

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Arylsulfatase (Pseudomonas aeruginosa) | BDBM50098109 (3-nitrophenyl sulfamate | CHEMBL283560 | Sulfamic ...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | 130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Melbourne Curated by ChEMBL | Assay Description Inhibition of Pseudomonas aeruginosa recombinant arylsulfatase A expressed in Escherichia coli | Bioorg Med Chem Lett 19: 477-80 (2008) Article DOI: 10.1016/j.bmcl.2008.11.059 BindingDB Entry DOI: 10.7270/Q2M61K4G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1,2-mannosidase, putative (Bacteroides thetaiotaomicron ) | BDBM36373 ((5R,6R,7S,8R)-5,6,7,8-Tetrahydro-5-(hydroxymethyl)...) | UniProtKB/TrEMBL GoogleScholar AffyNet | MMDB PC cid PC sid PDB UniChem Similars | Article PubMed | 400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Newcastle University Curated by ChEMBL | Assay Description Inhibition of Bacteroides thetaiotaomicron GH92 alpha-mannosidase 3965 assessed as reduction of mannose release using 4NP-mannopyranoside substrate | Nat Chem Biol 6: 125-32 (2010) Article DOI: 10.1038/nchembio.278 BindingDB Entry DOI: 10.7270/Q2ZW1PQB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1,2-mannosidase, putative (Bacteroides thetaiotaomicron ) | BDBM36373 ((5R,6R,7S,8R)-5,6,7,8-Tetrahydro-5-(hydroxymethyl)...) | UniProtKB/TrEMBL GoogleScholar AffyNet | MMDB PC cid PC sid PDB UniChem Similars | Article PubMed | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Newcastle University Curated by ChEMBL | Assay Description Inhibition of Bacteroides thetaiotaomicron GH92 alpha-mannosidase 3130 assessed as reduction of mannose release using 4NP-mannopyranoside substrate | Nat Chem Biol 6: 125-32 (2010) Article DOI: 10.1038/nchembio.278 BindingDB Entry DOI: 10.7270/Q2ZW1PQB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1,2-mannosidase, putative (Bacteroides thetaiotaomicron ) | BDBM50168995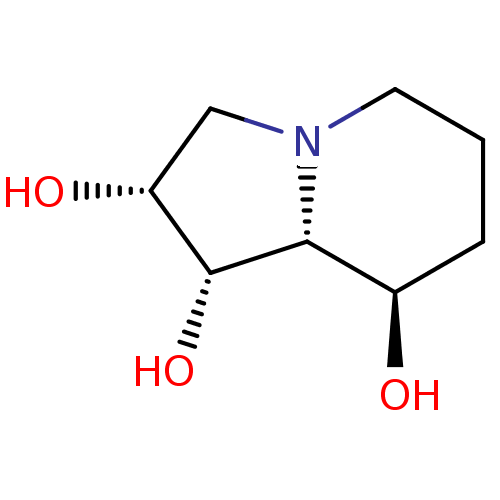 ((-)-swainsonine | (1S,2R,8R,8aR)-Octahydro-indoliz...) | UniProtKB/TrEMBL GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Newcastle University Curated by ChEMBL | Assay Description Inhibition of Bacteroides thetaiotaomicron GH92 alpha-mannosidase 3990 assessed as reduction of mannose release using 4NP-mannopyranoside substrate | Nat Chem Biol 6: 125-32 (2010) Article DOI: 10.1038/nchembio.278 BindingDB Entry DOI: 10.7270/Q2ZW1PQB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1,2-mannosidase, putative (Bacteroides thetaiotaomicron ) | BDBM50168995 ((-)-swainsonine | (1S,2R,8R,8aR)-Octahydro-indoliz...) | UniProtKB/TrEMBL GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 1.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Newcastle University Curated by ChEMBL | Assay Description Inhibition of Bacteroides thetaiotaomicron GH92 alpha-mannosidase 2199 assessed as reduction of mannose release using 4NP-mannopyranoside substrate | Nat Chem Biol 6: 125-32 (2010) Article DOI: 10.1038/nchembio.278 BindingDB Entry DOI: 10.7270/Q2ZW1PQB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1,2-mannosidase, putative (Bacteroides thetaiotaomicron ) | BDBM36373 ((5R,6R,7S,8R)-5,6,7,8-Tetrahydro-5-(hydroxymethyl)...) | UniProtKB/TrEMBL GoogleScholar AffyNet | MMDB PC cid PC sid PDB UniChem Similars | Article PubMed | 4.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Newcastle University Curated by ChEMBL | Assay Description Inhibition of Bacteroides thetaiotaomicron GH92 alpha-mannosidase 3994 assessed as reduction of mannose release using 4NP-mannopyranoside substrate | Nat Chem Biol 6: 125-32 (2010) Article DOI: 10.1038/nchembio.278 BindingDB Entry DOI: 10.7270/Q2ZW1PQB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1,2-mannosidase, putative (Bacteroides thetaiotaomicron ) | BDBM36373 ((5R,6R,7S,8R)-5,6,7,8-Tetrahydro-5-(hydroxymethyl)...) | UniProtKB/TrEMBL GoogleScholar AffyNet | MMDB PC cid PC sid PDB UniChem Similars | Article PubMed | 4.