 Found 57 hits with Last Name = 'whiteaker' and Initial = 'j'
Found 57 hits with Last Name = 'whiteaker' and Initial = 'j' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Enoyl-[acyl-carrier-protein] reductase [NADH]
(Mycobacterium tuberculosis (strain ATCC 25618 / H3...) | BDBM50370232

(BA-41166E | L-5103 | RIFAMPIN | Rifadin | Rifampic...)Show SMILES CO[C@H]1\C=C\O[C@@]2(C)Oc3c(C2=O)c2c(O)c(\C=N\N4CCN(C)CC4)c(NC(=O)\C(C)=C/C=C/[C@H](C)[C@H](O)[C@@H](C)[C@@H](O)[C@@H](C)[C@H](OC(C)=O)[C@@H]1C)c(O)c2c(O)c3C |r,c:33,t:3,35| Show InChI InChI=1S/C43H58N4O12/c1-21-12-11-13-22(2)42(55)45-33-28(20-44-47-17-15-46(9)16-18-47)37(52)30-31(38(33)53)36(51)26(6)40-32(30)41(54)43(8,59-40)57-19-14-29(56-10)23(3)39(58-27(7)48)25(5)35(50)24(4)34(21)49/h11-14,19-21,23-25,29,34-35,39,49-53H,15-18H2,1-10H3,(H,45,55)/b12-11+,19-14+,22-13-,44-20+/t21-,23+,24+,25+,29-,34-,35+,39+,43-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of Mycobacterium tuberculosis InhA using NADH and dodecyl coA substrate by LC-MS/MS method |
J Med Chem 57: 6572-82 (2014)
Article DOI: 10.1021/jm500833f
BindingDB Entry DOI: 10.7270/Q23J3FMN |
More data for this
Ligand-Target Pair | |
Adenosine receptor A1
(Homo sapiens (Human)) | BDBM50019514

(CHEMBL3290772)Show SMILES Cn1ncc(C(=O)N2CCC(CC2)Nc2cc(=O)n(C)c3ccccc23)c1Cl Show InChI InChI=1S/C20H22ClN5O2/c1-24-17-6-4-3-5-14(17)16(11-18(24)27)23-13-7-9-26(10-8-13)20(28)15-12-22-25(2)19(15)21/h3-6,11-13,23H,7-10H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of adenosine A1 receptor (unknown origin) |
J Med Chem 57: 5419-34 (2014)
Article DOI: 10.1021/jm5005978
BindingDB Entry DOI: 10.7270/Q25Q4XNM |
More data for this
Ligand-Target Pair | |
Adenosine receptor A1
(Homo sapiens (Human)) | BDBM50019519
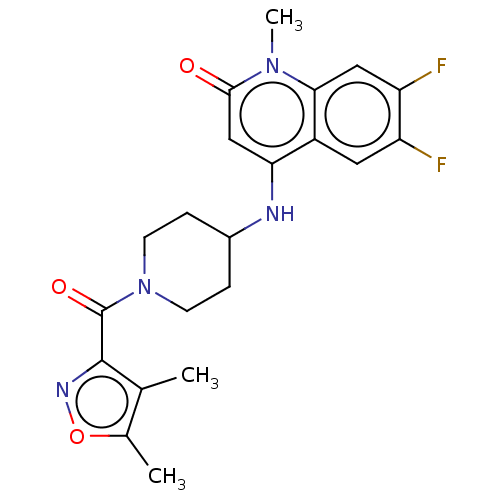
(CHEMBL3291056)Show SMILES Cc1onc(C(=O)N2CCC(CC2)Nc2cc(=O)n(C)c3cc(F)c(F)cc23)c1C Show InChI InChI=1S/C21H22F2N4O3/c1-11-12(2)30-25-20(11)21(29)27-6-4-13(5-7-27)24-17-10-19(28)26(3)18-9-16(23)15(22)8-14(17)18/h8-10,13,24H,4-7H2,1-3H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of adenosine A1 receptor (unknown origin) |
J Med Chem 57: 5419-34 (2014)
Article DOI: 10.1021/jm5005978
BindingDB Entry DOI: 10.7270/Q25Q4XNM |
More data for this
Ligand-Target Pair | |
Adenosine receptor A1
(Homo sapiens (Human)) | BDBM50019515

