

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50483552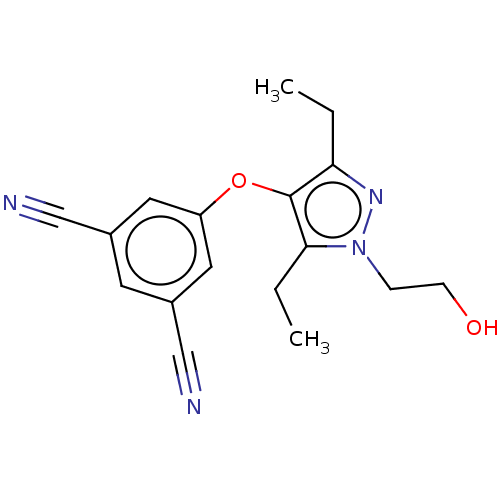 (Lersivirine | UK-453061) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | Article PubMed | 117 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Mixed non-competitive inhibition of HIV1 reverse transcriptase activity | Antimicrob Agents Chemother 54: 4451-63 (2010) Article DOI: 10.1128/AAC.01455-09 BindingDB Entry DOI: 10.7270/Q2WQ06MG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50334975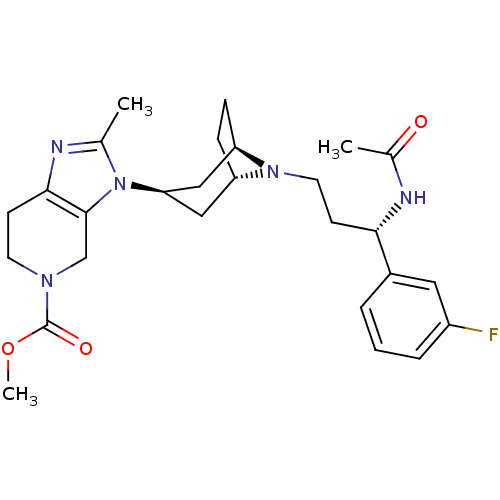 (CHEMBL1649924 | endo-methyl 3-(8-((S)-3-acetamido-...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Antagonist activity at CCR5 receptor expressed in HeLa-P4 cells co-expressing CD4 assessed as inhibition of infusion to HIV gp120 expressed in CHO-ta... | J Med Chem 54: 67-77 (2011) Article DOI: 10.1021/jm100978n BindingDB Entry DOI: 10.7270/Q2RV0PNV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50334986 (4,4-Difluoro-cyclohexanecarboxylic acid {(S)-3-[(1...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Antagonist activity at CCR5 receptor expressed in HeLa-P4 cells co-expressing CD4 assessed as inhibition of infusion to HIV gp120 expressed in CHO-ta... | J Med Chem 54: 67-77 (2011) Article DOI: 10.1021/jm100978n BindingDB Entry DOI: 10.7270/Q2RV0PNV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50334974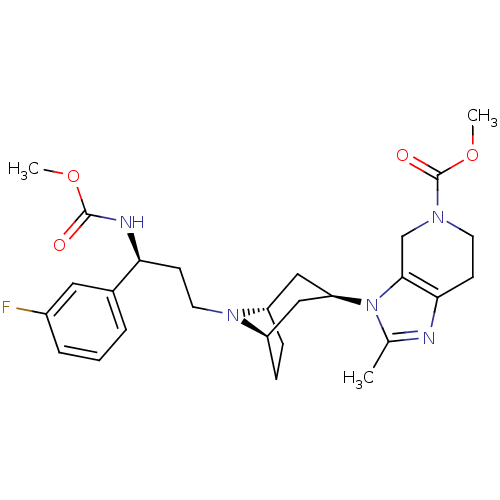 (CHEMBL1649927 | endo-methyl 3-(8-((S)-3-(3-fluorop...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Antagonist activity at CCR5 receptor expressed in HeLa-P4 cells co-expressing CD4 assessed as inhibition of infusion to HIV gp120 expressed in CHO-ta... | J Med Chem 54: 67-77 (2011) Article DOI: 10.1021/jm100978n BindingDB Entry DOI: 10.7270/Q2RV0PNV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50334973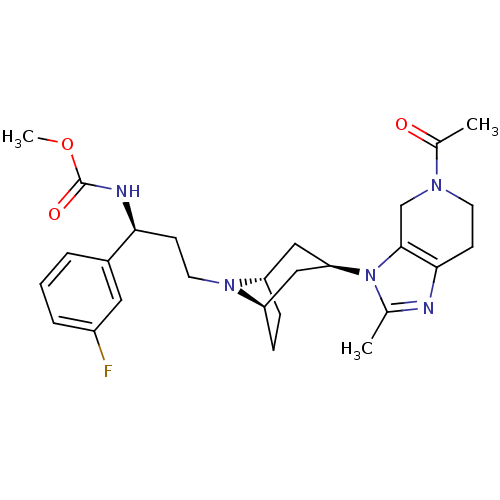 (CHEMBL1649926 | endo-methyl(S)-3-(3-(5-acetyl-2-me...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Antagonist activity at CCR5 receptor expressed in HeLa-P4 cells co-expressing CD4 assessed as inhibition of infusion to HIV gp120 expressed in CHO-ta... | J Med Chem 54: 67-77 (2011) Article DOI: 10.1021/jm100978n BindingDB Entry DOI: 10.7270/Q2RV0PNV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50334978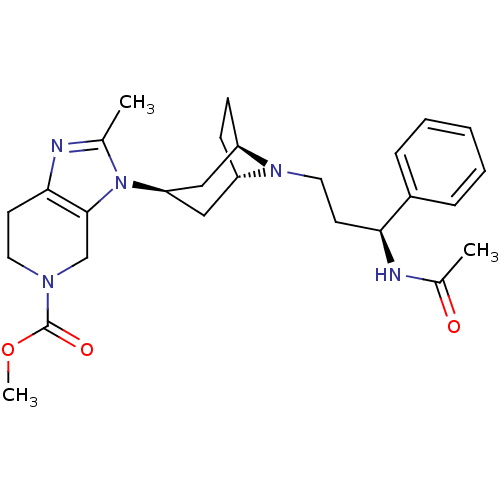 (CHEMBL1649922 | endo-methyl 3-(8-((S)-3-acetamido-...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Antagonist activity at CCR5 receptor expressed in HeLa-P4 cells co-expressing CD4 assessed as inhibition of infusion to HIV gp120 expressed in CHO-ta... | J Med Chem 54: 67-77 (2011) Article DOI: 10.1021/jm100978n BindingDB Entry DOI: 10.7270/Q2RV0PNV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50334979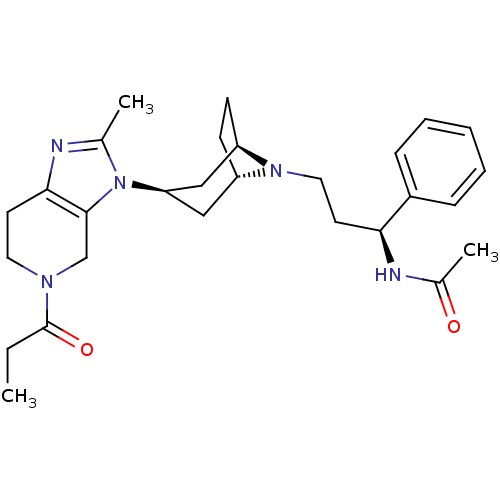 (CHEMBL1649920 | endo-N-((S)-3-(3-(2-methyl-5-propi...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Antagonist activity at CCR5 receptor expressed in HeLa-P4 cells co-expressing CD4 assessed as inhibition of infusion to HIV gp120 expressed in CHO-ta... | J Med Chem 54: 67-77 (2011) Article DOI: 10.1021/jm100978n BindingDB Entry DOI: 10.7270/Q2RV0PNV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50334977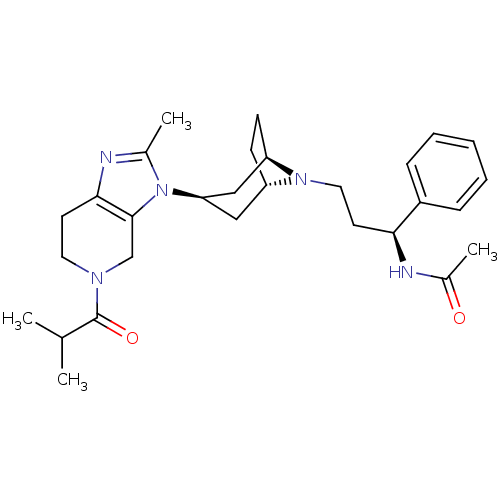 (CHEMBL1649921 | endo-N-((S)-3-(3-(5-isobutyryl-2-m...