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Newcastle University Curated by ChEMBL | Assay Description Inhibition of Bacteroides thetaiotaomicron GH92 alpha-mannosidase 3991 assessed as reduction of mannose release using 4NP-mannopyranoside substrate | Nat Chem Biol 6: 125-32 (2010) Article DOI: 10.1038/nchembio.278 BindingDB Entry DOI: 10.7270/Q2ZW1PQB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1,2-mannosidase, putative (Bacteroides thetaiotaomicron ) | BDBM36373 ((5R,6R,7S,8R)-5,6,7,8-Tetrahydro-5-(hydroxymethyl)...) | UniProtKB/TrEMBL GoogleScholar AffyNet | MMDB PC cid PC sid PDB UniChem Similars | Article PubMed | 7.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Newcastle University Curated by ChEMBL | Assay Description Inhibition of Bacteroides thetaiotaomicron GH92 alpha-mannosidase 2948 assessed as reduction of mannose release using 4NP-mannopyranoside substrate | Nat Chem Biol 6: 125-32 (2010) Article DOI: 10.1038/nchembio.278 BindingDB Entry DOI: 10.7270/Q2ZW1PQB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1,2-mannosidase, putative (Bacteroides thetaiotaomicron ) | BDBM36373 ((5R,6R,7S,8R)-5,6,7,8-Tetrahydro-5-(hydroxymethyl)...) | UniProtKB/TrEMBL GoogleScholar AffyNet | MMDB PC cid PC sid PDB UniChem Similars | Article PubMed | 9.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Newcastle University Curated by ChEMBL | Assay Description Inhibition of Bacteroides thetaiotaomicron GH92 alpha-mannosidase 2199 assessed as reduction of mannose release using 4NP-mannopyranoside substrate | Nat Chem Biol 6: 125-32 (2010) Article DOI: 10.1038/nchembio.278 BindingDB Entry DOI: 10.7270/Q2ZW1PQB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1,2-mannosidase, putative (Bacteroides thetaiotaomicron ) | BDBM36373 ((5R,6R,7S,8R)-5,6,7,8-Tetrahydro-5-(hydroxymethyl)...) | UniProtKB/TrEMBL GoogleScholar AffyNet | MMDB PC cid PC sid PDB UniChem Similars | Article PubMed | 9.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Newcastle University Curated by ChEMBL | Assay Description Inhibition of Bacteroides thetaiotaomicron GH92 alpha-mannosidase 3990 assessed as reduction of mannose release using 4NP-mannopyranoside substrate | Nat Chem Biol 6: 125-32 (2010) Article DOI: 10.1038/nchembio.278 BindingDB Entry DOI: 10.7270/Q2ZW1PQB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1,2-mannosidase, putative (Bacteroides thetaiotaomicron ) | BDBM50482721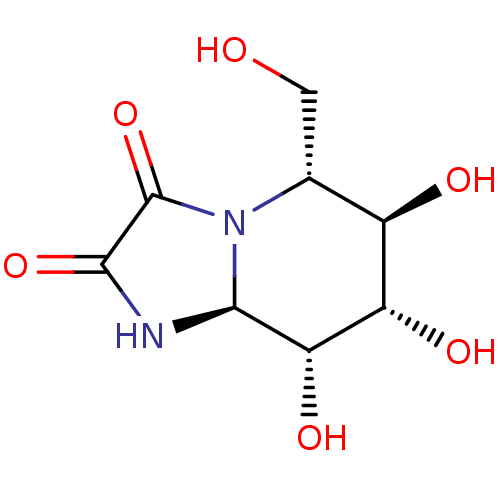 (Kifunensine) | UniProtKB/TrEMBL GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | Article PubMed | 1.40E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Newcastle University Curated by ChEMBL | Assay Description Inhibition of Bacteroides thetaiotaomicron GH92 alpha-mannosidase 3990 assessed as reduction of mannose release using 4NP-mannopyranoside substrate | Nat Chem Biol 6: 125-32 (2010) Article DOI: 10.1038/nchembio.278 BindingDB Entry DOI: 10.7270/Q2ZW1PQB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1,2-mannosidase, putative (Bacteroides thetaiotaomicron ) | BDBM50482721 (Kifunensine) | UniProtKB/TrEMBL GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | Article PubMed | 2.30E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Newcastle University Curated by ChEMBL | Assay Description Inhibition of Bacteroides thetaiotaomicron GH92 alpha-mannosidase 2199 assessed as reduction of mannose