(CHEMBL3291062)Show SMILES [H][C@]12CC[C@]([H])(C[C@@H](C1)Nc1cc(=O)n(C)c3cc(F)c(F)cc13)N2C(=O)c1noc(C)c1C |r,TLB:25:24:6.7.8:2.3,9:7:24:2.3| Show InChI InChI=1S/C23H24F2N4O3/c1-11-12(2)32-27-22(11)23(31)29-14-4-5-15(29)7-13(6-14)26-19-10-21(30)28(3)20-9-18(25)17(24)8-16(19)20/h8-10,13-15,26H,4-7H2,1-3H3/t13-,14+,15- | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 8.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of adenosine A1 receptor (unknown origin) |
J Med Chem 57: 5419-34 (2014)
Article DOI: 10.1021/jm5005978
BindingDB Entry DOI: 10.7270/Q25Q4XNM |
More data for this
Ligand-Target Pair | |
Adenosine receptor A1
(Homo sapiens (Human)) | BDBM50019516

(CHEMBL3291063)Show SMILES [H][C@]12CC[C@]([H])(C[C@@H](C1)Nc1n[nH]c3ccc(F)cc13)N2C(=O)c1cnn(C)c1Cl |r,TLB:21:20:2.3:7.6.8,9:7:20:2.3| Show InChI InChI=1S/C19H20ClFN6O/c1-26-17(20)15(9-22-26)19(28)27-12-3-4-13(27)8-11(7-12)23-18-14-6-10(21)2-5-16(14)24-25-18/h2,5-6,9,11-13H,3-4,7-8H2,1H3,(H2,23,24,25)/t11-,12+,13- | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 8.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of adenosine A1 receptor (unknown origin) |
J Med Chem 57: 5419-34 (2014)
Article DOI: 10.1021/jm5005978
BindingDB Entry DOI: 10.7270/Q25Q4XNM |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50019515

(CHEMBL3291062)Show SMILES [H][C@]12CC[C@]([H])(C[C@@H](C1)Nc1cc(=O)n(C)c3cc(F)c(F)cc13)N2C(=O)c1noc(C)c1C |r,TLB:25:24:6.7.8:2.3,9:7:24:2.3| Show InChI InChI=1S/C23H24F2N4O3/c1-11-12(2)32-27-22(11)23(31)29-14-4-5-15(29)7-13(6-14)26-19-10-21(30)28(3)20-9-18(25)17(24)8-16(19)20/h8-10,13-15,26H,4-7H2,1-3H3/t13-,14+,15- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human ERG by medium-throughput electrophysiology |
J Med Chem 57: 5419-34 (2014)
Article DOI: 10.1021/jm5005978
BindingDB Entry DOI: 10.7270/Q25Q4XNM |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50019511

(CHEMBL3290766)Show SMILES Cn1ncc(C(=O)N2CCC(CC2)Nc2cc(=O)[nH]c3ccccc23)c1Cl Show InChI InChI=1S/C19H20ClN5O2/c1-24-18(20)14(11-21-24)19(27)25-8-6-12(7-9-25)22-16-10-17(26)23-15-5-3-2-4-13(15)16/h2-5,10-12H,6-9H2,1H3,(H2,22,23,26) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.19E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of mu opioid receptor (unknown origin) |
J Med Chem 57: 5419-34 (2014)
Article DOI: 10.1021/jm5005978
BindingDB Entry DOI: 10.7270/Q25Q4XNM |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50019657

(CHEMBL3291424)Show SMILES Cc1cnc2c(cn(Cc3ncnc(OC(F)F)c3C)c2c1)C(=O)NCCO Show InChI InChI=1S/C18H19F2N5O3/c1-10-5-14-15(22-6-10)12(16(27)21-3-4-26)7-25(14)8-13-11(2)17(24-9-23-13)28-18(19)20/h5-7,9,18,26H,3-4,8H2,1-2H3,(H,21,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >3.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human ERG |
J Med Chem 57: 5728-37 (2014)
Article DOI: 10.1021/jm500571f
BindingDB Entry DOI: 10.7270/Q2NS0WHX |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50019658