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Antagonist activity at CCR5 receptor expressed in HeLa-P4 cells co-expressing CD4 assessed as inhibition of infusion to HIV gp120 expressed in CHO-ta... | J Med Chem 54: 67-77 (2011) Article DOI: 10.1021/jm100978n BindingDB Entry DOI: 10.7270/Q2RV0PNV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50334980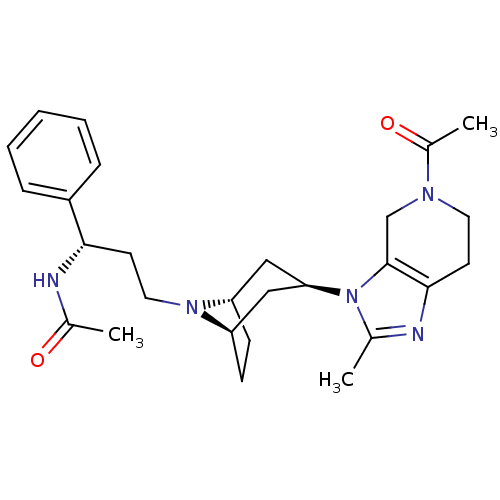 (CHEMBL1649919 | endo-N-((S)-3-(3-(5-acetyl-2-methy...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Antagonist activity at CCR5 receptor expressed in HeLa-P4 cells co-expressing CD4 assessed as inhibition of infusion to HIV gp120 expressed in CHO-ta... | J Med Chem 54: 67-77 (2011) Article DOI: 10.1021/jm100978n BindingDB Entry DOI: 10.7270/Q2RV0PNV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50334971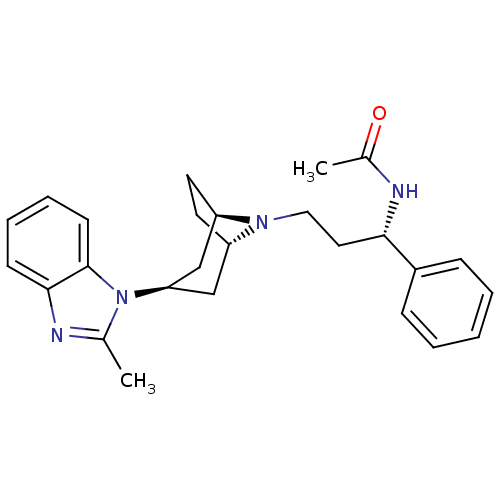 (CHEMBL1649913 | endo-N-((S)-3-(3-(2-methyl-1H-benz...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Antagonist activity at CCR5 receptor expressed in HeLa-P4 cells co-expressing CD4 assessed as inhibition of infusion to HIV gp120 expressed in CHO-ta... | J Med Chem 54: 67-77 (2011) Article DOI: 10.1021/jm100978n BindingDB Entry DOI: 10.7270/Q2RV0PNV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50334976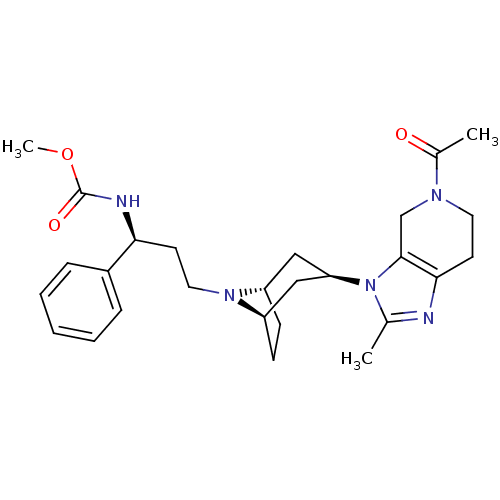 (CHEMBL1649925 | endo-methyl(S)-3-(3-(5-acetyl-2-me...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Antagonist activity at CCR5 receptor expressed in HeLa-P4 cells co-expressing CD4 assessed as inhibition of infusion to HIV gp120 expressed in CHO-ta... | J Med Chem 54: 67-77 (2011) Article DOI: 10.1021/jm100978n BindingDB Entry DOI: 10.7270/Q2RV0PNV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50334972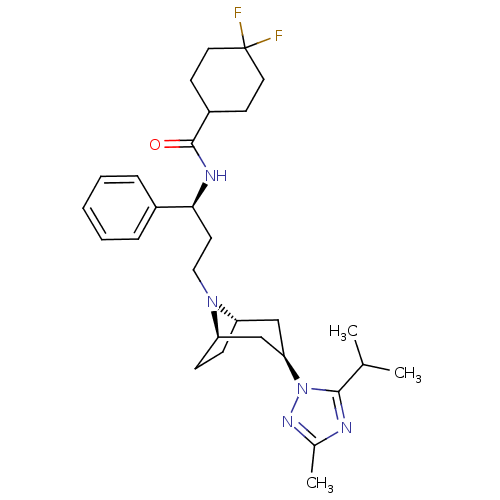 (CHEMBL1649912 | endo-4,4-difluoro-N-((S)-3-(3-(5-i...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Antagonist activity at CCR5 receptor expressed in HeLa-P4 cells co-expressing CD4 assessed as inhibition of infusion to HIV gp120 expressed in CHO-ta... | J Med Chem 54: 67-77 (2011) Article DOI: 10.1021/jm100978n BindingDB Entry DOI: 10.7270/Q2RV0PNV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50334982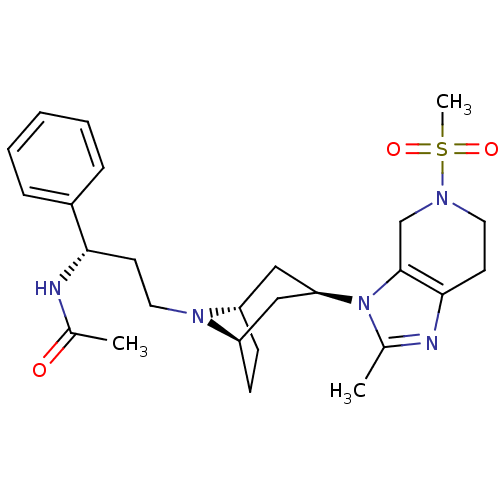 (CHEMBL1649918 | endo-N-((S)-3-(3-(2-methyl-5-(meth...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Antagonist activity at CCR5 receptor expressed in HeLa-P4 cells co-expressing CD4 assessed as inhibition of infusion to HIV gp120 expressed in CHO-ta... | J Med Chem 54: 67-77 (2011) Article DOI: 10.1021/jm100978n BindingDB Entry DOI: 10.7270/Q2RV0PNV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50334981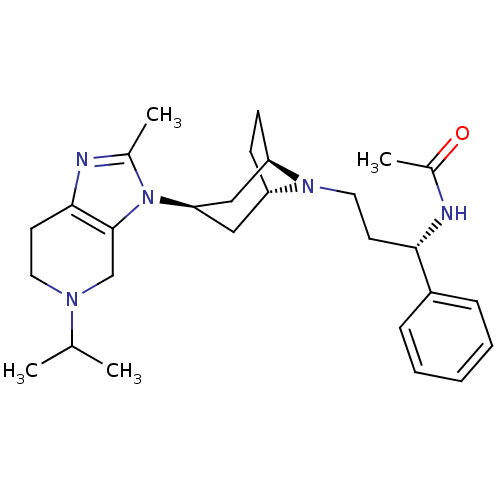 (CHEMBL1649917 | endo-N-((S)-3-(3-(5-isopropyl-2-me...