release using 4NP-mannopyranoside substrate | Nat Chem Biol 6: 125-32 (2010) Article DOI: 10.1038/nchembio.278 BindingDB Entry DOI: 10.7270/Q2ZW1PQB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1,2-mannosidase, putative (Bacteroides thetaiotaomicron ) | BDBM36373 ((5R,6R,7S,8R)-5,6,7,8-Tetrahydro-5-(hydroxymethyl)...) | UniProtKB/TrEMBL GoogleScholar AffyNet | MMDB PC cid PC sid PDB UniChem Similars | Article PubMed | 2.60E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Newcastle University Curated by ChEMBL | Assay Description Inhibition of Bacteroides thetaiotaomicron GH92 alpha-mannosidase 3858 assessed as reduction of mannose release using 4NP-mannopyranoside substrate | Nat Chem Biol 6: 125-32 (2010) Article DOI: 10.1038/nchembio.278 BindingDB Entry DOI: 10.7270/Q2ZW1PQB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1,2-mannosidase, putative (Bacteroides thetaiotaomicron ) | BDBM36373 ((5R,6R,7S,8R)-5,6,7,8-Tetrahydro-5-(hydroxymethyl)...) | UniProtKB/TrEMBL GoogleScholar AffyNet | MMDB PC cid PC sid PDB UniChem Similars | Article PubMed | 2.70E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Newcastle University Curated by ChEMBL | Assay Description Inhibition of Bacteroides thetaiotaomicron GH92 alpha-mannosidase 3962 assessed as reduction of mannose release using 4NP-mannopyranoside substrate | Nat Chem Biol 6: 125-32 (2010) Article DOI: 10.1038/nchembio.278 BindingDB Entry DOI: 10.7270/Q2ZW1PQB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1,2-mannosidase, putative (Bacteroides thetaiotaomicron ) | BDBM36373 ((5R,6R,7S,8R)-5,6,7,8-Tetrahydro-5-(hydroxymethyl)...) | UniProtKB/TrEMBL GoogleScholar AffyNet | MMDB PC cid PC sid PDB UniChem Similars | Article PubMed | 3.60E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Newcastle University Curated by ChEMBL | Assay Description Inhibition of Bacteroides thetaiotaomicron GH92 alpha-mannosidase 4073 assessed as reduction of mannose release using 4NP-mannopyranoside substrate | Nat Chem Biol 6: 125-32 (2010) Article DOI: 10.1038/nchembio.278 BindingDB Entry DOI: 10.7270/Q2ZW1PQB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1,2-mannosidase/Alpha-1,2-mannosidase family protein/Alpha-1,2-mannosidase, putative/Glycoside hydrolase family 92/Putative alpha-1,2-mannosidase (Bacteroides thetaiotaomicron ) | BDBM36373 ((5R,6R,7S,8R)-5,6,7,8-Tetrahydro-5-(hydroxymethyl)...) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | MMDB PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 6.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Newcastle University Curated by ChEMBL | Assay Description Inhibition of Bacteroides thetaiotaomicron GH92 alpha-mannosidase 1032 assessed as reduction of mannose release using 4NP-mannopyranoside substrate | Nat Chem Biol 6: 125-32 (2010) Article DOI: 10.1038/nchembio.278 BindingDB Entry DOI: 10.7270/Q2ZW1PQB | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Alpha-1,2-mannosidase, putative (Bacteroides thetaiotaomicron ) | BDBM36373 ((5R,6R,7S,8R)-5,6,7,8-Tetrahydro-5-(hydroxymethyl)...) | UniProtKB/TrEMBL GoogleScholar AffyNet | MMDB PC cid PC sid PDB UniChem Similars | Article PubMed | 7.90E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Newcastle University Curated by ChEMBL | Assay Description Inhibition of Bacteroides thetaiotaomicron GH92 alpha-mannosidase 3963 assessed as reduction of mannose release using 4NP-mannopyranoside substrate | Nat Chem Biol 6: 125-32 (2010) Article DOI: 10.1038/nchembio.278 BindingDB Entry DOI: 10.7270/Q2ZW1PQB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1,2-mannosidase, putative (Bacteroides thetaiotaomicron ) | BDBM36373 ((5R,6R,7S,8R)-5,6,7,8-Tetrahydro-5-(hydroxymethyl)...) | UniProtKB/TrEMBL GoogleScholar AffyNet | MMDB PC cid PC sid PDB UniChem Similars | Article PubMed | 8.60E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Newcastle University Curated by ChEMBL | Assay Description Inhibition of Bacteroides thetaiotaomicron GH92 alpha-mannosidase 4093 assessed as reduction of mannose release using 4NP-mannopyranoside substrate | Nat Chem Biol 6: 125-32 (2010) Article DOI: 10.1038/nchembio.278 BindingDB Entry DOI: 10.7270/Q2ZW1PQB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1,2-mannosidase, putative (Bacteroides thetaiotaomicron ) | BDBM36373 ((5R,6R,7S,8R)-5,6,7,8-Tetrahydro-5-(hydroxymethyl)...) | UniProtKB/TrEMBL GoogleScholar AffyNet | MMDB PC cid PC sid PDB UniChem Similars | Article PubMed | 1.60E+6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Newcastle University Curated by ChEMBL | Assay Description Inhibition of Bacteroides thetaiotaomicron GH92 alpha-mannosidase 4092 assessed as reduction of mannose release using 4NP-mannopyranoside substrate | Nat Chem Biol 6: 125-32 (2010) Article DOI: 10.1038/nchembio.278 BindingDB Entry DOI: 10.7270/Q2ZW1PQB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1,2-mannosidase, putative (Bacteroides thetaiotaomicron ) | BDBM36373 ((5R,6R,7S,8R)-5,6,7,8-Tetrahydro-5-(hydroxymethyl)...) | UniProtKB/TrEMBL GoogleScholar AffyNet | MMDB PC cid PC sid PDB UniChem Similars | Article PubMed | 1.80E+6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Newcastle University Curated by ChEMBL | Assay Description Inhibition of Bacteroides thetaiotaomicron GH92 alpha-mannosidase 1878 assessed as reduction of mannose release using 4NP-mannopyranoside substrate | Nat Chem Biol 6: 125-32 (2010) Article DOI: 10.1038/nchembio.278 BindingDB Entry DOI: 10.7270/Q2ZW1PQB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1,2-mannosidase, putative (Bacteroides thetaiotaomicron ) | BDBM36373 ((5R,6R,7S,8R)-5,6,7,8-Tetrahydro-5-(hydroxymethyl)...) | UniProtKB/TrEMBL GoogleScholar AffyNet | MMDB PC cid PC sid PDB UniChem Similars | Article PubMed | 5.80E+6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Newcastle University Curated by ChEMBL | Assay Description Inhibition of Bacteroides thetaiotaomicron GH92 alpha-mannosidase 3784 assessed as reduction of mannose release using 4NP-mannopyranoside substrate | Nat Chem Biol 6: 125-32 (2010) Article DOI: 10.1038/nchembio.278 BindingDB Entry DOI: 10.7270/Q2ZW1PQB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1,2-mannosidase, putative (Bacteroides thetaiotaomicron ) | BDBM50065259 ((2R,3R,4R,5R)-2-(Hydroxymethyl)piperidine-3,4,5-tr...) | UniProtKB/TrEMBL GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid UniChem Patents Similars | Article PubMed | 1.20E+7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Newcastle University Curated by ChEMBL | Assay Description Inhibition of Bacteroides thetaiotaomicron GH92 alpha-mannosidase 3990 assessed as reduction of mannose release using 4NP-mannopyranoside substrate | Nat Chem Biol 6: 125-32 (2010) Article DOI: 10.1038/nchembio.278 BindingDB Entry DOI: 10.7270/Q2ZW1PQB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1,2-mannosidase, putative (Bacteroides thetaiotaomicron ) | BDBM50065259 ((2R,3R,4R,5R)-2-(Hydroxymethyl)piperidine-3,4,5-tr...) | UniProtKB/TrEMBL GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid UniChem Patents Similars | Article PubMed | 1.30E+7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Newcastle University Curated by ChEMBL | Assay Description Inhibition of Bacteroides thetaiotaomicron GH92 alpha-mannosidase 2199 assessed as reduction of mannose release using 4NP-mannopyranoside substrate | Nat Chem Biol 6: 125-32 (2010) Article DOI: 10.1038/nchembio.278 BindingDB Entry DOI: 10.7270/Q2ZW1PQB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor (RAT) | BDBM50169743 ((13S,17S)-13-Methyl-7-[9-(4,4,5,5,5-pentafluoro-pe...