 Found 32 hits with Last Name = 'winiski' and Initial = 'ap'
Found 32 hits with Last Name = 'winiski' and Initial = 'ap' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Steryl-sulfatase
(Homo sapiens (Human)) | BDBM50134329

(CHEMBL122708 | Sulfamic acid (11R,12S,15S,16S)-13-...)Show SMILES C[C@]12CC[C@H]3[C@@H](CCc4cc(OS(N)(=O)=O)ccc34)[C@@H]1CCC2=O Show InChI InChI=1S/C18H23NO4S/c1-18-9-8-14-13-5-3-12(23-24(19,21)22)10-11(13)2-4-15(14)16(18)6-7-17(18)20/h3,5,10,14-16H,2,4,6-9H2,1H3,(H2,19,21,22)/t14-,15-,16+,18+/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| 470 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Forschungsinstitut
Curated by ChEMBL
| Assay Description
Inhibitory constant against human steroid sulfatase in CHO cells |
Bioorg Med Chem Lett 13: 4313-6 (2003)
BindingDB Entry DOI: 10.7270/Q28S4QGH |
More data for this
Ligand-Target Pair | |
Steryl-sulfatase
(Homo sapiens (Human)) | BDBM50136297
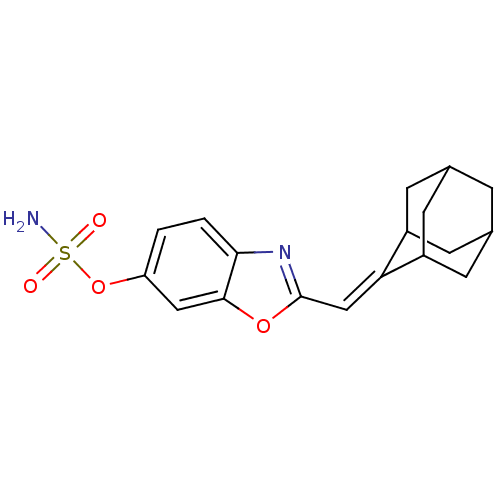
(CHEMBL136112 | Sulfamic acid 2-adamantan-2-ylidene...)Show SMILES NS(=O)(=O)Oc1ccc2nc(C=C3C4CC5CC(C4)CC3C5)oc2c1 |TLB:21:20:18:15.14.16,THB:21:15:12.20.19:18,16:15:12:17.19.18,16:17:12:15.14.21,11:12:18:15.14.16,(-1.25,-4.19,;.08,-3.42,;-.69,-2.08,;.85,-2.08,;1.41,-4.19,;2.76,-3.42,;2.76,-1.87,;4.09,-1.1,;5.42,-1.86,;6.91,-1.38,;7.82,-2.63,;9.36,-2.63,;10.13,-1.29,;9.45,.09,;10.55,1.03,;11.96,1.18,;12.47,2.87,;11.3,1.82,;9.8,1.7,;12.03,.4,;11.65,-1.18,;12.63,-.14,;6.91,-3.9,;5.42,-3.42,;4.09,-4.19,)| Show InChI InChI=1S/C18H20N2O4S/c19-25(21,22)24-14-1-2-16-17(8-14)23-18(20-16)9-15-12-4-10-3-11(6-12)7-13(15)5-10/h1-2,8-13H,3-7H2,(H2,19,21,22)/b15-9- | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 6.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Forschungsinstitut
Curated by ChEMBL
| Assay Description
Inhibitory constant against human steroid sulfatase in CHO cells |
Bioorg Med Chem Lett 13: 4313-6 (2003)
BindingDB Entry DOI: 10.7270/Q28S4QGH |
More data for this
Ligand-Target Pair | |
Steryl-sulfatase
(Homo sapiens (Human)) | BDBM50136297