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Antagonist activity at CCR5 receptor expressed in HeLa-P4 cells co-expressing CD4 assessed as inhibition of infusion to HIV gp120 expressed in CHO-ta... | J Med Chem 54: 67-77 (2011) Article DOI: 10.1021/jm100978n BindingDB Entry DOI: 10.7270/Q2RV0PNV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50334983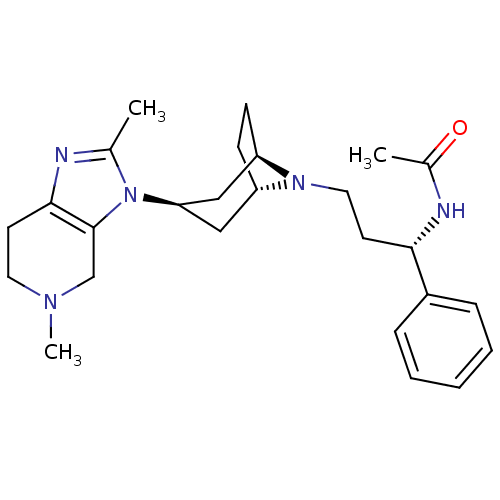 (CHEMBL1649916 | endo-N-((S)-3-(3-(2,5-dimethyl-4,5...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Antagonist activity at CCR5 receptor expressed in HeLa-P4 cells co-expressing CD4 assessed as inhibition of infusion to HIV gp120 expressed in CHO-ta... | J Med Chem 54: 67-77 (2011) Article DOI: 10.1021/jm100978n BindingDB Entry DOI: 10.7270/Q2RV0PNV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM2483 ((4S)-6-chloro-4-(2-cyclopropylethynyl)-4-(trifluor...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 7.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of HIV1 reverse transcriptase activity by primer extension assay | Antimicrob Agents Chemother 54: 4451-63 (2010) Article DOI: 10.1128/AAC.01455-09 BindingDB Entry DOI: 10.7270/Q2WQ06MG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50334985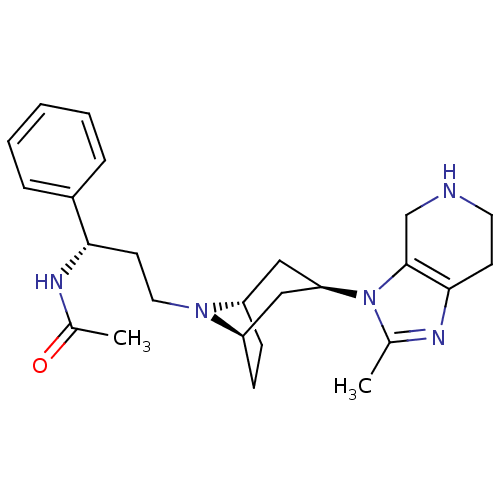 (CHEMBL1649915 | endo-N-((S)-3-(3-(2-methyl-4,5,6,7...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Antagonist activity at CCR5 receptor expressed in HeLa-P4 cells co-expressing CD4 assessed as inhibition of infusion to HIV gp120 expressed in CHO-ta... | J Med Chem 54: 67-77 (2011) Article DOI: 10.1021/jm100978n BindingDB Entry DOI: 10.7270/Q2RV0PNV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM50334969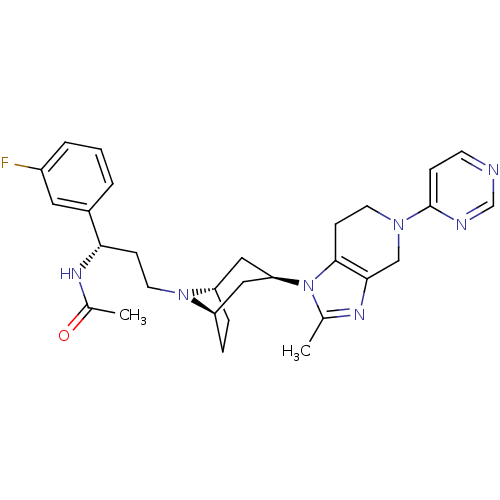 (CHEMBL1649928 | endo-N-((S)-1-(3-fluorophenyl)-3-(...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Displacement of [3H]-dofetilide from human ERG expressed in HEK293 cells | J Med Chem 54: 67-77 (2011) Article DOI: 10.1021/jm100978n BindingDB Entry DOI: 10.7270/Q2RV0PNV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [588-1027] (Human immunodeficiency virus type 1 group M subtyp...) | BDBM2483 ((4S)-6-chloro-4-(2-cyclopropylethynyl)-4-(trifluor...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article PubMed | n/a | n/a | 14 | n/a | n/a | n/a | n/a | 7.8 | 37 |
Pfizer | Assay Description The inhibition of the RNA-dependent DNA polymerase activity of the HIV-1 reverse transcriptase enzyme was measured using a primer extension assay. Th... | J Med Chem 52: 1219-23 (2009) Article DOI: 10.1021/jm801322h BindingDB Entry DOI: 10.7270/Q2707ZRW | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Gag-Pol polyprotein [588-1027] (Human immunodeficiency virus type 1 group M subtyp...) | BDBM27577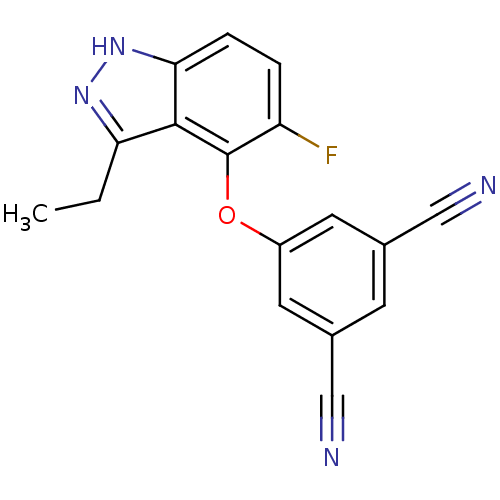 (5-[(3-ethyl-5-fluoro-1H-indazol-4-yl)oxy]benzene-1...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 25 | n/a | n/a | n/a | n/a | 7.8 | 37 |