(CHEMBL3291427)Show InChI InChI=1S/C18H21N5O4/c1-11-14(21-10-22-18(11)27-3)9-23-8-13(17(25)19-4-5-24)16-15(23)6-12(26-2)7-20-16/h6-8,10,24H,4-5,9H2,1-3H3,(H,19,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >3.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human ERG |
J Med Chem 57: 5728-37 (2014)
Article DOI: 10.1021/jm500571f
BindingDB Entry DOI: 10.7270/Q2NS0WHX |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50019659
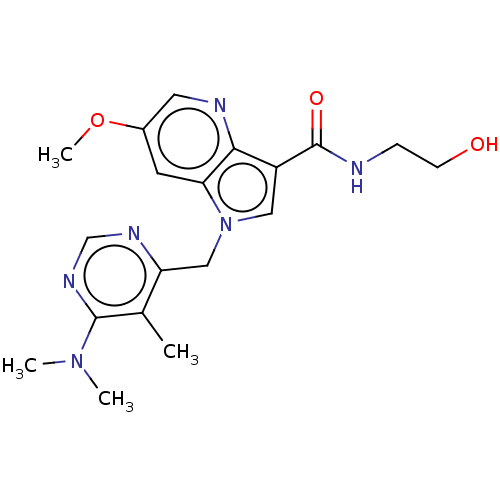
(CHEMBL3291430)Show SMILES COc1cnc2c(cn(Cc3ncnc(N(C)C)c3C)c2c1)C(=O)NCCO Show InChI InChI=1S/C19H24N6O3/c1-12-15(22-11-23-18(12)24(2)3)10-25-9-14(19(27)20-5-6-26)17-16(25)7-13(28-4)8-21-17/h7-9,11,26H,5-6,10H2,1-4H3,(H,20,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >3.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human ERG |
J Med Chem 57: 5728-37 (2014)
Article DOI: 10.1021/jm500571f
BindingDB Entry DOI: 10.7270/Q2NS0WHX |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50019514

(CHEMBL3290772)Show SMILES Cn1ncc(C(=O)N2CCC(CC2)Nc2cc(=O)n(C)c3ccccc23)c1Cl Show InChI InChI=1S/C20H22ClN5O2/c1-24-17-6-4-3-5-14(17)16(11-18(24)27)23-13-7-9-26(10-8-13)20(28)15-12-22-25(2)19(15)21/h3-6,11-13,23H,7-10H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >3.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human ERG by medium-throughput electrophysiology |
J Med Chem 57: 5419-34 (2014)
Article DOI: 10.1021/jm5005978
BindingDB Entry DOI: 10.7270/Q25Q4XNM |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50019516

(CHEMBL3291063)Show SMILES [H][C@]12CC[C@]([H])(C[C@@H](C1)Nc1n[nH]c3ccc(F)cc13)N2C(=O)c1cnn(C)c1Cl |r,TLB:21:20:2.3:7.6.8,9:7:20:2.3| Show InChI InChI=1S/C19H20ClFN6O/c1-26-17(20)15(9-22-26)19(28)27-12-3-4-13(27)8-11(7-12)23-18-14-6-10(21)2-5-16(14)24-25-18/h2,5-6,9,11-13H,3-4,7-8H2,1H3,(H2,23,24,25)/t11-,12+,13- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >3.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human ERG by medium-throughput electrophysiology |
J Med Chem 57: 5419-34 (2014)
Article DOI: 10.1021/jm5005978
BindingDB Entry DOI: 10.7270/Q25Q4XNM |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50019517

(CHEMBL3291065)Show SMILES [H][C@]12CC[C@]([H])(C[C@@H](C1)Nc1cc(=O)n(C)c3ncccc13)N2C(=O)c1cnn(C)c1Cl |r,TLB:23:22:2.3:7.6.8,9:7:22:2.3| Show InChI InChI=1S/C21H23ClN6O2/c1-26-18(29)10-17(15-4-3-7-23-20(15)26)25-12-8-13-5-6-14(9-12)28(13)21(30)16-11-24-27(2)19(16)22/h3-4,7,10-14,25H,5-6,8-9H2,1-2H3/t12-,13+,14- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >3.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human ERG by medium-throughput electrophysiology |
J Med Chem 57: 5419-34 (2014)
Article DOI: 10.1021/jm5005978
BindingDB Entry DOI: 10.7270/Q25Q4XNM |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50019652

(CHEMBL3109801)Show InChI InChI=1S/C18H20FN5O2/c1-11-6-15-16(21-7-11)13(17(25)20-5-4-19)8-24(15)9-14-12(2)18(26-3)23-10-22-14/h6-8,10H,4-5,9H2,1-3H3,(H,20,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >3.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human ERG |
J Med Chem 57: 5728-37 (2014)
Article DOI: 10.1021/jm500571f
BindingDB Entry DOI: 10.7270/Q2NS0WHX |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50019654

(CHEMBL3109802)Show InChI InChI=1S/C18H21N5O3/c1-11-6-15-16(20-7-11)13(17(25)19-4-5-24)8-23(15)9-14-12(2)18(26-3)22-10-21-14/h6-8,10,24H,4-5,9H2,1-3H3,(H,19,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >3.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human ERG |
J Med Chem 57: 5728-37 (2014)
Article DOI: 10.1021/jm500571f
BindingDB Entry DOI: 10.7270/Q2NS0WHX |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50019518