(CHEMBL136112 | Sulfamic acid 2-adamantan-2-ylidene...)Show SMILES NS(=O)(=O)Oc1ccc2nc(C=C3C4CC5CC(C4)CC3C5)oc2c1 |TLB:21:20:18:15.14.16,THB:21:15:12.20.19:18,16:15:12:17.19.18,16:17:12:15.14.21,11:12:18:15.14.16,(-1.25,-4.19,;.08,-3.42,;-.69,-2.08,;.85,-2.08,;1.41,-4.19,;2.76,-3.42,;2.76,-1.87,;4.09,-1.1,;5.42,-1.86,;6.91,-1.38,;7.82,-2.63,;9.36,-2.63,;10.13,-1.29,;9.45,.09,;10.55,1.03,;11.96,1.18,;12.47,2.87,;11.3,1.82,;9.8,1.7,;12.03,.4,;11.65,-1.18,;12.63,-.14,;6.91,-3.9,;5.42,-3.42,;4.09,-4.19,)| Show InChI InChI=1S/C18H20N2O4S/c19-25(21,22)24-14-1-2-16-17(8-14)23-18(20-16)9-15-12-4-10-3-11(6-12)7-13(15)5-10/h1-2,8-13H,3-7H2,(H2,19,21,22)/b15-9- | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 0.150 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Forschungsinstitut
Curated by ChEMBL
| Assay Description
Inhibitory activity against human steroid sulfatase in sebocytes |
Bioorg Med Chem Lett 13: 4313-6 (2003)
BindingDB Entry DOI: 10.7270/Q28S4QGH |
More data for this
Ligand-Target Pair | |
Steryl-sulfatase
(Homo sapiens (Human)) | BDBM50118560
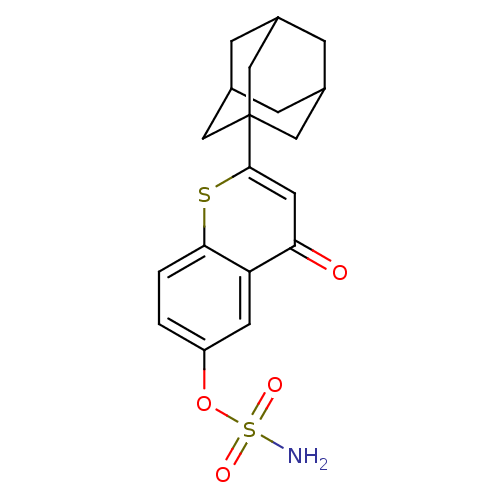
(CHEMBL262050 | Sulfamic acid 2-adamantan-1-yl-4-ox...)Show SMILES NS(=O)(=O)Oc1ccc2sc(cc(=O)c2c1)C12CC3CC(CC(C3)C1)C2 |TLB:23:22:25:17.18.19,23:18:25:24.22.21,THB:21:22:17:25.20.19,21:20:17:24.22.23| Show InChI InChI=1S/C19H21NO4S2/c20-26(22,23)24-14-1-2-17-15(6-14)16(21)7-18(25-17)19-8-11-3-12(9-19)5-13(4-11)10-19/h1-2,6-7,11-13H,3-5,8-10H2,(H2,20,22,23) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.340 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Research Institute Vienna
Curated by ChEMBL
| Assay Description
Inhibition of steroid sulfatase |
J Med Chem 46: 5091-4 (2003)
Article DOI: 10.1021/jm030926s
BindingDB Entry DOI: 10.7270/Q2RF5VR1 |
More data for this
Ligand-Target Pair | |
Steryl-sulfatase
(Homo sapiens (Human)) | BDBM50136297

(CHEMBL136112 | Sulfamic acid 2-adamantan-2-ylidene...)Show SMILES NS(=O)(=O)Oc1ccc2nc(C=C3C4CC5CC(C4)CC3C5)oc2c1 |TLB:21:20:18:15.14.16,THB:21:15:12.20.19:18,16:15:12:17.19.18,16:17:12:15.14.21,11:12:18:15.14.16,(-1.25,-4.19,;.08,-3.42,;-.69,-2.08,;.85,-2.08,;1.41,-4.19,;2.76,-3.42,;2.76,-1.87,;4.09,-1.1,;5.42,-1.86,;6.91,-1.38,;7.82,-2.63,;9.36,-2.63,;10.13,-1.29,;9.45,.09,;10.55,1.03,;11.96,1.18,;12.47,2.87,;11.3,1.82,;9.8,1.7,;12.03,.4,;11.65,-1.18,;12.63,-.14,;6.91,-3.9,;5.42,-3.42,;4.09,-4.19,)| Show InChI InChI=1S/C18H20N2O4S/c19-25(21,22)24-14-1-2-16-17(8-14)23-18(20-16)9-15-12-4-10-3-11(6-12)7-13(15)5-10/h1-2,8-13H,3-7H2,(H2,19,21,22)/b15-9- | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 0.75 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Forschungsinstitut
Curated by ChEMBL
| Assay Description
Inhibitory activity against human steroid sulfatase expressing in CHO cells |
Bioorg Med Chem Lett 13: 4313-6 (2003)
BindingDB Entry DOI: 10.7270/Q28S4QGH |
More data for this
Ligand-Target Pair | |
Steryl-sulfatase
(Homo sapiens (Human)) | BDBM50136297

(CHEMBL136112 | Sulfamic acid 2-adamantan-2-ylidene...)Show SMILES NS(=O)(=O)Oc1ccc2nc(C=C3C4CC5CC(C4)CC3C5)oc2c1 |TLB:21:20:18:15.14.16,THB:21:15:12.20.19:18,16:15:12:17.19.18,16:17:12:15.14.21,11:12:18:15.14.16,(-1.25,-4.19,;.08,-3.42,;-.69,-2.08,;.85,-2.08,;1.41,-4.19,;2.76,-3.42,;2.76,-1.87,;4.09,-1.1,;5.42,-1.86,;6.91,-1.38,;7.82,-2.63,;9.36,-2.63,;10.13,-1.29,;9.45,.09,;10.55,1.03,;11.96,1.18,;12.47,2.87,;11.3,1.82,;9.8,1.7,;12.03,.4,;11.65,-1.18,;12.63,-.14,;6.91,-3.9,;5.42,-3.42,;4.09,-4.19,)| Show InChI InChI=1S/C18H20N2O4S/c19-25(21,22)24-14-1-2-16-17(8-14)23-18(20-16)9-15-12-4-10-3-11(6-12)7-13(15)5-10/h1-2,8-13H,3-7H2,(H2,19,21,22)/b15-9- | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 0.770 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Forschungsinstitut
Curated by ChEMBL
| Assay Description
Inhibition of human STS in keratinocytes |
Bioorg Med Chem Lett 13: 4313-6 (2003)
BindingDB Entry DOI: 10.7270/Q28S4QGH |
More data for this
Ligand-Target Pair | |
Steryl-sulfatase
(Homo sapiens (Human)) | BDBM50136297