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 5.89 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Melbourne Curated by ChEMBL | Assay Description Displacement of [3H]estradiol from rat uterine cytosolic estrogen receptor | Bioorg Med Chem 20: 2353-61 (2012) Article DOI: 10.1016/j.bmc.2012.02.008 BindingDB Entry DOI: 10.7270/Q2F76DT2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50266017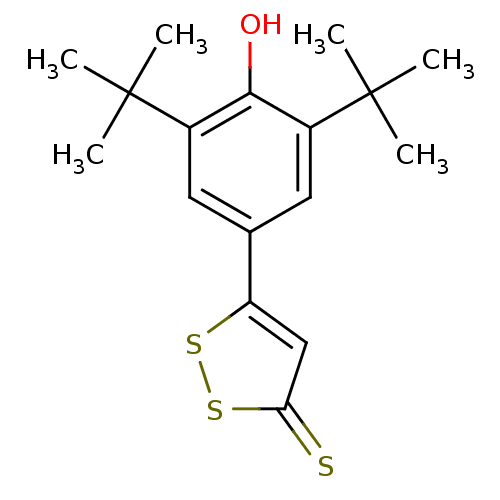 (5-(3,5-di-tert-butyl-4-hydroxyphenyl)-3H-1,2-dithi...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute Curated by ChEMBL | Assay Description Inhibition of human recombinant COX2 expressed in Sf21 cells assessed as effect on prostaglandin E2 production by ELISA | Bioorg Med Chem Lett 19: 459-61 (2008) Article DOI: 10.1016/j.bmcl.2008.11.045 BindingDB Entry DOI: 10.7270/Q2QZ29T1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 1 (Homo sapiens (Human)) | BDBM17638 (2-{1-[(4-chlorophenyl)carbonyl]-5-methoxy-2-methyl...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 28 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute Curated by ChEMBL | Assay Description Inhibition of human platelet COX1 assessed as effect on prostaglandin E2 production by ELISA | Bioorg Med Chem Lett 19: 459-61 (2008) Article DOI: 10.1016/j.bmcl.2008.11.045 BindingDB Entry DOI: 10.7270/Q2QZ29T1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50266016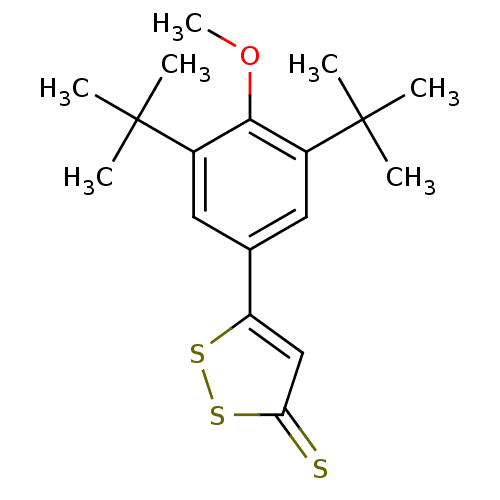 (5-(3,5-di-tert-butyl-4-methoxyphenyl)-3H-1,2-dithi...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 47 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute Curated by ChEMBL | Assay Description Inhibition of human recombinant COX2 expressed in Sf21 cells assessed as effect on prostaglandin E2 production by ELISA | Bioorg Med Chem Lett 19: 459-61 (2008) Article DOI: 10.1016/j.bmcl.2008.11.045 BindingDB Entry DOI: 10.7270/Q2QZ29T1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM22369 (4-(4-methanesulfonylphenyl)-3-phenyl-2,5-dihydrofu...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 75 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute Curated by ChEMBL | Assay Description Inhibition of human recombinant COX2 expressed in Sf21 cells assessed as effect on prostaglandin E2 production by ELISA | Bioorg Med Chem Lett 19: 459-61 (2008) Article DOI: 10.1016/j.bmcl.2008.11.045 BindingDB Entry DOI: 10.7270/Q2QZ29T1 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Sodium channel protein type 8 subunit alpha (Homo sapiens (Human)) | BDBM432084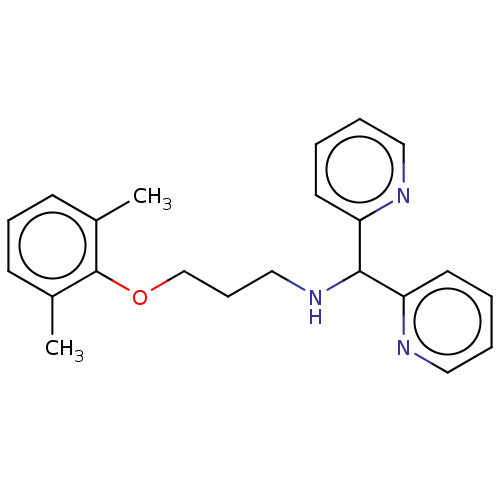 (US10577308, Compound 562) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
The Florey Institute; The University of Melbourne US Patent | Assay Description Test Protocol for Determining the Potency of Compounds on Nav1.2 and Nav1.6 Subtypes of Voltage-Gated Sodium Ion Channels Expressed in Mammalian Cell... | US Patent US10577308 (2020) BindingDB Entry DOI: 10.7270/Q2CZ39JQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium channel protein type 8 subunit alpha (Homo sapiens (Human)) | BDBM432084 (US10577308, Compound 562) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
The Florey Institute; The University of Melbourne US Patent | Assay Description Test Protocol for Determining the Potency of Compounds on Nav1.2 and Nav1.6 Subtypes of Voltage-Gated Sodium Ion Channels Expressed in Mammalian Cell... | US Patent US10577308 (2020) BindingDB Entry DOI: 10.7270/Q2CZ39JQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium channel protein type 2 subunit alpha (Homo sapiens (Human)) | BDBM432084 (US10577308, Compound 562) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