Pfizer | Assay Description The inhibition of the RNA-dependent DNA polymerase activity of the HIV-1 reverse transcriptase enzyme was measured using a primer extension assay. Th... | J Med Chem 52: 1219-23 (2009) Article DOI: 10.1021/jm801322h BindingDB Entry DOI: 10.7270/Q2707ZRW | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50334984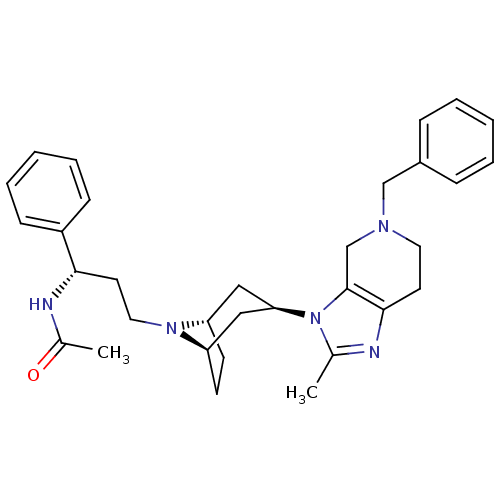 (CHEMBL1649914 | endo-N-((S)-3-(3-(5-benzyl-2-methy...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 29 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Antagonist activity at CCR5 receptor expressed in HeLa-P4 cells co-expressing CD4 assessed as inhibition of infusion to HIV gp120 expressed in CHO-ta... | J Med Chem 54: 67-77 (2011) Article DOI: 10.1021/jm100978n BindingDB Entry DOI: 10.7270/Q2RV0PNV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [588-1127,Y768C]/[588-1147,Y768C] (Human immunodeficiency virus type 1) | BDBM27577 (5-[(3-ethyl-5-fluoro-1H-indazol-4-yl)oxy]benzene-1...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 32 | n/a | n/a | n/a | n/a | 7.8 | 37 |
Pfizer | Assay Description The inhibition of the RNA-dependent DNA polymerase activity of the HIV-1 reverse transcriptase enzyme was measured using a primer extension assay. Th... | J Med Chem 52: 1219-23 (2009) Article DOI: 10.1021/jm801322h BindingDB Entry DOI: 10.7270/Q2707ZRW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [588-1127,Y768C]/[588-1147,Y768C] (Human immunodeficiency virus type 1) | BDBM2483 ((4S)-6-chloro-4-(2-cyclopropylethynyl)-4-(trifluor...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article PubMed | n/a | n/a | 40 | n/a | n/a | n/a | n/a | 7.8 | 37 |
Pfizer | Assay Description The inhibition of the RNA-dependent DNA polymerase activity of the HIV-1 reverse transcriptase enzyme was measured using a primer extension assay. Th... | J Med Chem 52: 1219-23 (2009) Article DOI: 10.1021/jm801322h BindingDB Entry DOI: 10.7270/Q2707ZRW | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Gag-Pol polyprotein [588-1027] (Human immunodeficiency virus type 1 group M subtyp...) | BDBM27579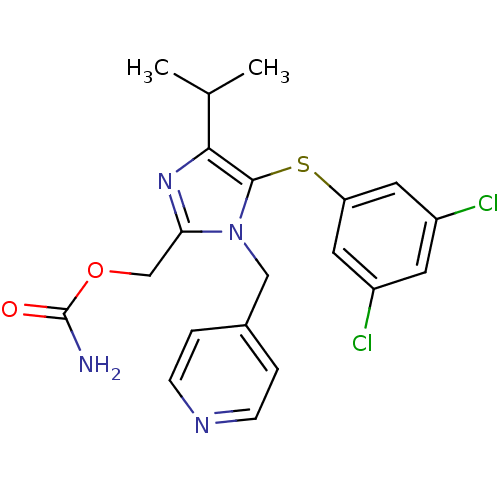 (AG 1549 | CPV | Capravirine | {5-[(3,5-dichlorophe...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | DrugBank MMDB PC cid PC sid PDB UniChem Patents | MMDB PDB Article PubMed | n/a | n/a | 47 | n/a | n/a | n/a | n/a | 7.8 | 37 |
Pfizer | Assay Description The inhibition of the RNA-dependent DNA polymerase activity of the HIV-1 reverse transcriptase enzyme was measured using a primer extension assay. Th... | J Med Chem 52: 1219-23 (2009) Article DOI: 10.1021/jm801322h BindingDB Entry DOI: 10.7270/Q2707ZRW | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Gag-Pol polyprotein [588-1027] (Human immunodeficiency virus type 1 group M subtyp...) | BDBM27576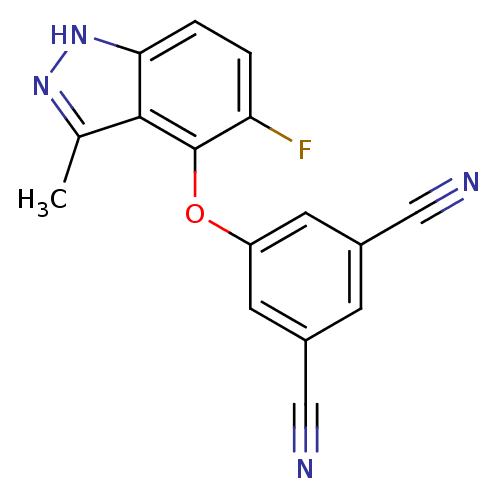 (5-[(5-fluoro-3-methyl-1H-indazol-4-yl)oxy]benzene-...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | DrugBank MMDB PC cid PC sid PDB UniChem Similars | MMDB PDB Article PubMed | n/a | n/a | 50 | n/a | n/a | n/a | n/a | 7.8 | 37 |
Pfizer | Assay Description The inhibition of the RNA-dependent DNA polymerase activity of the HIV-1 reverse transcriptase enzyme was measured using a primer extension assay. Th... | J Med Chem 52: 1219-23 (2009) Article DOI: 10.1021/jm801322h BindingDB Entry DOI: 10.7270/Q2707ZRW | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Gag-Pol polyprotein [588-1127,Y768C]/[588-1147,Y768C] (Human immunodeficiency virus type 1) | BDBM27579 (AG 1549 | CPV | Capravirine | {5-[(3,5-dichlorophe...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | DrugBank MMDB PC cid PC sid PDB UniChem Patents | MMDB PDB Article PubMed | n/a | n/a | 61 | n/a | n/a | n/a | n/a | 7.8 | 37 |