(CHEMBL136112 | Sulfamic acid 2-adamantan-2-ylidene...)Show SMILES NS(=O)(=O)Oc1ccc2nc(C=C3C4CC5CC(C4)CC3C5)oc2c1 |TLB:21:20:18:15.14.16,THB:21:15:12.20.19:18,16:15:12:17.19.18,16:17:12:15.14.21,11:12:18:15.14.16,(-1.25,-4.19,;.08,-3.42,;-.69,-2.08,;.85,-2.08,;1.41,-4.19,;2.76,-3.42,;2.76,-1.87,;4.09,-1.1,;5.42,-1.86,;6.91,-1.38,;7.82,-2.63,;9.36,-2.63,;10.13,-1.29,;9.45,.09,;10.55,1.03,;11.96,1.18,;12.47,2.87,;11.3,1.82,;9.8,1.7,;12.03,.4,;11.65,-1.18,;12.63,-.14,;6.91,-3.9,;5.42,-3.42,;4.09,-4.19,)| Show InChI InChI=1S/C18H20N2O4S/c19-25(21,22)24-14-1-2-16-17(8-14)23-18(20-16)9-15-12-4-10-3-11(6-12)7-13(15)5-10/h1-2,8-13H,3-7H2,(H2,19,21,22)/b15-9- | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Forschungsinstitut
Curated by ChEMBL
| Assay Description
Inhibitory activity against STS in human MCF-7 breast cancer cells |
Bioorg Med Chem Lett 13: 4313-6 (2003)
BindingDB Entry DOI: 10.7270/Q28S4QGH |
More data for this
Ligand-Target Pair | |
Steryl-sulfatase
(Homo sapiens (Human)) | BDBM50135162
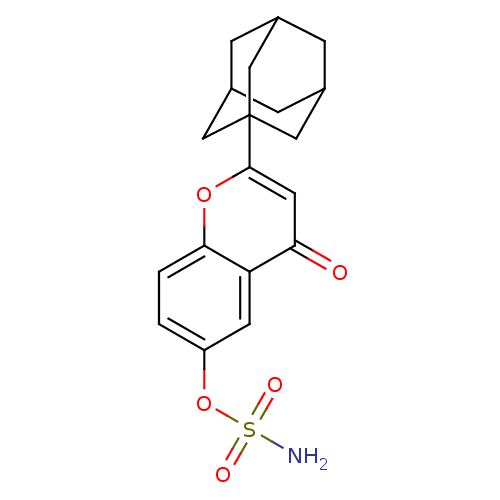
(CHEMBL147705 | Sulfamic acid 2-adamantan-1-yl-4-ox...)Show SMILES NS(=O)(=O)Oc1ccc2oc(cc(=O)c2c1)C12CC3CC(CC(C3)C1)C2 |TLB:23:18:25:24.22.21,23:22:25:17.18.19,THB:21:20:17:24.22.23,21:22:17:25.20.19| Show InChI InChI=1S/C19H21NO5S/c20-26(22,23)25-14-1-2-17-15(6-14)16(21)7-18(24-17)19-8-11-3-12(9-19)5-13(4-11)10-19/h1-2,6-7,11-13H,3-5,8-10H2,(H2,20,22,23) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Research Institute Vienna
Curated by ChEMBL
| Assay Description
Inhibition of steroid sulfatase |
J Med Chem 46: 5091-4 (2003)
Article DOI: 10.1021/jm030926s
BindingDB Entry DOI: 10.7270/Q2RF5VR1 |
More data for this
Ligand-Target Pair | |
Steryl-sulfatase
(Homo sapiens (Human)) | BDBM50135161
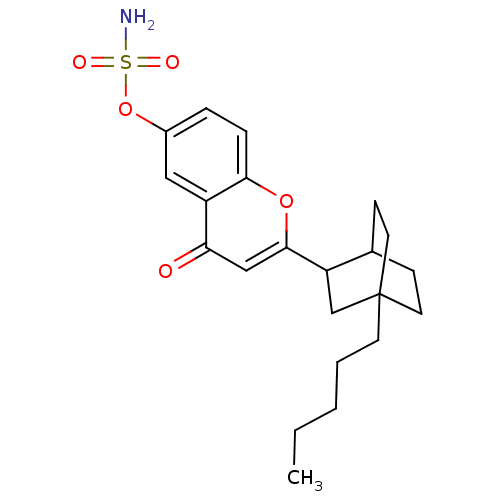
(CHEMBL359199 | Sulfamic acid 4-oxo-2-(4-pentyl-bic...)Show SMILES CCCCCC12CCC(CC1)C(C2)c1cc(=O)c2cc(OS(N)(=O)=O)ccc2o1 |TLB:13:11:7.6:9.10,(10.64,3.66,;9.31,2.89,;9.32,1.35,;7.99,.58,;8,-.96,;6.98,-2.11,;6.54,-.56,;5.14,-.56,;5.58,-2.18,;7.43,-2.69,;8.59,-2.41,;4.74,-3.45,;6.18,-3.37,;3.41,-4.22,;3.41,-5.76,;2.08,-6.53,;2.08,-8.06,;.75,-5.76,;-.58,-6.53,;-1.91,-5.76,;-3.26,-6.54,;-4.61,-5.77,;-5.97,-5,;-5.38,-7.12,;-3.84,-4.43,;-1.91,-4.22,;-.58,-3.45,;.75,-4.22,;2.08,-3.45,)| Show InChI InChI=1S/C22H29NO5S/c1-2-3-4-9-22-10-7-15(8-11-22)18(14-22)21-13-19(24)17-12-16(28-29(23,25)26)5-6-20(17)27-21/h5-6,12-13,15,18H,2-4,7-11,14H2,1H3,(H2,23,25,26) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Research Institute Vienna
Curated by ChEMBL
| Assay Description
Inhibition of steroid sulfatase |
J Med Chem 46: 5091-4 (2003)
Article DOI: 10.1021/jm030926s
BindingDB Entry DOI: 10.7270/Q2RF5VR1 |
More data for this
Ligand-Target Pair | |
Steryl-sulfatase
(Homo sapiens (Human)) | BDBM50118551