(CHEMBL3291066)Show SMILES [H][C@]12CC[C@]([H])(C[C@@H](C1)Nc1cc(=O)n(C)c3nccnc13)N2C(=O)c1noc(C)c1C |r,TLB:23:22:6.7.8:2.3,9:7:22:2.3| Show InChI InChI=1S/C21H24N6O3/c1-11-12(2)30-25-18(11)21(29)27-14-4-5-15(27)9-13(8-14)24-16-10-17(28)26(3)20-19(16)22-6-7-23-20/h6-7,10,13-15,24H,4-5,8-9H2,1-3H3/t13-,14+,15- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >3.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human ERG by medium-throughput electrophysiology |
J Med Chem 57: 5419-34 (2014)
Article DOI: 10.1021/jm5005978
BindingDB Entry DOI: 10.7270/Q25Q4XNM |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50019656
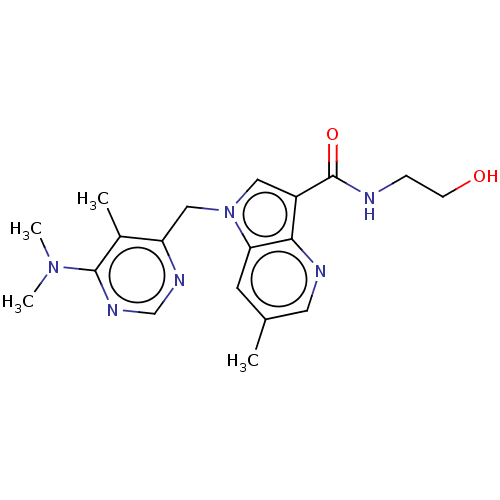
(CHEMBL3291423)Show SMILES CN(C)c1ncnc(Cn2cc(C(=O)NCCO)c3ncc(C)cc23)c1C Show InChI InChI=1S/C19H24N6O2/c1-12-7-16-17(21-8-12)14(19(27)20-5-6-26)9-25(16)10-15-13(2)18(24(3)4)23-11-22-15/h7-9,11,26H,5-6,10H2,1-4H3,(H,20,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >3.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human ERG |
J Med Chem 57: 5728-37 (2014)
Article DOI: 10.1021/jm500571f
BindingDB Entry DOI: 10.7270/Q2NS0WHX |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50019655

(CHEMBL3291422)Show SMILES COc1ncnc(Cn2cc(C(=O)NCC(F)F)c3ncc(C)cc23)c1C Show InChI InChI=1S/C18H19F2N5O2/c1-10-4-14-16(21-5-10)12(17(26)22-6-15(19)20)7-25(14)8-13-11(2)18(27-3)24-9-23-13/h4-5,7,9,15H,6,8H2,1-3H3,(H,22,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >3.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human ERG |
J Med Chem 57: 5728-37 (2014)
Article DOI: 10.1021/jm500571f
BindingDB Entry DOI: 10.7270/Q2NS0WHX |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C9
(Homo sapiens (Human)) | BDBM50019658

(CHEMBL3291427)Show InChI InChI=1S/C18H21N5O4/c1-11-14(21-10-22-18(11)27-3)9-23-8-13(17(25)19-4-5-24)16-15(23)6-12(26-2)7-20-16/h6-8,10,24H,4-5,9H2,1-3H3,(H,19,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human CYP2C9 |
J Med Chem 57: 5728-37 (2014)
Article DOI: 10.1021/jm500571f
BindingDB Entry DOI: 10.7270/Q2NS0WHX |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C9
(Homo sapiens (Human)) | BDBM50019659

(CHEMBL3291430)Show SMILES COc1cnc2c(cn(Cc3ncnc(N(C)C)c3C)c2c1)C(=O)NCCO Show InChI InChI=1S/C19H24N6O3/c1-12-15(22-11-23-18(12)24(2)3)10-25-9-14(19(27)20-5-6-26)17-16(25)7-13(28-4)8-21-17/h7-9,11,26H,5-6,10H2,1-4H3,(H,20,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human CYP2C9 |
J Med Chem 57: 5728-37 (2014)
Article DOI: 10.1021/jm500571f
BindingDB Entry DOI: 10.7270/Q2NS0WHX |
More data for this
Ligand-Target Pair | |
Cytochrome P450 1A2
(Homo sapiens (Human)) | BDBM50019652

(CHEMBL3109801)Show InChI InChI=1S/C18H20FN5O2/c1-11-6-15-16(21-7-11)13(17(25)20-5-4-19)8-24(15)9-14-12(2)18(26-3)23-10-22-14/h6-8,10H,4-5,9H2,1-3H3,(H,20,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human CYP1A2 |
J Med Chem 57: 5728-37 (2014)
Article DOI: 10.1021/jm500571f
BindingDB Entry DOI: 10.7270/Q2NS0WHX |
More data for this
Ligand-Target Pair | |
Cytochrome P450 1A2
(Homo sapiens (Human)) | BDBM50019654

