 Found 51 hits with Last Name = 'wu' and Initial = 'jd'
Found 51 hits with Last Name = 'wu' and Initial = 'jd' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Replicase polyprotein 1ab
(Human SARS coronavirus (SARS-CoV) (Severe acute re...) | BDBM11233

(N-[(benzyloxy)carbonyl]-O-(tert-butyl)-L-threonyl-...)Show SMILES C[C@H](OC(C)(C)C)[C@H](NC(=O)OCc1ccccc1)C(=O)N[C@@H](CC1CCCCC1)C(=O)N[C@@H](C[C@@H]1CCNC1=O)C=O |r| Show InChI InChI=1S/C32H48N4O7/c1-21(43-32(2,3)4)27(36-31(41)42-20-23-13-9-6-10-14-23)30(40)35-26(17-22-11-7-5-8-12-22)29(39)34-25(19-37)18-24-15-16-33-28(24)38/h6,9-10,13-14,19,21-22,24-27H,5,7-8,11-12,15-18,20H2,1-4H3,(H,33,38)(H,34,39)(H,35,40)(H,36,41)/t21-,24-,25-,26-,27-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 53 | -41.5 | n/a | n/a | n/a | n/a | n/a | 7.5 | 25 |
TaiGen Biotechnology Co.
| Assay Description
The effects of compound on enzyme activity were measured by using peptide cleavage assay. Cleavage products were resolved and analyzed with a reverse... |
J Med Chem 49: 4971-80 (2006)
Article DOI: 10.1021/jm0603926
BindingDB Entry DOI: 10.7270/Q24B2ZJT |
More data for this
Ligand-Target Pair | |
Replicase polyprotein 1ab
(Human SARS coronavirus (SARS-CoV) (Severe acute re...) | BDBM11232
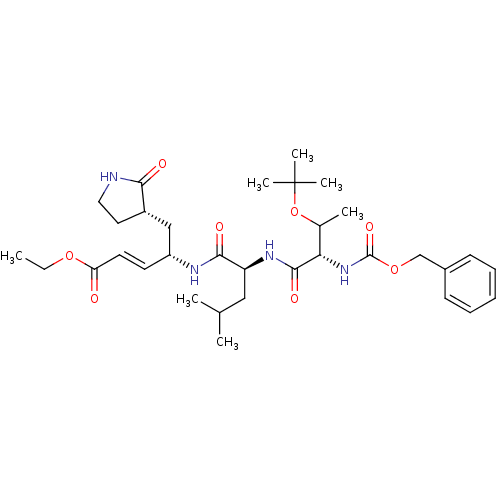
(N-[(benzyloxy)carbonyl]-O-(tert-butyl)-L-threonyl-...)Show SMILES CCOC(=O)\C=C\[C@H](C[C@@H]1CCNC1=O)NC(=O)[C@H](CC(C)C)NC(=O)[C@@H](NC(=O)OCc1ccccc1)C(C)OC(C)(C)C |r| Show InChI InChI=1S/C33H50N4O8/c1-8-43-27(38)15-14-25(19-24-16-17-34-29(24)39)35-30(40)26(18-21(2)3)36-31(41)28(22(4)45-33(5,6)7)37-32(42)44-20-23-12-10-9-11-13-23/h9-15,21-22,24-26,28H,8,16-20H2,1-7H3,(H,34,39)(H,35,40)(H,36,41)(H,37,42)/b15-14+/t22?,24-,25+,26-,28-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 58 | -41.3 | n/a | n/a | n/a | n/a | n/a | 7.5 | 25 |
TaiGen Biotechnology Co.