(CHEMBL137392 | Sulfamic acid 2-tert-butyl-4-oxo-4H...)Show InChI InChI=1S/C13H15NO5S/c1-13(2,3)12-7-10(15)9-6-8(19-20(14,16)17)4-5-11(9)18-12/h4-7H,1-3H3,(H2,14,16,17) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Research Institute Vienna
Curated by ChEMBL
| Assay Description
Inhibition of steroid sulfatase |
J Med Chem 46: 5091-4 (2003)
Article DOI: 10.1021/jm030926s
BindingDB Entry DOI: 10.7270/Q2RF5VR1 |
More data for this
Ligand-Target Pair | |
Steryl-sulfatase
(Homo sapiens (Human)) | BDBM50136297

(CHEMBL136112 | Sulfamic acid 2-adamantan-2-ylidene...)Show SMILES NS(=O)(=O)Oc1ccc2nc(C=C3C4CC5CC(C4)CC3C5)oc2c1 |TLB:21:20:18:15.14.16,THB:21:15:12.20.19:18,16:15:12:17.19.18,16:17:12:15.14.21,11:12:18:15.14.16,(-1.25,-4.19,;.08,-3.42,;-.69,-2.08,;.85,-2.08,;1.41,-4.19,;2.76,-3.42,;2.76,-1.87,;4.09,-1.1,;5.42,-1.86,;6.91,-1.38,;7.82,-2.63,;9.36,-2.63,;10.13,-1.29,;9.45,.09,;10.55,1.03,;11.96,1.18,;12.47,2.87,;11.3,1.82,;9.8,1.7,;12.03,.4,;11.65,-1.18,;12.63,-.14,;6.91,-3.9,;5.42,-3.42,;4.09,-4.19,)| Show InChI InChI=1S/C18H20N2O4S/c19-25(21,22)24-14-1-2-16-17(8-14)23-18(20-16)9-15-12-4-10-3-11(6-12)7-13(15)5-10/h1-2,8-13H,3-7H2,(H2,19,21,22)/b15-9- | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 32 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Forschungsinstitut
Curated by ChEMBL
| Assay Description
Inhibitory constant against human steroid sulfatase in CHO cells |
Bioorg Med Chem Lett 13: 4313-6 (2003)
BindingDB Entry DOI: 10.7270/Q28S4QGH |
More data for this
Ligand-Target Pair | |
Steryl-sulfatase
(Homo sapiens (Human)) | BDBM50118575

(CHEMBL137391 | Sulfamic acid 4-oxo-2-(2,2,3,3-tetr...)Show SMILES CC1(C)C(c2cc(=O)c3cc(OS(N)(=O)=O)ccc3o2)C1(C)C Show InChI InChI=1S/C16H19NO5S/c1-15(2)14(16(15,3)4)13-8-11(18)10-7-9(22-23(17,19)20)5-6-12(10)21-13/h5-8,14H,1-4H3,(H2,17,19,20) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 45 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Research Institute Vienna
Curated by ChEMBL
| Assay Description
Inhibition of steroid sulfatase |
J Med Chem 46: 5091-4 (2003)
Article DOI: 10.1021/jm030926s
BindingDB Entry DOI: 10.7270/Q2RF5VR1 |
More data for this
Ligand-Target Pair | |
Steryl-sulfatase
(Homo sapiens (Human)) | BDBM50118549

(CHEMBL137289 | Sulfamic acid 2-cyclododecyl-4-oxo-...)Show SMILES NS(=O)(=O)Oc1ccc2oc(cc(=O)c2c1)C1CCCCCCCCCCC1 Show InChI InChI=1S/C21H29NO5S/c22-28(24,25)27-17-12-13-20-18(14-17)19(23)15-21(26-20)16-10-8-6-4-2-1-3-5-7-9-11-16/h12-16H,1-11H2,(H2,22,24,25) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 45 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Research Institute Vienna
Curated by ChEMBL
| Assay Description
Inhibition of steroid sulfatase |
J Med Chem 46: 5091-4 (2003)
Article DOI: 10.1021/jm030926s
BindingDB Entry DOI: 10.7270/Q2RF5VR1 |
More data for this
Ligand-Target Pair | |
Steryl-sulfatase
(Homo sapiens (Human)) | BDBM50134329