(CHEMBL3109802)Show InChI InChI=1S/C18H21N5O3/c1-11-6-15-16(20-7-11)13(17(25)19-4-5-24)8-23(15)9-14-12(2)18(26-3)22-10-21-14/h6-8,10,24H,4-5,9H2,1-3H3,(H,19,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human CYP1A2 |
J Med Chem 57: 5728-37 (2014)
Article DOI: 10.1021/jm500571f
BindingDB Entry DOI: 10.7270/Q2NS0WHX |
More data for this
Ligand-Target Pair | |
Cytochrome P450 1A2
(Homo sapiens (Human)) | BDBM50019655

(CHEMBL3291422)Show SMILES COc1ncnc(Cn2cc(C(=O)NCC(F)F)c3ncc(C)cc23)c1C Show InChI InChI=1S/C18H19F2N5O2/c1-10-4-14-16(21-5-10)12(17(26)22-6-15(19)20)7-25(14)8-13-11(2)18(27-3)24-9-23-13/h4-5,7,9,15H,6,8H2,1-3H3,(H,22,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human CYP1A2 |
J Med Chem 57: 5728-37 (2014)
Article DOI: 10.1021/jm500571f
BindingDB Entry DOI: 10.7270/Q2NS0WHX |
More data for this
Ligand-Target Pair | |
Cytochrome P450 1A2
(Homo sapiens (Human)) | BDBM50019656

(CHEMBL3291423)Show SMILES CN(C)c1ncnc(Cn2cc(C(=O)NCCO)c3ncc(C)cc23)c1C Show InChI InChI=1S/C19H24N6O2/c1-12-7-16-17(21-8-12)14(19(27)20-5-6-26)9-25(16)10-15-13(2)18(24(3)4)23-11-22-15/h7-9,11,26H,5-6,10H2,1-4H3,(H,20,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human CYP1A2 |
J Med Chem 57: 5728-37 (2014)
Article DOI: 10.1021/jm500571f
BindingDB Entry DOI: 10.7270/Q2NS0WHX |
More data for this
Ligand-Target Pair | |
Cytochrome P450 1A2
(Homo sapiens (Human)) | BDBM50019657

(CHEMBL3291424)Show SMILES Cc1cnc2c(cn(Cc3ncnc(OC(F)F)c3C)c2c1)C(=O)NCCO Show InChI InChI=1S/C18H19F2N5O3/c1-10-5-14-15(22-6-10)12(16(27)21-3-4-26)7-25(14)8-13-11(2)17(24-9-23-13)28-18(19)20/h5-7,9,18,26H,3-4,8H2,1-2H3,(H,21,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human CYP1A2 |
J Med Chem 57: 5728-37 (2014)
Article DOI: 10.1021/jm500571f
BindingDB Entry DOI: 10.7270/Q2NS0WHX |
More data for this
Ligand-Target Pair | |
Cytochrome P450 1A2
(Homo sapiens (Human)) | BDBM50019658

(CHEMBL3291427)Show InChI InChI=1S/C18H21N5O4/c1-11-14(21-10-22-18(11)27-3)9-23-8-13(17(25)19-4-5-24)16-15(23)6-12(26-2)7-20-16/h6-8,10,24H,4-5,9H2,1-3H3,(H,19,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human CYP1A2 |
J Med Chem 57: 5728-37 (2014)
Article DOI: 10.1021/jm500571f
BindingDB Entry DOI: 10.7270/Q2NS0WHX |
More data for this
Ligand-Target Pair | |
Cytochrome P450 1A2
(Homo sapiens (Human)) | BDBM50019659

(CHEMBL3291430)Show SMILES COc1cnc2c(cn(Cc3ncnc(N(C)C)c3C)c2c1)C(=O)NCCO Show InChI InChI=1S/C19H24N6O3/c1-12-15(22-11-23-18(12)24(2)3)10-25-9-14(19(27)20-5-6-26)17-16(25)7-13(28-4)8-21-17/h7-9,11,26H,5-6,10H2,1-4H3,(H,20,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human CYP1A2 |
J Med Chem 57: 5728-37 (2014)
Article DOI: 10.1021/jm500571f
BindingDB Entry DOI: 10.7270/Q2NS0WHX |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50019652

(CHEMBL3109801)Show InChI InChI=1S/C18H20FN5O2/c1-11-6-15-16(21-7-11)13(17(25)20-5-4-19)8-24(15)9-14-12(2)18(26-3)23-10-22-14/h6-8,10H,4-5,9H2,1-3H3,(H,20,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human CYP3A4 |
J Med Chem 57: 5728-37 (2014)
Article DOI: 10.1021/jm500571f
BindingDB Entry DOI: 10.7270/Q2NS0WHX |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50019654