Pfizer | Assay Description The inhibition of the RNA-dependent DNA polymerase activity of the HIV-1 reverse transcriptase enzyme was measured using a primer extension assay. Th... | J Med Chem 52: 1219-23 (2009) Article DOI: 10.1021/jm801322h BindingDB Entry DOI: 10.7270/Q2707ZRW | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Gag-Pol polyprotein [588-1027,K691N]/[588-1147,K690N] (Human immunodeficiency virus type 1) | BDBM27579 (AG 1549 | CPV | Capravirine | {5-[(3,5-dichlorophe...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | DrugBank MMDB PC cid PC sid PDB UniChem Patents | MMDB PDB Article PubMed | n/a | n/a | 68 | n/a | n/a | n/a | n/a | 7.8 | 37 |
Pfizer | Assay Description The inhibition of the RNA-dependent DNA polymerase activity of the HIV-1 reverse transcriptase enzyme was measured using a primer extension assay. Th... | J Med Chem 52: 1219-23 (2009) Article DOI: 10.1021/jm801322h BindingDB Entry DOI: 10.7270/Q2707ZRW | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Gag-Pol polyprotein [588-1027] (Human immunodeficiency virus type 1 group M subtyp...) | BDBM27578 (5-{[7-(hydroxymethyl)-3-methyl-1H-indazol-4-yl]oxy...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 71 | n/a | n/a | n/a | n/a | 7.8 | 37 |
Pfizer | Assay Description The inhibition of the RNA-dependent DNA polymerase activity of the HIV-1 reverse transcriptase enzyme was measured using a primer extension assay. Th... | J Med Chem 52: 1219-23 (2009) Article DOI: 10.1021/jm801322h BindingDB Entry DOI: 10.7270/Q2707ZRW | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Gag-Pol polyprotein [588-1027] (Human immunodeficiency virus type 1 group M subtyp...) | BDBM27572 (5-[(3-ethyl-1H-indazol-4-yl)oxy]benzene-1,3-dicarb...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 112 | n/a | n/a | n/a | n/a | 7.8 | 37 |
Pfizer | Assay Description The inhibition of the RNA-dependent DNA polymerase activity of the HIV-1 reverse transcriptase enzyme was measured using a primer extension assay. Th... | J Med Chem 52: 1219-23 (2009) Article DOI: 10.1021/jm801322h BindingDB Entry DOI: 10.7270/Q2707ZRW | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50483552 (Lersivirine | UK-453061) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | Article PubMed | n/a | n/a | 118 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of HIV1 reverse transcriptase activity by primer extension assay | Antimicrob Agents Chemother 54: 4451-63 (2010) Article DOI: 10.1128/AAC.01455-09 BindingDB Entry DOI: 10.7270/Q2WQ06MG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [588-1127,Y768C]/[588-1147,Y768C] (Human immunodeficiency virus type 1) | BDBM27576 (5-[(5-fluoro-3-methyl-1H-indazol-4-yl)oxy]benzene-...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | DrugBank MMDB PC cid PC sid PDB UniChem Similars | MMDB PDB Article PubMed | n/a | n/a | 145 | n/a | n/a | n/a | n/a | 7.8 | 37 |