(CHEMBL122708 | Sulfamic acid (11R,12S,15S,16S)-13-...)Show SMILES C[C@]12CC[C@H]3[C@@H](CCc4cc(OS(N)(=O)=O)ccc34)[C@@H]1CCC2=O Show InChI InChI=1S/C18H23NO4S/c1-18-9-8-14-13-5-3-12(23-24(19,21)22)10-11(13)2-4-15(14)16(18)6-7-17(18)20/h3,5,10,14-16H,2,4,6-9H2,1H3,(H2,19,21,22)/t14-,15-,16+,18+/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 56 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Research Institute Vienna
Curated by ChEMBL
| Assay Description
Inhibition of steroid sulfatase |
J Med Chem 46: 5091-4 (2003)
Article DOI: 10.1021/jm030926s
BindingDB Entry DOI: 10.7270/Q2RF5VR1 |
More data for this
Ligand-Target Pair | |
Steryl-sulfatase
(Homo sapiens (Human)) | BDBM50118570
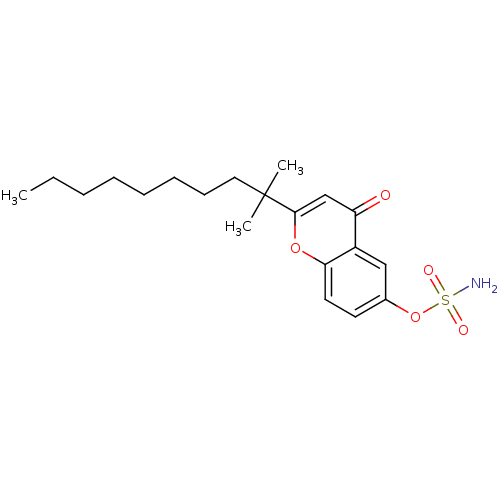
(CHEMBL137707 | Sulfamic acid 2-(1,1-dimethyl-nonyl...)Show SMILES CCCCCCCCC(C)(C)c1cc(=O)c2cc(OS(N)(=O)=O)ccc2o1 Show InChI InChI=1S/C20H29NO5S/c1-4-5-6-7-8-9-12-20(2,3)19-14-17(22)16-13-15(26-27(21,23)24)10-11-18(16)25-19/h10-11,13-14H,4-9,12H2,1-3H3,(H2,21,23,24) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 78 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Research Institute Vienna
Curated by ChEMBL
| Assay Description
Inhibition of steroid sulfatase |
J Med Chem 46: 5091-4 (2003)
Article DOI: 10.1021/jm030926s
BindingDB Entry DOI: 10.7270/Q2RF5VR1 |
More data for this
Ligand-Target Pair | |
Steryl-sulfatase
(Homo sapiens (Human)) | BDBM50118564

(CHEMBL136663 | Sulfamic acid 2-adamantan-1-yl-4-ox...)Show SMILES NS(=O)(=O)Oc1ccc2OC(CC(=O)c2c1)C12CC3CC(CC(C3)C1)C2 |TLB:23:22:25:17.18.19,23:18:25:24.22.21,THB:21:22:17:25.20.19,21:20:17:24.22.23| Show InChI InChI=1S/C19H23NO5S/c20-26(22,23)25-14-1-2-17-15(6-14)16(21)7-18(24-17)19-8-11-3-12(9-19)5-13(4-11)10-19/h1-2,6,11-13,18H,3-5,7-10H2,(H2,20,22,23) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 140 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Research Institute Vienna
Curated by ChEMBL
| Assay Description
Inhibition of steroid sulfatase |
J Med Chem 46: 5091-4 (2003)
Article DOI: 10.1021/jm030926s
BindingDB Entry DOI: 10.7270/Q2RF5VR1 |
More data for this
Ligand-Target Pair | |
Steryl-sulfatase
(Homo sapiens (Human)) | BDBM50118574

(CHEMBL137119 | Sulfamic acid 4-oxo-2-phenethyl-4H-...)Show InChI InChI=1S/C17H15NO5S/c18-24(20,21)23-14-8-9-17-15(10-14)16(19)11-13(22-17)7-6-12-4-2-1-3-5-12/h1-5,8-11H,6-7H2,(H2,18,20,21) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 190 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Research Institute Vienna
Curated by ChEMBL
| Assay Description
Inhibition of steroid sulfatase |
J Med Chem 46: 5091-4 (2003)
Article DOI: 10.1021/jm030926s
BindingDB Entry DOI: 10.7270/Q2RF5VR1 |
More data for this
Ligand-Target Pair | |
Steryl-sulfatase
(Homo sapiens (Human)) | BDBM50118567
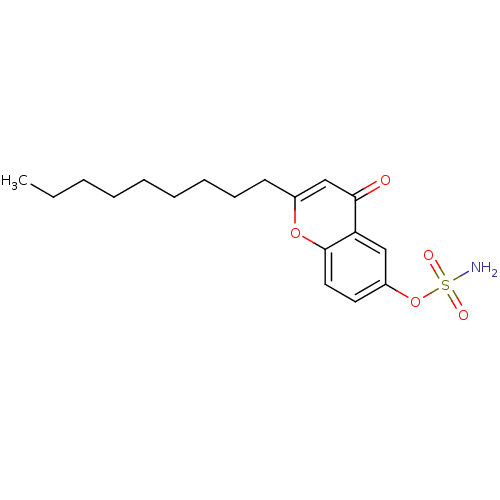
(CHEMBL137692 | Sulfamic acid 2-nonyl-4-oxo-4H-chro...)Show InChI InChI=1S/C18H25NO5S/c1-2-3-4-5-6-7-8-9-14-13-17(20)16-12-15(24-25(19,21)22)10-11-18(16)23-14/h10-13H,2-9H2,1H3,(H2,19,21,22) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 403 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Research Institute Vienna
Curated by ChEMBL
| Assay Description
Inhibition of steroid sulfatase |
J Med Chem 46: 5091-4 (2003)
Article DOI: 10.1021/jm030926s
BindingDB Entry DOI: 10.7270/Q2RF5VR1 |
More data for this
Ligand-Target Pair | |
Tubulin beta chain
(Sus scrofa) | BDBM50035218
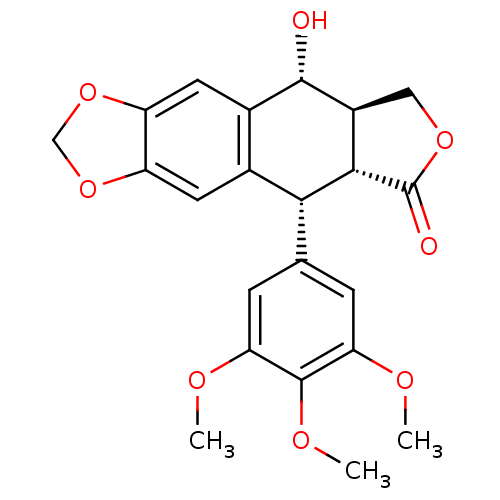
(CHEMBL61 | PODOFILOX | Podophyllinic acid lactone ...)Show SMILES COc1cc(cc(OC)c1OC)[C@H]1[C@@H]2[C@H](COC2=O)[C@@H](O)c2cc3OCOc3cc12 |r| Show InChI InChI=1S/C22H22O8/c1-25-16-4-10(5-17(26-2)21(16)27-3)18-11-6-14-15(30-9-29-14)7-12(11)20(23)13-8-28-22(24)19(13)18/h4-7,13,18-20,23H,8-9H2,1-3H3/t13-,18+,19-,20-/m0/s1 | PDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 560 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Binding affinity to pig brain tubulin at 10 uM after 15 mins relative to control |
Bioorg Med Chem 16: 7552-60 (2008)
Article DOI: 10.1016/j.bmc.2008.07.039
BindingDB Entry DOI: 10.7270/Q2FN1909 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Tubulin beta chain
(Sus scrofa) | BDBM50038200