(CHEMBL3109802)Show InChI InChI=1S/C18H21N5O3/c1-11-6-15-16(20-7-11)13(17(25)19-4-5-24)8-23(15)9-14-12(2)18(26-3)22-10-21-14/h6-8,10,24H,4-5,9H2,1-3H3,(H,19,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human CYP3A4 |
J Med Chem 57: 5728-37 (2014)
Article DOI: 10.1021/jm500571f
BindingDB Entry DOI: 10.7270/Q2NS0WHX |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50019655

(CHEMBL3291422)Show SMILES COc1ncnc(Cn2cc(C(=O)NCC(F)F)c3ncc(C)cc23)c1C Show InChI InChI=1S/C18H19F2N5O2/c1-10-4-14-16(21-5-10)12(17(26)22-6-15(19)20)7-25(14)8-13-11(2)18(27-3)24-9-23-13/h4-5,7,9,15H,6,8H2,1-3H3,(H,22,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human CYP3A4 |
J Med Chem 57: 5728-37 (2014)
Article DOI: 10.1021/jm500571f
BindingDB Entry DOI: 10.7270/Q2NS0WHX |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50019656

(CHEMBL3291423)Show SMILES CN(C)c1ncnc(Cn2cc(C(=O)NCCO)c3ncc(C)cc23)c1C Show InChI InChI=1S/C19H24N6O2/c1-12-7-16-17(21-8-12)14(19(27)20-5-6-26)9-25(16)10-15-13(2)18(24(3)4)23-11-22-15/h7-9,11,26H,5-6,10H2,1-4H3,(H,20,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human CYP3A4 |
J Med Chem 57: 5728-37 (2014)
Article DOI: 10.1021/jm500571f
BindingDB Entry DOI: 10.7270/Q2NS0WHX |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50019657

(CHEMBL3291424)Show SMILES Cc1cnc2c(cn(Cc3ncnc(OC(F)F)c3C)c2c1)C(=O)NCCO Show InChI InChI=1S/C18H19F2N5O3/c1-10-5-14-15(22-6-10)12(16(27)21-3-4-26)7-25(14)8-13-11(2)17(24-9-23-13)28-18(19)20/h5-7,9,18,26H,3-4,8H2,1-2H3,(H,21,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human CYP3A4 |
J Med Chem 57: 5728-37 (2014)
Article DOI: 10.1021/jm500571f
BindingDB Entry DOI: 10.7270/Q2NS0WHX |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50019658

(CHEMBL3291427)Show InChI InChI=1S/C18H21N5O4/c1-11-14(21-10-22-18(11)27-3)9-23-8-13(17(25)19-4-5-24)16-15(23)6-12(26-2)7-20-16/h6-8,10,24H,4-5,9H2,1-3H3,(H,19,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human CYP3A4 |
J Med Chem 57: 5728-37 (2014)
Article DOI: 10.1021/jm500571f
BindingDB Entry DOI: 10.7270/Q2NS0WHX |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50019659

(CHEMBL3291430)Show SMILES COc1cnc2c(cn(Cc3ncnc(N(C)C)c3C)c2c1)C(=O)NCCO Show InChI InChI=1S/C19H24N6O3/c1-12-15(22-11-23-18(12)24(2)3)10-25-9-14(19(27)20-5-6-26)17-16(25)7-13(28-4)8-21-17/h7-9,11,26H,5-6,10H2,1-4H3,(H,20,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human CYP3A4 |
J Med Chem 57: 5728-37 (2014)
Article DOI: 10.1021/jm500571f
BindingDB Entry DOI: 10.7270/Q2NS0WHX |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C9
(Homo sapiens (Human)) | BDBM50019657

(CHEMBL3291424)Show SMILES Cc1cnc2c(cn(Cc3ncnc(OC(F)F)c3C)c2c1)C(=O)NCCO Show InChI InChI=1S/C18H19F2N5O3/c1-10-5-14-15(22-6-10)12(16(27)21-3-4-26)7-25(14)8-13-11(2)17(24-9-23-13)28-18(19)20/h5-7,9,18,26H,3-4,8H2,1-2H3,(H,21,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human CYP2C9 |
J Med Chem 57: 5728-37 (2014)
Article DOI: 10.1021/jm500571f
BindingDB Entry DOI: 10.7270/Q2NS0WHX |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C9
(Homo sapiens (Human)) | BDBM50019656