Pfizer | Assay Description The inhibition of the RNA-dependent DNA polymerase activity of the HIV-1 reverse transcriptase enzyme was measured using a primer extension assay. Th... | J Med Chem 52: 1219-23 (2009) Article DOI: 10.1021/jm801322h BindingDB Entry DOI: 10.7270/Q2707ZRW | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Gag-Pol polyprotein [588-1027,K691N]/[588-1147,K690N] (Human immunodeficiency virus type 1) | BDBM27577 (5-[(3-ethyl-5-fluoro-1H-indazol-4-yl)oxy]benzene-1...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 183 | n/a | n/a | n/a | n/a | 7.8 | 37 |
Pfizer | Assay Description The inhibition of the RNA-dependent DNA polymerase activity of the HIV-1 reverse transcriptase enzyme was measured using a primer extension assay. Th... | J Med Chem 52: 1219-23 (2009) Article DOI: 10.1021/jm801322h BindingDB Entry DOI: 10.7270/Q2707ZRW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase protein (Human immunodeficiency virus 1) | BDBM50483552 (Lersivirine | UK-453061) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | Article PubMed | n/a | n/a | 215 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of HIV1 HXB2 reverse transcriptase K103N mutant activity by primer extension assay | Antimicrob Agents Chemother 54: 4451-63 (2010) Article DOI: 10.1128/AAC.01455-09 BindingDB Entry DOI: 10.7270/Q2WQ06MG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [588-1027] (Human immunodeficiency virus type 1 group M subtyp...) | BDBM27571 (5-[(3-methyl-1H-indazol-4-yl)oxy]benzene-1,3-dicar...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 332 | n/a | n/a | n/a | n/a | 7.8 | 37 |
Pfizer | Assay Description The inhibition of the RNA-dependent DNA polymerase activity of the HIV-1 reverse transcriptase enzyme was measured using a primer extension assay. Th... | J Med Chem 52: 1219-23 (2009) Article DOI: 10.1021/jm801322h BindingDB Entry DOI: 10.7270/Q2707ZRW | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Gag-Pol polyprotein [588-1027,K691N]/[588-1147,K690N] (Human immunodeficiency virus type 1) | BDBM2483 ((4S)-6-chloro-4-(2-cyclopropylethynyl)-4-(trifluor...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article PubMed | n/a | n/a | 364 | n/a | n/a | n/a | n/a | 7.8 | 37 |
Pfizer | Assay Description The inhibition of the RNA-dependent DNA polymerase activity of the HIV-1 reverse transcriptase enzyme was measured using a primer extension assay. Th... | J Med Chem 52: 1219-23 (2009) Article DOI: 10.1021/jm801322h BindingDB Entry DOI: 10.7270/Q2707ZRW | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Gag-Pol polyprotein [588-1027,K691N]/[588-1147,K690N] (Human immunodeficiency virus type 1) | BDBM27576 (5-[(5-fluoro-3-methyl-1H-indazol-4-yl)oxy]benzene-...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | DrugBank MMDB PC cid PC sid PDB UniChem Similars | MMDB PDB Article PubMed | n/a | n/a | 384 | n/a | n/a | n/a | n/a | 7.8 | 37 |