(5-[2-(2,5-Dimethoxy-phenyl)-ethyl]-2-hydroxy-benzo...)Show InChI InChI=1S/C18H20O5/c1-21-14-7-9-17(22-2)13(11-14)6-4-12-5-8-16(19)15(10-12)18(20)23-3/h5,7-11,19H,4,6H2,1-3H3 | PDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of pig brain tubulin polymerization |
Bioorg Med Chem 16: 7552-60 (2008)
Article DOI: 10.1016/j.bmc.2008.07.039
BindingDB Entry DOI: 10.7270/Q2FN1909 |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50038199
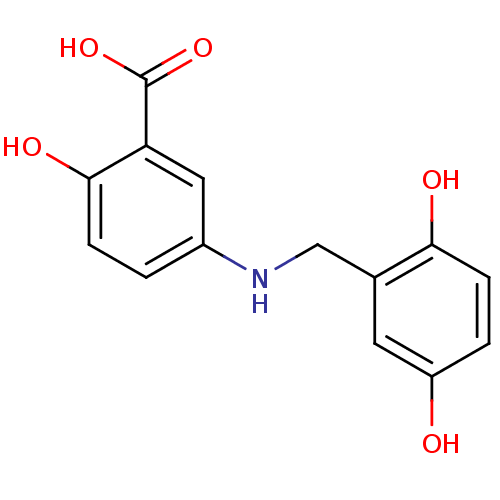
(5-(2,5-Dihydroxy-benzylamino)-2-hydroxy-benzoic ac...)Show InChI InChI=1S/C14H13NO5/c16-10-2-4-12(17)8(5-10)7-15-9-1-3-13(18)11(6-9)14(19)20/h1-6,15-18H,7H2,(H,19,20) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 2.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
SANDOZ Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of EGF-R Tyrosine kinase (TK) |
J Med Chem 37: 4079-84 (1995)
BindingDB Entry DOI: 10.7270/Q25D8QWP |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50038203
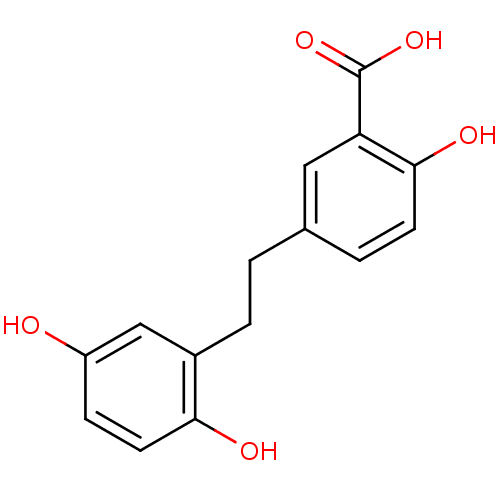
(5-[2-(2,5-Dihydroxy-phenyl)-ethyl]-2-hydroxy-benzo...)Show InChI InChI=1S/C15H14O5/c16-11-4-6-13(17)10(8-11)3-1-9-2-5-14(18)12(7-9)15(19)20/h2,4-8,16-18H,1,3H2,(H,19,20) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 3.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
SANDOZ Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of EGF-R Tyrosine kinase (TK) |
J Med Chem 37: 4079-84 (1995)
BindingDB Entry DOI: 10.7270/Q25D8QWP |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50038202

(5-((2,5-dihydroxybenzyl)(2-hydroxybenzyl)amino)-2-...)Show SMILES OC(=O)c1cc(ccc1O)N(Cc1ccccc1O)Cc1cc(O)ccc1O Show InChI InChI=1S/C21H19NO6/c23-16-6-8-19(25)14(9-16)12-22(11-13-3-1-2-4-18(13)24)15-5-7-20(26)17(10-15)21(27)28/h1-10,23-26H,11-12H2,(H,27,28) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 4.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
SANDOZ Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of EGF-R Tyrosine kinase (TK) |
J Med Chem 37: 4079-84 (1995)
BindingDB Entry DOI: 10.7270/Q25D8QWP |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50038205