(CHEMBL3291423)Show SMILES CN(C)c1ncnc(Cn2cc(C(=O)NCCO)c3ncc(C)cc23)c1C Show InChI InChI=1S/C19H24N6O2/c1-12-7-16-17(21-8-12)14(19(27)20-5-6-26)9-25(16)10-15-13(2)18(24(3)4)23-11-22-15/h7-9,11,26H,5-6,10H2,1-4H3,(H,20,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human CYP2C9 |
J Med Chem 57: 5728-37 (2014)
Article DOI: 10.1021/jm500571f
BindingDB Entry DOI: 10.7270/Q2NS0WHX |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C9
(Homo sapiens (Human)) | BDBM50019655

(CHEMBL3291422)Show SMILES COc1ncnc(Cn2cc(C(=O)NCC(F)F)c3ncc(C)cc23)c1C Show InChI InChI=1S/C18H19F2N5O2/c1-10-4-14-16(21-5-10)12(17(26)22-6-15(19)20)7-25(14)8-13-11(2)18(27-3)24-9-23-13/h4-5,7,9,15H,6,8H2,1-3H3,(H,22,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human CYP2C9 |
J Med Chem 57: 5728-37 (2014)
Article DOI: 10.1021/jm500571f
BindingDB Entry DOI: 10.7270/Q2NS0WHX |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C9
(Homo sapiens (Human)) | BDBM50019652

(CHEMBL3109801)Show InChI InChI=1S/C18H20FN5O2/c1-11-6-15-16(21-7-11)13(17(25)20-5-4-19)8-24(15)9-14-12(2)18(26-3)23-10-22-14/h6-8,10H,4-5,9H2,1-3H3,(H,20,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human CYP2C9 |
J Med Chem 57: 5728-37 (2014)
Article DOI: 10.1021/jm500571f
BindingDB Entry DOI: 10.7270/Q2NS0WHX |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C19
(Homo sapiens (Human)) | BDBM50019659

(CHEMBL3291430)Show SMILES COc1cnc2c(cn(Cc3ncnc(N(C)C)c3C)c2c1)C(=O)NCCO Show InChI InChI=1S/C19H24N6O3/c1-12-15(22-11-23-18(12)24(2)3)10-25-9-14(19(27)20-5-6-26)17-16(25)7-13(28-4)8-21-17/h7-9,11,26H,5-6,10H2,1-4H3,(H,20,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human CYP2C19 |
J Med Chem 57: 5728-37 (2014)
Article DOI: 10.1021/jm500571f
BindingDB Entry DOI: 10.7270/Q2NS0WHX |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C19
(Homo sapiens (Human)) | BDBM50019658

(CHEMBL3291427)Show InChI InChI=1S/C18H21N5O4/c1-11-14(21-10-22-18(11)27-3)9-23-8-13(17(25)19-4-5-24)16-15(23)6-12(26-2)7-20-16/h6-8,10,24H,4-5,9H2,1-3H3,(H,19,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human CYP2C19 |
J Med Chem 57: 5728-37 (2014)
Article DOI: 10.1021/jm500571f
BindingDB Entry DOI: 10.7270/Q2NS0WHX |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C19
(Homo sapiens (Human)) | BDBM50019657

(CHEMBL3291424)Show SMILES Cc1cnc2c(cn(Cc3ncnc(OC(F)F)c3C)c2c1)C(=O)NCCO Show InChI InChI=1S/C18H19F2N5O3/c1-10-5-14-15(22-6-10)12(16(27)21-3-4-26)7-25(14)8-13-11(2)17(24-9-23-13)28-18(19)20/h5-7,9,18,26H,3-4,8H2,1-2H3,(H,21,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human CYP2C19 |
J Med Chem 57: 5728-37 (2014)
Article DOI: 10.1021/jm500571f
BindingDB Entry DOI: 10.7270/Q2NS0WHX |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C19
(Homo sapiens (Human)) | BDBM50019656

(CHEMBL3291423)Show SMILES CN(C)c1ncnc(Cn2cc(C(=O)NCCO)c3ncc(C)cc23)c1C Show InChI InChI=1S/C19H24N6O2/c1-12-7-16-17(21-8-12)14(19(27)20-5-6-26)9-25(16)10-15-13(2)18(24(3)4)23-11-22-15/h7-9,11,26H,5-6,10H2,1-4H3,(H,20,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human CYP2C19 |
J Med Chem 57: 5728-37 (2014)
Article DOI: 10.1021/jm500571f
BindingDB Entry DOI: 10.7270/Q2NS0WHX |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C19
(Homo sapiens (Human)) | BDBM50019655