Pfizer | Assay Description The inhibition of the RNA-dependent DNA polymerase activity of the HIV-1 reverse transcriptase enzyme was measured using a primer extension assay. Th... | J Med Chem 52: 1219-23 (2009) Article DOI: 10.1021/jm801322h BindingDB Entry DOI: 10.7270/Q2707ZRW | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Gag-Pol polyprotein [588-1027] (Human immunodeficiency virus type 1 group M subtyp...) | BDBM27575 (5-[(5-chloro-3-methyl-1H-indazol-4-yl)oxy]benzene-...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 399 | n/a | n/a | n/a | n/a | 7.8 | 37 |
Pfizer | Assay Description The inhibition of the RNA-dependent DNA polymerase activity of the HIV-1 reverse transcriptase enzyme was measured using a primer extension assay. Th... | J Med Chem 52: 1219-23 (2009) Article DOI: 10.1021/jm801322h BindingDB Entry DOI: 10.7270/Q2707ZRW | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Reverse transcriptase protein (Human immunodeficiency virus 1) | BDBM2483 ((4S)-6-chloro-4-(2-cyclopropylethynyl)-4-(trifluor...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 414 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of HIV1 HXB2 reverse transcriptase K103N mutant activity by primer extension assay | Antimicrob Agents Chemother 54: 4451-63 (2010) Article DOI: 10.1128/AAC.01455-09 BindingDB Entry DOI: 10.7270/Q2WQ06MG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM50334970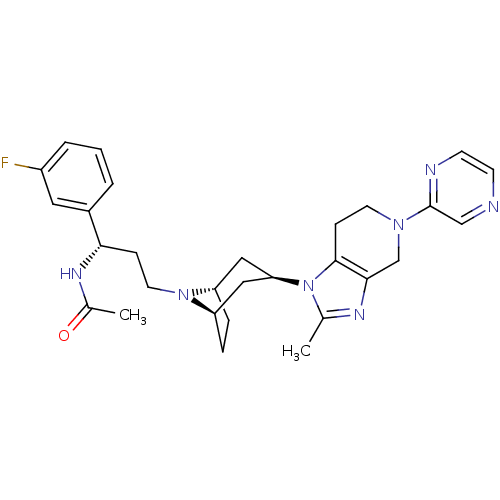 (CHEMBL1649929 | endo-N-((S)-1-(3-fluorophenyl)-3-(...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Displacement of [3H]-dofetilide from human ERG expressed in HEK293 cells | J Med Chem 54: 67-77 (2011) Article DOI: 10.1021/jm100978n BindingDB Entry DOI: 10.7270/Q2RV0PNV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [588-1027,K691N]/[588-1147,K690N] (Human immunodeficiency virus type 1) | BDBM27578 (5-{[7-(hydroxymethyl)-3-methyl-1H-indazol-4-yl]oxy...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.27E+3 | n/a | n/a | n/a | n/a | 7.8 | 37 |
Pfizer | Assay Description The inhibition of the RNA-dependent DNA polymerase activity of the HIV-1 reverse transcriptase enzyme was measured using a primer extension assay. Th... | J Med Chem 52: 1219-23 (2009) Article DOI: 10.1021/jm801322h BindingDB Entry DOI: 10.7270/Q2707ZRW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM50334971 (CHEMBL1649913 | endo-N-((S)-3-(3-(2-methyl-1H-benz...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Displacement of [3H]-dofetilide from human ERG expressed in HEK293 cells | J Med Chem 54: 67-77 (2011) Article DOI: 10.1021/jm100978n BindingDB Entry DOI: 10.7270/Q2RV0PNV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [588-1027] (Human immunodeficiency virus type 1 group M subtyp...) | BDBM27574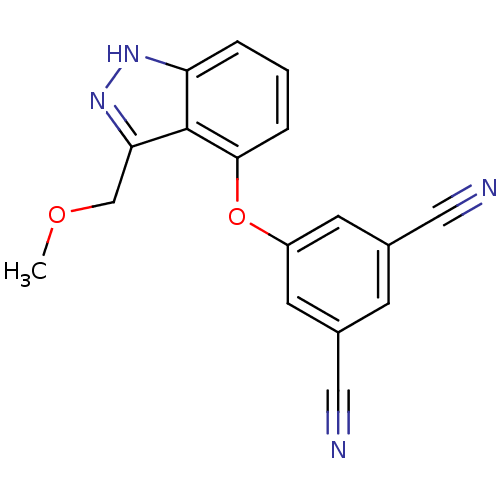 (5-{[3-(methoxymethyl)-1H-indazol-4-yl]oxy}benzene-...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.67E+3 | n/a | n/a | n/a | n/a | 7.8 | 37 |