The Florey Institute; The University of Melbourne US Patent | Assay Description Test Protocol for Determining the Potency of Compounds on Nav1.2 and Nav1.6 Subtypes of Voltage-Gated Sodium Ion Channels Expressed in Mammalian Cell... | US Patent US10577308 (2020) BindingDB Entry DOI: 10.7270/Q2CZ39JQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium channel protein type 2 subunit alpha (Homo sapiens (Human)) | BDBM432084 (US10577308, Compound 562) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
The Florey Institute; The University of Melbourne US Patent | Assay Description Test Protocol for Determining the Potency of Compounds on Nav1.2 and Nav1.6 Subtypes of Voltage-Gated Sodium Ion Channels Expressed in Mammalian Cell... | US Patent US10577308 (2020) BindingDB Entry DOI: 10.7270/Q2CZ39JQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium channel protein type 2 subunit alpha (Homo sapiens (Human)) | BDBM432087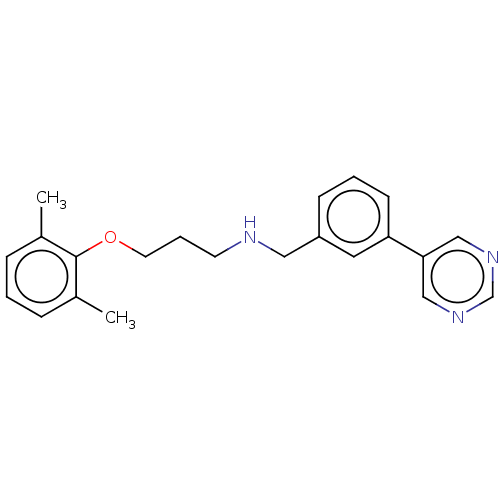 (US10577308, Compound 558) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
The Florey Institute; The University of Melbourne US Patent | Assay Description Test Protocol for Determining the Potency of Compounds on Nav1.2 and Nav1.6 Subtypes of Voltage-Gated Sodium Ion Channels Expressed in Mammalian Cell... | US Patent US10577308 (2020) BindingDB Entry DOI: 10.7270/Q2CZ39JQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium channel protein type 2 subunit alpha (Homo sapiens (Human)) | BDBM432082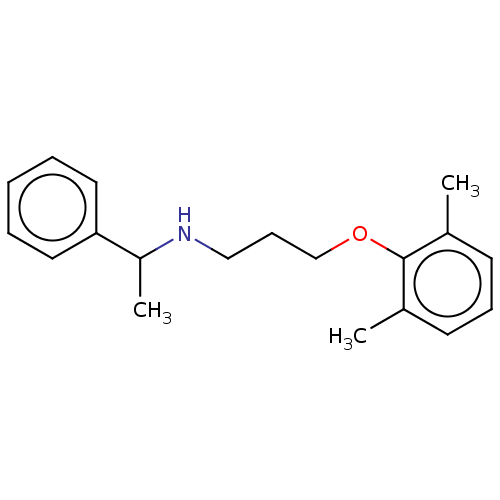 (US10577308, Compound 553) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
The Florey Institute; The University of Melbourne US Patent | Assay Description Test Protocol for Determining the Potency of Compounds on Nav1.2 and Nav1.6 Subtypes of Voltage-Gated Sodium Ion Channels Expressed in Mammalian Cell... | US Patent US10577308 (2020) BindingDB Entry DOI: 10.7270/Q2CZ39JQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium channel protein type 2 subunit alpha (Homo sapiens (Human)) | BDBM432087 (US10577308, Compound 558) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
The Florey Institute; The University of Melbourne US Patent | Assay Description Test Protocol for Determining the Potency of Compounds on Nav1.2 and Nav1.6 Subtypes of Voltage-Gated Sodium Ion Channels Expressed in Mammalian Cell... | US Patent US10577308 (2020) BindingDB Entry DOI: 10.7270/Q2CZ39JQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium channel protein type 2 subunit alpha (Homo sapiens (Human)) | BDBM432082 (US10577308, Compound 553) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
The Florey Institute; The University of Melbourne US Patent | Assay Description Test Protocol for Determining the Potency of Compounds on Nav1.2 and Nav1.6 Subtypes of Voltage-Gated Sodium Ion Channels Expressed in Mammalian Cell... | US Patent US10577308 (2020) BindingDB Entry DOI: 10.7270/Q2CZ39JQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor (RAT) | BDBM50166895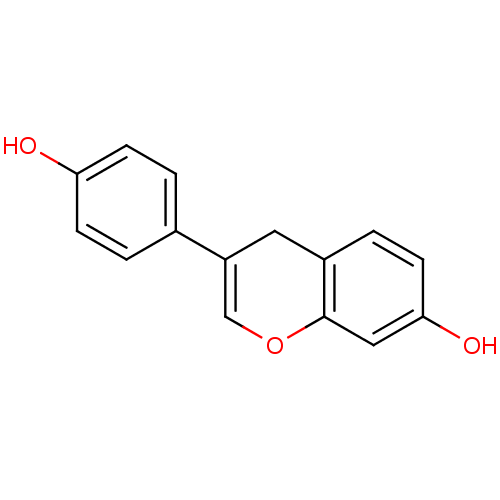 (3-(4-Hydroxy-phenyl)-4H-chromen-7-ol | CHEMBL19556...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 234 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Melbourne Curated by ChEMBL | Assay Description Displacement of [3H]estradiol from rat uterine cytosolic estrogen receptor | Bioorg Med Chem 20: 2353-61 (2012) Article DOI: 10.1016/j.bmc.2012.02.008 BindingDB Entry DOI: 10.7270/Q2F76DT2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium channel protein type 8 subunit alpha (Homo sapiens (Human)) | BDBM432087 (US10577308, Compound 558) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 300 | n/a | n/a | n/a | n/a | n/a | n/a |