| Assay Description
The effects of compound on enzyme activity were measured by using peptide cleavage assay. Cleavage products were resolved and analyzed with a reverse... |
J Med Chem 49: 4971-80 (2006)
Article DOI: 10.1021/jm0603926
BindingDB Entry DOI: 10.7270/Q24B2ZJT |
More data for this
Ligand-Target Pair | |
Replicase polyprotein 1ab
(Human SARS coronavirus (SARS-CoV) (Severe acute re...) | BDBM11231

(N-[(benzyloxy)carbonyl]-L-valyl-N1-((1S,2E)-4-etho...)Show SMILES CCOC(=O)\C=C\[C@H](C[C@@H]1CCNC1=O)NC(=O)[C@H](CC(C)C)NC(=O)[C@@H](NC(=O)OCc1ccccc1)C(C)C |r| Show InChI InChI=1S/C30H44N4O7/c1-6-40-25(35)13-12-23(17-22-14-15-31-27(22)36)32-28(37)24(16-19(2)3)33-29(38)26(20(4)5)34-30(39)41-18-21-10-8-7-9-11-21/h7-13,19-20,22-24,26H,6,14-18H2,1-5H3,(H,31,36)(H,32,37)(H,33,38)(H,34,39)/b13-12+/t22-,23+,24-,26-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 660 | -35.3 | n/a | n/a | n/a | n/a | n/a | 7.5 | 25 |
TaiGen Biotechnology Co.
| Assay Description
The effects of compound on enzyme activity were measured by using peptide cleavage assay. Cleavage products were resolved and analyzed with a reverse... |
J Med Chem 49: 4971-80 (2006)
Article DOI: 10.1021/jm0603926
BindingDB Entry DOI: 10.7270/Q24B2ZJT |
More data for this
Ligand-Target Pair | |
Replicase polyprotein 1ab
(Human SARS coronavirus (SARS-CoV) (Severe acute re...) | BDBM11230
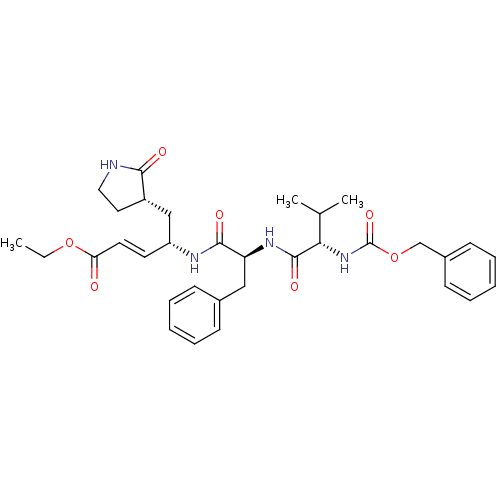
(AG7088 analogue 2d | CHEMBL277716 | N-[(benzyloxy)...)Show SMILES CCOC(=O)\C=C\[C@H](C[C@@H]1CCNC1=O)NC(=O)[C@H](Cc1ccccc1)NC(=O)[C@@H](NC(=O)OCc1ccccc1)C(C)C |r| Show InChI InChI=1S/C33H42N4O7/c1-4-43-28(38)16-15-26(20-25-17-18-34-30(25)39)35-31(40)27(19-23-11-7-5-8-12-23)36-32(41)29(22(2)3)37-33(42)44-21-24-13-9-6-10-14-24/h5-16,22,25-27,29H,4,17-21H2,1-3H3,(H,34,39)(H,35,40)(H,36,41)(H,37,42)/b16-15+/t25-,26+,27-,29-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 2.26E+3 | -32.2 | n/a | n/a | n/a | n/a | n/a | 7.5 | 25 |
TaiGen Biotechnology Co.