Pfizer | Assay Description The inhibition of the RNA-dependent DNA polymerase activity of the HIV-1 reverse transcriptase enzyme was measured using a primer extension assay. Th... | J Med Chem 52: 1219-23 (2009) Article DOI: 10.1021/jm801322h BindingDB Entry DOI: 10.7270/Q2707ZRW | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM50334968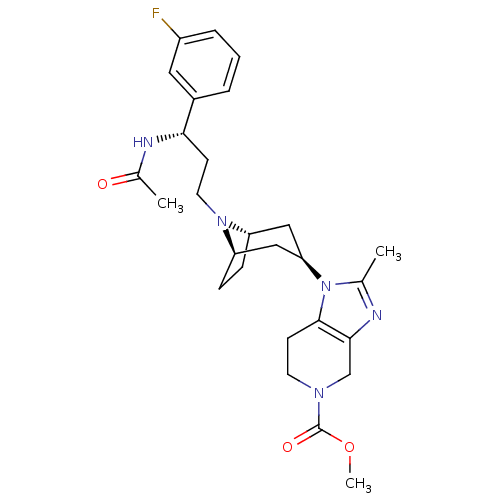 (CHEMBL1649910 | Methyl 1-endo-{8-[(3S)-3-(Acetamid...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Displacement of [3H]-dofetilide from human ERG expressed in HEK293 cells | J Med Chem 54: 67-77 (2011) Article DOI: 10.1021/jm100978n BindingDB Entry DOI: 10.7270/Q2RV0PNV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM50334972 (CHEMBL1649912 | endo-4,4-difluoro-N-((S)-3-(3-(5-i...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.23E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Displacement of [3H]-dofetilide from human ERG expressed in HEK293 cells | J Med Chem 54: 67-77 (2011) Article DOI: 10.1021/jm100978n BindingDB Entry DOI: 10.7270/Q2RV0PNV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM50334967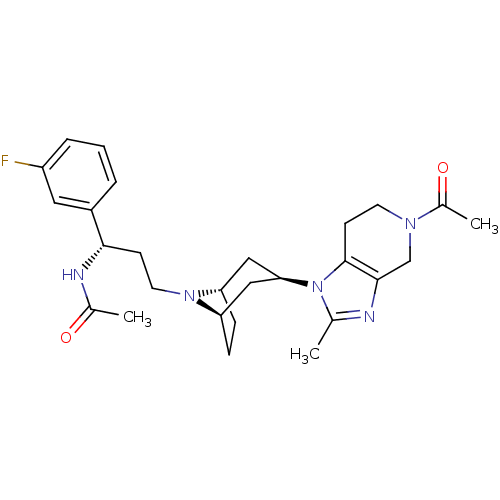 (CHEMBL1649911 | endo-N-((S)-3-(3-(5-acetyl-2-methy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Displacement of [3H]-dofetilide from human ERG expressed in HEK293 cells | J Med Chem 54: 67-77 (2011) Article DOI: 10.1021/jm100978n BindingDB Entry DOI: 10.7270/Q2RV0PNV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM50334966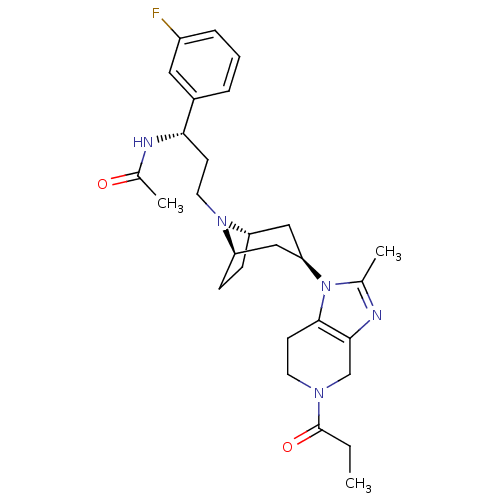 (CHEMBL1649930 | endo-N-((S)-1-(3-fluorophenyl)-3-(...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Displacement of [3H]-dofetilide from human ERG expressed in HEK293 cells | J Med Chem 54: 67-77 (2011) Article DOI: 10.1021/jm100978n BindingDB Entry DOI: 10.7270/Q2RV0PNV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM50334963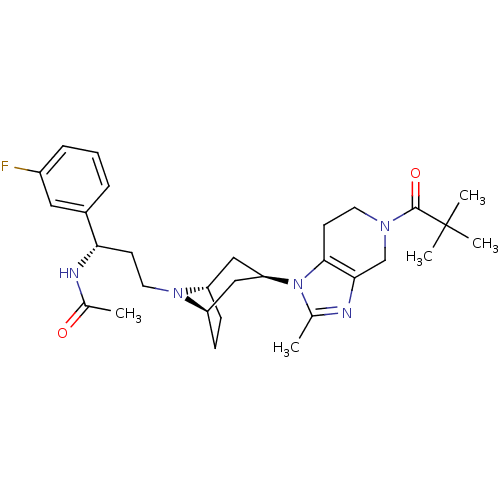 (CHEMBL1649933 | endo-N-((S)-1-(3-fluorophenyl)-3-(...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Displacement of [3H]-dofetilide from human ERG expressed in HEK293 cells | J Med Chem 54: 67-77 (2011) Article DOI: 10.1021/jm100978n BindingDB Entry DOI: 10.7270/Q2RV0PNV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM50334965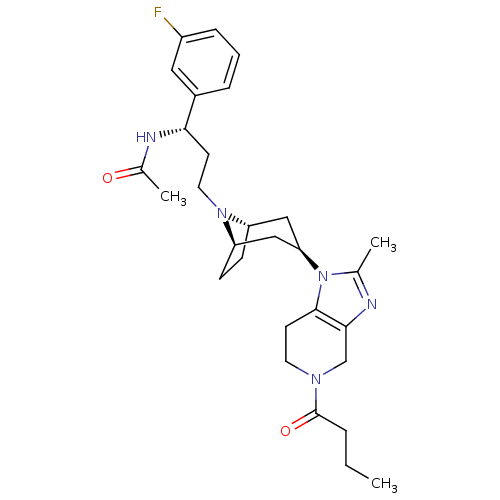 (CHEMBL1649932 | endo-N-((S)-3-(3-(5-butyryl-2-meth...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 6.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Displacement of [3H]-dofetilide from human ERG expressed in HEK293 cells | J Med Chem 54: 67-77 (2011) Article DOI: 10.1021/jm100978n BindingDB Entry DOI: 10.7270/Q2RV0PNV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM50334964 (CHEMBL1649931 | N-{(1S)-3-[3-endo-(5-Isobutyryl-2-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Displacement of [3H]-dofetilide from human ERG expressed in HEK293 cells | J Med Chem 54: 67-77 (2011) Article DOI: 10.1021/jm100978n BindingDB Entry DOI: 10.7270/Q2RV0PNV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [588-1027] (Human immunodeficiency virus type 1 group M subtyp...) | BDBM27573 (5-({3-[(methylamino)methyl]-1H-indazol-4-yl}oxy)be...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | 7.8 | 37 |
Pfizer | Assay Description The inhibition of the RNA-dependent DNA polymerase activity of the HIV-1 reverse transcriptase enzyme was measured using a primer extension assay. Th... | J Med Chem 52: 1219-23 (2009) Article DOI: 10.1021/jm801322h BindingDB Entry DOI: 10.7270/Q2707ZRW | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Displayed 1 to 50 (of 51 total ) | Next | Last >> |