(5-(2,5-Dihydroxy-benzylamino)-2-hydroxy-benzoic ac...)Show InChI InChI=1S/C15H15NO5/c1-21-15(20)12-7-10(2-4-14(12)19)16-8-9-6-11(17)3-5-13(9)18/h2-7,16-19H,8H2,1H3 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 2.44E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
SANDOZ Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of EGF-R Tyrosine kinase (TK) |
J Med Chem 37: 4079-84 (1995)
BindingDB Entry DOI: 10.7270/Q25D8QWP |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50038200

(5-[2-(2,5-Dimethoxy-phenyl)-ethyl]-2-hydroxy-benzo...)Show InChI InChI=1S/C18H20O5/c1-21-14-7-9-17(22-2)13(11-14)6-4-12-5-8-16(19)15(10-12)18(20)23-3/h5,7-11,19H,4,6H2,1-3H3 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
SANDOZ Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of EGF-R Tyrosine kinase (TK) |
J Med Chem 37: 4079-84 (1995)
BindingDB Entry DOI: 10.7270/Q25D8QWP |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50038206
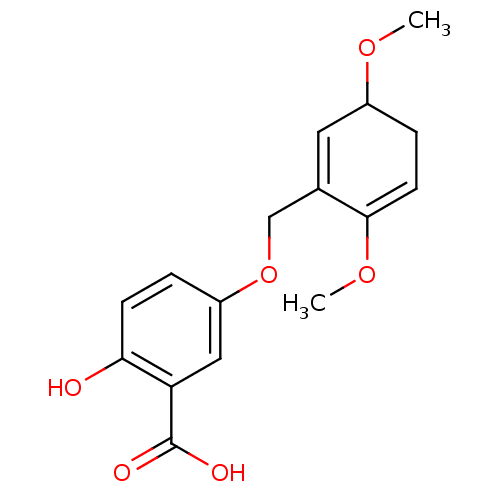
(5-(3,6-Dimethoxy-cyclohexa-1,5-dienylmethoxy)-2-hy...)Show SMILES COC1CC=C(OC)C(COc2ccc(O)c(c2)C(O)=O)=C1 |c:21,t:4| Show InChI InChI=1S/C16H18O6/c1-20-11-4-6-15(21-2)10(7-11)9-22-12-3-5-14(17)13(8-12)16(18)19/h3,5-8,11,17H,4,9H2,1-2H3,(H,18,19) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
SANDOZ Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of EGF-R Tyrosine kinase (TK) |
J Med Chem 37: 4079-84 (1995)
BindingDB Entry DOI: 10.7270/Q25D8QWP |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50038197
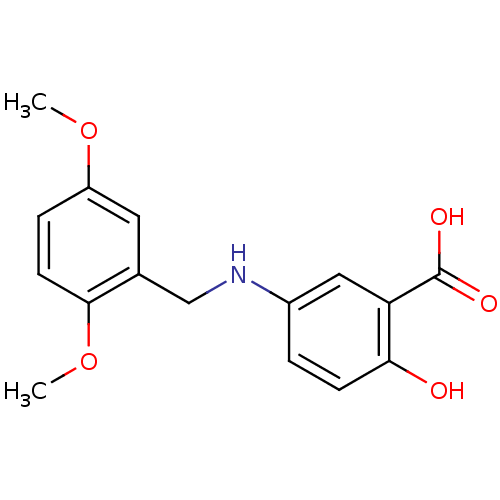
(5-(2,5-Dimethoxy-benzylamino)-2-hydroxy-benzoic ac...)Show InChI InChI=1S/C16H17NO5/c1-21-12-4-6-15(22-2)10(7-12)9-17-11-3-5-14(18)13(8-11)16(19)20/h3-8,17-18H,9H2,1-2H3,(H,19,20) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
SANDOZ Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of EGF-R Tyrosine kinase (TK) |
J Med Chem 37: 4079-84 (1995)
BindingDB Entry DOI: 10.7270/Q25D8QWP |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50038201
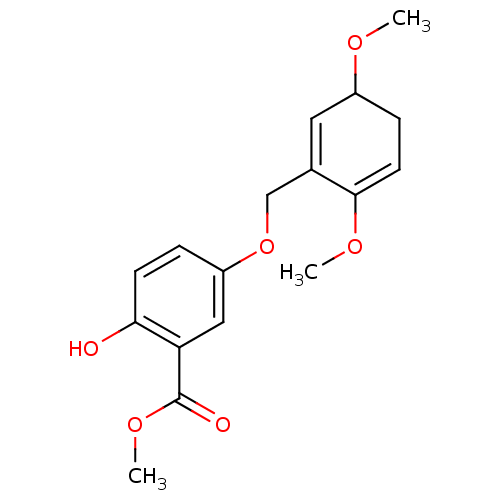
(5-(3,6-Dimethoxy-cyclohexa-1,5-dienylmethoxy)-2-hy...)Show SMILES COC1CC=C(OC)C(COc2ccc(O)c(c2)C(=O)OC)=C1 |c:22,t:4| Show InChI InChI=1S/C17H20O6/c1-20-12-5-7-16(21-2)11(8-12)10-23-13-4-6-15(18)14(9-13)17(19)22-3/h4,6-9,12,18H,5,10H2,1-3H3 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
SANDOZ Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of EGF-R Tyrosine kinase (TK) |
J Med Chem 37: 4079-84 (1995)
BindingDB Entry DOI: 10.7270/Q25D8QWP |
More data for this
Ligand-Target Pair | |
Estrogen receptor beta
(Homo sapiens (Human)) | BDBM50136297

