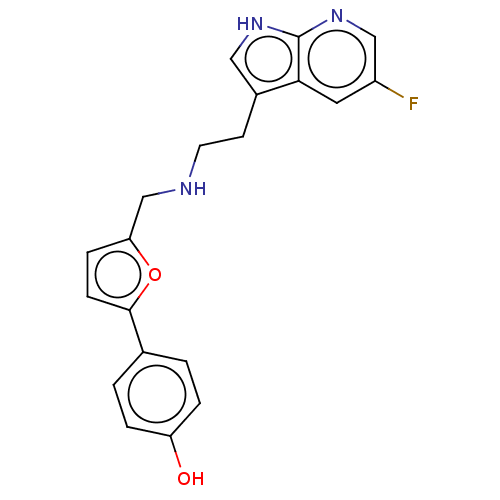
(CHEMBL4442855)Show SMILES Oc1ccc(cc1)-c1ccc(CNCCc2c[nH]c3ncc(F)cc23)o1 Show InChI InChI=1S/C20H18FN3O2/c21-15-9-18-14(10-23-20(18)24-11-15)7-8-22-12-17-5-6-19(26-17)13-1-3-16(25)4-2-13/h1-6,9-11,22,25H,7-8,12H2,(H,23,24) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| 25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Polish Academy of Sciences
Curated by ChEMBL
| Assay Description
Displacement of [3H]-LSD from human 5-HT6R expressed in HEK293 cells after 1 hr by microbeta plate reader analysis |
Eur J Med Chem 185: (2020)
Article DOI: 10.1016/j.ejmech.2019.111857
BindingDB Entry DOI: 10.7270/Q2833W92 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50562703
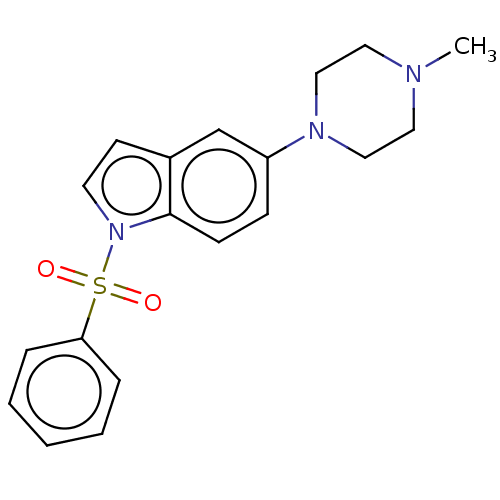
(CHEMBL4753562)Show SMILES CN1CCN(CC1)c1ccc2n(ccc2c1)S(=O)(=O)c1ccccc1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 27 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]-LSD from human 5-HT6 receptor incubated for 1 hr by microbeta counting method |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.112916
BindingDB Entry DOI: 10.7270/Q22R3WD3 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50232667

(CHEMBL4060194)Show SMILES CCN(CC)Cc1cc2cn(c3cccc(o1)c23)S(=O)(=O)c1ccccc1 Show InChI InChI=1S/C21H22N2O3S/c1-3-22(4-2)15-17-13-16-14-23(19-11-8-12-20(26-17)21(16)19)27(24,25)18-9-6-5-7-10-18/h5-14H,3-4,15H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 28 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Polish Academy of Sciences
Curated by ChEMBL
| Assay Description
Displacement of [3H]-LSD from 5-HT6 receptor (unknown origin) |
ACS Med Chem Lett 8: 390-394 (2017)
Article DOI: 10.1021/acsmedchemlett.6b00482
BindingDB Entry DOI: 10.7270/Q2DN479B |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Homo sapiens (Human)) | BDBM50504834
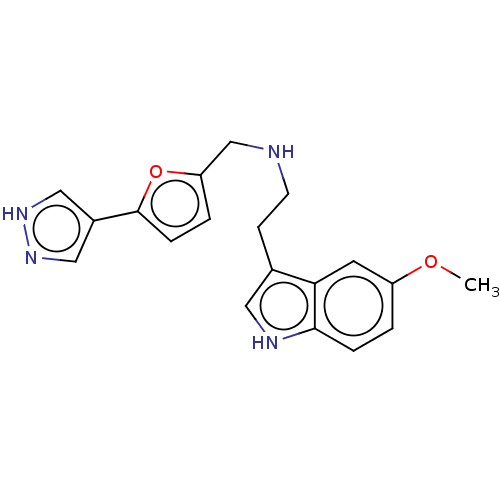
(CHEMBL4591410)Show SMILES COc1ccc2[nH]cc(CCNCc3ccc(o3)-c3cn[nH]c3)c2c1 Show InChI InChI=1S/C19H20N4O2/c1-24-15-2-4-18-17(8-15)13(9-21-18)6-7-20-12-16-3-5-19(25-16)14-10-22-23-11-14/h2-5,8-11,20-21H,6-7,12H2,1H3,(H,22,23) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 28 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Polish Academy of Sciences
Curated by ChEMBL
| Assay Description
Binding affinity to human 5-HT2AR expressed in HEK293 cells by competitive binding assay |
Eur J Med Chem 185: (2020)
Article DOI: 10.1016/j.ejmech.2019.111857
BindingDB Entry DOI: 10.7270/Q2833W92 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data