The Florey Institute; The University of Melbourne US Patent | Assay Description Test Protocol for Determining the Potency of Compounds on Nav1.2 and Nav1.6 Subtypes of Voltage-Gated Sodium Ion Channels Expressed in Mammalian Cell... | US Patent US10577308 (2020) BindingDB Entry DOI: 10.7270/Q2CZ39JQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium channel protein type 8 subunit alpha (Homo sapiens (Human)) | BDBM432087 (US10577308, Compound 558) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 300 | n/a | n/a | n/a | n/a | n/a | n/a |
The Florey Institute; The University of Melbourne US Patent | Assay Description Test Protocol for Determining the Potency of Compounds on Nav1.2 and Nav1.6 Subtypes of Voltage-Gated Sodium Ion Channels Expressed in Mammalian Cell... | US Patent US10577308 (2020) BindingDB Entry DOI: 10.7270/Q2CZ39JQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium channel protein type 8 subunit alpha (Homo sapiens (Human)) | BDBM432082 (US10577308, Compound 553) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 300 | n/a | n/a | n/a | n/a | n/a | n/a |
The Florey Institute; The University of Melbourne US Patent | Assay Description Test Protocol for Determining the Potency of Compounds on Nav1.2 and Nav1.6 Subtypes of Voltage-Gated Sodium Ion Channels Expressed in Mammalian Cell... | US Patent US10577308 (2020) BindingDB Entry DOI: 10.7270/Q2CZ39JQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium channel protein type 8 subunit alpha (Homo sapiens (Human)) | BDBM432082 (US10577308, Compound 553) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 300 | n/a | n/a | n/a | n/a | n/a | n/a |
The Florey Institute; The University of Melbourne US Patent | Assay Description Test Protocol for Determining the Potency of Compounds on Nav1.2 and Nav1.6 Subtypes of Voltage-Gated Sodium Ion Channels Expressed in Mammalian Cell... | US Patent US10577308 (2020) BindingDB Entry DOI: 10.7270/Q2CZ39JQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium channel protein type 8 subunit alpha (Homo sapiens (Human)) | BDBM432087 (US10577308, Compound 558) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 400 | n/a | n/a | n/a | n/a | n/a | n/a |
The Florey Institute; The University of Melbourne US Patent | Assay Description Test Protocol for Determining the Potency of Compounds on Nav1.2 and Nav1.6 Subtypes of Voltage-Gated Sodium Ion Channels Expressed in Mammalian Cell... | US Patent US10577308 (2020) BindingDB Entry DOI: 10.7270/Q2CZ39JQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium channel protein type 2 subunit alpha (Homo sapiens (Human)) | BDBM432070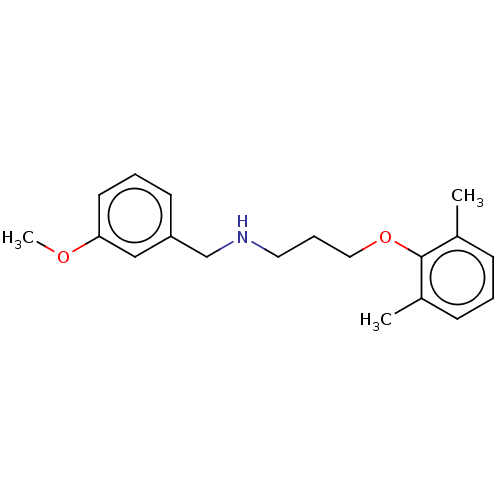 (US10577308, Compound 525) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 400 | n/a | n/a | n/a | n/a | n/a | n/a |
The Florey Institute; The University of Melbourne US Patent | Assay Description Test Protocol for Determining the Potency of Compounds on Nav1.2 and Nav1.6 Subtypes of Voltage-Gated Sodium Ion Channels Expressed in Mammalian Cell... | US Patent US10577308 (2020) BindingDB Entry DOI: 10.7270/Q2CZ39JQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium channel protein type 2 subunit alpha (Homo sapiens (Human)) | BDBM432075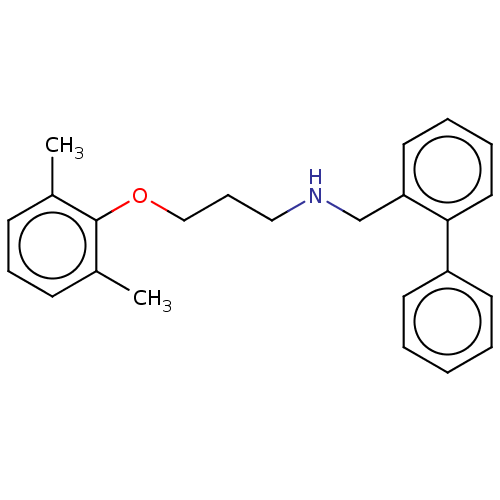 (US10577308, Compound 536) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 400 | n/a | n/a | n/a | n/a | n/a | n/a |