(CHEMBL3291422)Show SMILES COc1ncnc(Cn2cc(C(=O)NCC(F)F)c3ncc(C)cc23)c1C Show InChI InChI=1S/C18H19F2N5O2/c1-10-4-14-16(21-5-10)12(17(26)22-6-15(19)20)7-25(14)8-13-11(2)18(27-3)24-9-23-13/h4-5,7,9,15H,6,8H2,1-3H3,(H,22,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human CYP2C19 |
J Med Chem 57: 5728-37 (2014)
Article DOI: 10.1021/jm500571f
BindingDB Entry DOI: 10.7270/Q2NS0WHX |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C19
(Homo sapiens (Human)) | BDBM50019654

(CHEMBL3109802)Show InChI InChI=1S/C18H21N5O3/c1-11-6-15-16(20-7-11)13(17(25)19-4-5-24)8-23(15)9-14-12(2)18(26-3)22-10-21-14/h6-8,10,24H,4-5,9H2,1-3H3,(H,19,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human CYP2C19 |
J Med Chem 57: 5728-37 (2014)
Article DOI: 10.1021/jm500571f
BindingDB Entry DOI: 10.7270/Q2NS0WHX |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C19
(Homo sapiens (Human)) | BDBM50019652

(CHEMBL3109801)Show InChI InChI=1S/C18H20FN5O2/c1-11-6-15-16(21-7-11)13(17(25)20-5-4-19)8-24(15)9-14-12(2)18(26-3)23-10-22-14/h6-8,10H,4-5,9H2,1-3H3,(H,20,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human CYP2C19 |
J Med Chem 57: 5728-37 (2014)
Article DOI: 10.1021/jm500571f
BindingDB Entry DOI: 10.7270/Q2NS0WHX |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM50019659

(CHEMBL3291430)Show SMILES COc1cnc2c(cn(Cc3ncnc(N(C)C)c3C)c2c1)C(=O)NCCO Show InChI InChI=1S/C19H24N6O3/c1-12-15(22-11-23-18(12)24(2)3)10-25-9-14(19(27)20-5-6-26)17-16(25)7-13(28-4)8-21-17/h7-9,11,26H,5-6,10H2,1-4H3,(H,20,27) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human CYP2D6 |
J Med Chem 57: 5728-37 (2014)
Article DOI: 10.1021/jm500571f
BindingDB Entry DOI: 10.7270/Q2NS0WHX |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM50019658

(CHEMBL3291427)Show InChI InChI=1S/C18H21N5O4/c1-11-14(21-10-22-18(11)27-3)9-23-8-13(17(25)19-4-5-24)16-15(23)6-12(26-2)7-20-16/h6-8,10,24H,4-5,9H2,1-3H3,(H,19,25) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human CYP2D6 |
J Med Chem 57: 5728-37 (2014)
Article DOI: 10.1021/jm500571f
BindingDB Entry DOI: 10.7270/Q2NS0WHX |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM50019657

(CHEMBL3291424)Show SMILES Cc1cnc2c(cn(Cc3ncnc(OC(F)F)c3C)c2c1)C(=O)NCCO Show InChI InChI=1S/C18H19F2N5O3/c1-10-5-14-15(22-6-10)12(16(27)21-3-4-26)7-25(14)8-13-11(2)17(24-9-23-13)28-18(19)20/h5-7,9,18,26H,3-4,8H2,1-2H3,(H,21,27) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human CYP2D6 |
J Med Chem 57: 5728-37 (2014)
Article DOI: 10.1021/jm500571f
BindingDB Entry DOI: 10.7270/Q2NS0WHX |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM50019656

(CHEMBL3291423)Show SMILES CN(C)c1ncnc(Cn2cc(C(=O)NCCO)c3ncc(C)cc23)c1C Show InChI InChI=1S/C19H24N6O2/c1-12-7-16-17(21-8-12)14(19(27)20-5-6-26)9-25(16)10-15-13(2)18(24(3)4)23-11-22-15/h7-9,11,26H,5-6,10H2,1-4H3,(H,20,27) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human CYP2D6 |
J Med Chem 57: 5728-37 (2014)
Article DOI: 10.1021/jm500571f
BindingDB Entry DOI: 10.7270/Q2NS0WHX |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM50019655

(CHEMBL3291422)Show SMILES COc1ncnc(Cn2cc(C(=O)NCC(F)F)c3ncc(C)cc23)c1C Show InChI InChI=1S/C18H19F2N5O2/c1-10-4-14-16(21-5-10)12(17(26)22-6-15(19)20)7-25(14)8-13-11(2)18(27-3)24-9-23-13/h4-5,7,9,15H,6,8H2,1-3H3,(H,22,26) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human CYP2D6 |
J Med Chem 57: 5728-37 (2014)
Article DOI: 10.1021/jm500571f
BindingDB Entry DOI: 10.7270/Q2NS0WHX |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data