(CHEMBL136112 | Sulfamic acid 2-adamantan-2-ylidene...)Show SMILES NS(=O)(=O)Oc1ccc2nc(C=C3C4CC5CC(C4)CC3C5)oc2c1 |TLB:21:20:18:15.14.16,THB:21:15:12.20.19:18,16:15:12:17.19.18,16:17:12:15.14.21,11:12:18:15.14.16,(-1.25,-4.19,;.08,-3.42,;-.69,-2.08,;.85,-2.08,;1.41,-4.19,;2.76,-3.42,;2.76,-1.87,;4.09,-1.1,;5.42,-1.86,;6.91,-1.38,;7.82,-2.63,;9.36,-2.63,;10.13,-1.29,;9.45,.09,;10.55,1.03,;11.96,1.18,;12.47,2.87,;11.3,1.82,;9.8,1.7,;12.03,.4,;11.65,-1.18,;12.63,-.14,;6.91,-3.9,;5.42,-3.42,;4.09,-4.19,)| Show InChI InChI=1S/C18H20N2O4S/c19-25(21,22)24-14-1-2-16-17(8-14)23-18(20-16)9-15-12-4-10-3-11(6-12)7-13(15)5-10/h1-2,8-13H,3-7H2,(H2,19,21,22)/b15-9- | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a |
Novartis Forschungsinstitut
Curated by ChEMBL
| Assay Description
Affinity for human estrogen receptor beta |
Bioorg Med Chem Lett 13: 4313-6 (2003)
BindingDB Entry DOI: 10.7270/Q28S4QGH |
More data for this
Ligand-Target Pair | |
Estrogen receptor
(Homo sapiens (Human)) | BDBM50136297

(CHEMBL136112 | Sulfamic acid 2-adamantan-2-ylidene...)Show SMILES NS(=O)(=O)Oc1ccc2nc(C=C3C4CC5CC(C4)CC3C5)oc2c1 |TLB:21:20:18:15.14.16,THB:21:15:12.20.19:18,16:15:12:17.19.18,16:17:12:15.14.21,11:12:18:15.14.16,(-1.25,-4.19,;.08,-3.42,;-.69,-2.08,;.85,-2.08,;1.41,-4.19,;2.76,-3.42,;2.76,-1.87,;4.09,-1.1,;5.42,-1.86,;6.91,-1.38,;7.82,-2.63,;9.36,-2.63,;10.13,-1.29,;9.45,.09,;10.55,1.03,;11.96,1.18,;12.47,2.87,;11.3,1.82,;9.8,1.7,;12.03,.4,;11.65,-1.18,;12.63,-.14,;6.91,-3.9,;5.42,-3.42,;4.09,-4.19,)| Show InChI InChI=1S/C18H20N2O4S/c19-25(21,22)24-14-1-2-16-17(8-14)23-18(20-16)9-15-12-4-10-3-11(6-12)7-13(15)5-10/h1-2,8-13H,3-7H2,(H2,19,21,22)/b15-9- | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a |
Novartis Forschungsinstitut
Curated by ChEMBL
| Assay Description
Affinity for human estrogen receptor alpha |
Bioorg Med Chem Lett 13: 4313-6 (2003)
BindingDB Entry DOI: 10.7270/Q28S4QGH |
More data for this
Ligand-Target Pair | |
Estrogen receptor beta
(Homo sapiens (Human)) | BDBM50134329

(CHEMBL122708 | Sulfamic acid (11R,12S,15S,16S)-13-...)Show SMILES C[C@]12CC[C@H]3[C@@H](CCc4cc(OS(N)(=O)=O)ccc34)[C@@H]1CCC2=O Show InChI InChI=1S/C18H23NO4S/c1-18-9-8-14-13-5-3-12(23-24(19,21)22)10-11(13)2-4-15(14)16(18)6-7-17(18)20/h3,5,10,14-16H,2,4,6-9H2,1H3,(H2,19,21,22)/t14-,15-,16+,18+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| n/a | n/a | n/a | n/a | 3.70E+3 | n/a | n/a | n/a | n/a |
Novartis Forschungsinstitut
Curated by ChEMBL
| Assay Description
Affinity for human estrogen receptor beta |
Bioorg Med Chem Lett 13: 4313-6 (2003)
BindingDB Entry DOI: 10.7270/Q28S4QGH |
More data for this
Ligand-Target Pair | |
Estrogen receptor
(Homo sapiens (Human)) | BDBM50134329

(CHEMBL122708 | Sulfamic acid (11R,12S,15S,16S)-13-...)Show SMILES C[C@]12CC[C@H]3[C@@H](CCc4cc(OS(N)(=O)=O)ccc34)[C@@H]1CCC2=O Show InChI InChI=1S/C18H23NO4S/c1-18-9-8-14-13-5-3-12(23-24(19,21)22)10-11(13)2-4-15(14)16(18)6-7-17(18)20/h3,5,10,14-16H,2,4,6-9H2,1H3,(H2,19,21,22)/t14-,15-,16+,18+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| n/a | n/a | n/a | n/a | 2.60E+3 | n/a | n/a | n/a | n/a |
Novartis Forschungsinstitut
Curated by ChEMBL
| Assay Description
Affinity for human estrogen receptor alpha |
Bioorg Med Chem Lett 13: 4313-6 (2003)
BindingDB Entry DOI: 10.7270/Q28S4QGH |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data