The Florey Institute; The University of Melbourne US Patent | Assay Description Test Protocol for Determining the Potency of Compounds on Nav1.2 and Nav1.6 Subtypes of Voltage-Gated Sodium Ion Channels Expressed in Mammalian Cell... | US Patent US10577308 (2020) BindingDB Entry DOI: 10.7270/Q2CZ39JQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium channel protein type 2 subunit alpha (Homo sapiens (Human)) | BDBM432075 (US10577308, Compound 536) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 400 | n/a | n/a | n/a | n/a | n/a | n/a |
The Florey Institute; The University of Melbourne US Patent | Assay Description Test Protocol for Determining the Potency of Compounds on Nav1.2 and Nav1.6 Subtypes of Voltage-Gated Sodium Ion Channels Expressed in Mammalian Cell... | US Patent US10577308 (2020) BindingDB Entry DOI: 10.7270/Q2CZ39JQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium channel protein type 2 subunit alpha (Homo sapiens (Human)) | BDBM432070 (US10577308, Compound 525) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 400 | n/a | n/a | n/a | n/a | n/a | n/a |
The Florey Institute; The University of Melbourne US Patent | Assay Description Test Protocol for Determining the Potency of Compounds on Nav1.2 and Nav1.6 Subtypes of Voltage-Gated Sodium Ion Channels Expressed in Mammalian Cell... | US Patent US10577308 (2020) BindingDB Entry DOI: 10.7270/Q2CZ39JQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium channel protein type 2 subunit alpha (Homo sapiens (Human)) | BDBM432058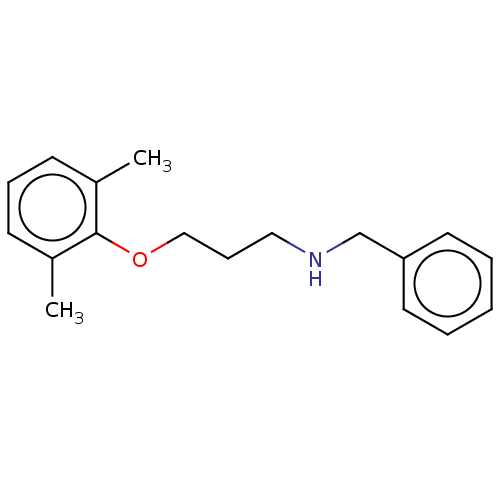 (US10577308, Compound 518) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 500 | n/a | n/a | n/a | n/a | n/a | n/a |
The Florey Institute; The University of Melbourne US Patent | Assay Description Test Protocol for Determining the Potency of Compounds on Nav1.2 and Nav1.6 Subtypes of Voltage-Gated Sodium Ion Channels Expressed in Mammalian Cell... | US Patent US10577308 (2020) BindingDB Entry DOI: 10.7270/Q2CZ39JQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium channel protein type 2 subunit alpha (Homo sapiens (Human)) | BDBM432058 (US10577308, Compound 518) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 500 | n/a | n/a | n/a | n/a | n/a | n/a |
The Florey Institute; The University of Melbourne US Patent | Assay Description Test Protocol for Determining the Potency of Compounds on Nav1.2 and Nav1.6 Subtypes of Voltage-Gated Sodium Ion Channels Expressed in Mammalian Cell... | US Patent US10577308 (2020) BindingDB Entry DOI: 10.7270/Q2CZ39JQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor (RAT) | BDBM50419932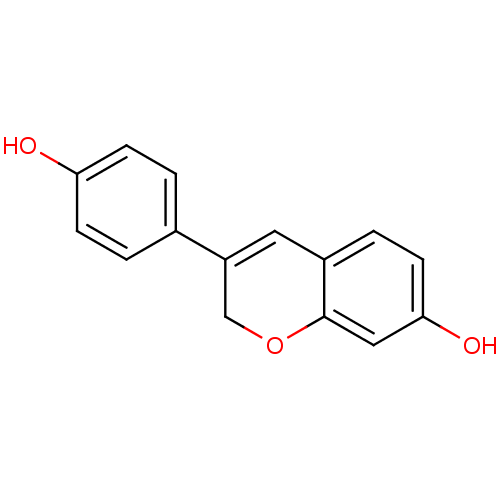 (IDRONOXIL) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase DrugBank MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 661 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Melbourne Curated by ChEMBL | Assay Description Displacement of [3H]estradiol from rat uterine cytosolic estrogen receptor | Bioorg Med Chem 20: 2353-61 (2012) Article DOI: 10.1016/j.bmc.2012.02.008 BindingDB Entry DOI: 10.7270/Q2F76DT2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium channel protein type 8 subunit alpha (Homo sapiens (Human)) | BDBM432077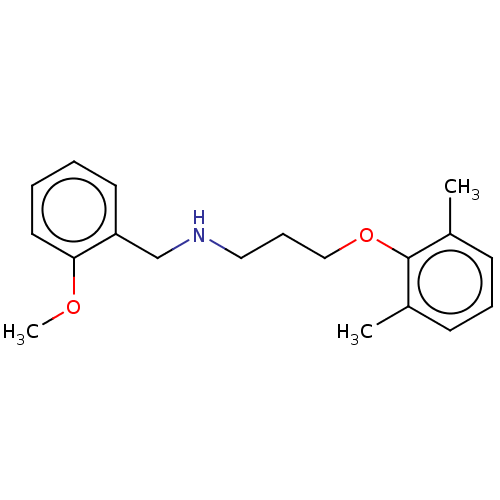 (US10577308, Compound 530) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 800 | n/a | n/a | n/a | n/a | n/a | n/a |
The Florey Institute; The University of Melbourne US Patent | Assay Description Test Protocol for Determining the Potency of Compounds on Nav1.2 and Nav1.6 Subtypes of Voltage-Gated Sodium Ion Channels Expressed in Mammalian Cell... | US Patent US10577308 (2020) BindingDB Entry DOI: 10.7270/Q2CZ39JQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 251 total ) | Next | Last >> |