| Assay Description
The effects of compound on enzyme activity were measured by using peptide cleavage assay. Cleavage products were resolved and analyzed with a reverse... |
J Med Chem 49: 4971-80 (2006)
Article DOI: 10.1021/jm0603926
BindingDB Entry DOI: 10.7270/Q24B2ZJT |
More data for this
Ligand-Target Pair | |
Replicase polyprotein 1ab
(Human SARS coronavirus (SARS-CoV) (Severe acute re...) | BDBM11229
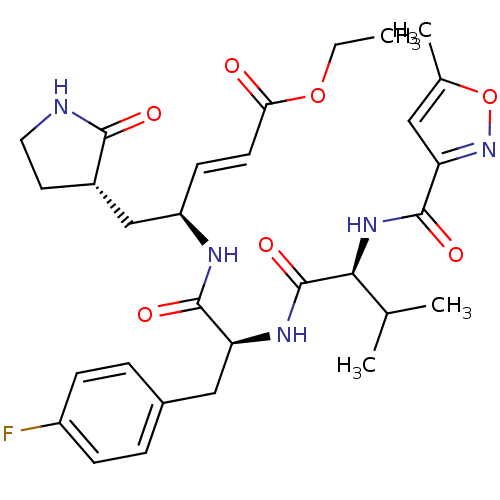
(AG7088 analogue 2a | CHEMBL20636 | N-[(5-methyliso...)Show SMILES CCOC(=O)\C=C\[C@H](C[C@@H]1CCNC1=O)NC(=O)[C@H](Cc1ccc(F)cc1)NC(=O)[C@@H](NC(=O)c1cc(C)on1)C(C)C |r| Show InChI InChI=1S/C30H38FN5O7/c1-5-42-25(37)11-10-22(16-20-12-13-32-27(20)38)33-28(39)23(15-19-6-8-21(31)9-7-19)34-30(41)26(17(2)3)35-29(40)24-14-18(4)43-36-24/h6-11,14,17,20,22-23,26H,5,12-13,15-16H2,1-4H3,(H,32,38)(H,33,39)(H,34,41)(H,35,40)/b11-10+/t20-,22+,23-,26-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| >1.00E+4 | >-28.5 | n/a | n/a | n/a | n/a | n/a | 7.5 | 25 |
TaiGen Biotechnology Co.
| Assay Description
The effects of compound on enzyme activity were measured by using peptide cleavage assay. Cleavage products were resolved and analyzed with a reverse... |
J Med Chem 49: 4971-80 (2006)
Article DOI: 10.1021/jm0603926
BindingDB Entry DOI: 10.7270/Q24B2ZJT |
More data for this
Ligand-Target Pair | |
Histone deacetylase 6
(Homo sapiens (Human)) | BDBM50579375
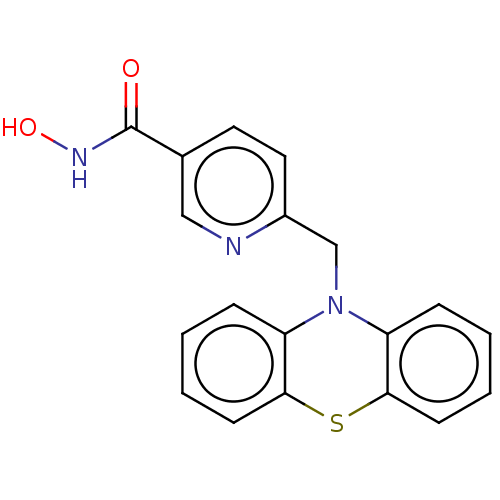
(CHEMBL4858133) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human HDAC6 using fluorogenic substrate incubated for 1 hrs by fluorescence plate reader assay |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113821
BindingDB Entry DOI: 10.7270/Q2HX1HHW |
More data for this
Ligand-Target Pair | |
Histone deacetylase 6
(Homo sapiens (Human)) | BDBM50579375

(CHEMBL4858133) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human HDAC6 using fluorogenic substrate incubated for 1 hrs by fluorescence plate reader assay |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113821
BindingDB Entry DOI: 10.7270/Q2HX1HHW |
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3 beta
(Homo sapiens (Human)) | BDBM2579

((2S,3R,4R,6R)-3-methoxy-2-methyl-4-(methylamino)-2...)Show SMILES CN[C@@H]1C[C@H]2O[C@@](C)([C@@H]1OC)n1c3ccccc3c3c4CNC(=O)c4c4c5ccccc5n2c4c13 |r| Show InChI InChI=1S/C28H26N4O3/c1-28-26(34-3)17(29-2)12-20(35-28)31-18-10-6-4-8-14(18)22-23-16(13-30-27(23)33)21-15-9-5-7-11-19(15)32(28)25(21)24(22)31/h4-11,17,20,26,29H,12-13H2,1-3H3,(H,30,33)/t17-,20-,26-,28+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 24 | n/a | n/a | n/a | n/a | n/a | n/a |
Shandong University
Curated by ChEMBL
| Assay Description
Inhibition of GSK-3beta (unknown origin) using FAM-labeled peptide as substrate preincubated for 10 mins followed by substrate addition by caliper as... |
Eur J Med Chem 167: 211-225 (2019)
Article DOI: 10.1016/j.ejmech.2019.02.001
BindingDB Entry DOI: 10.7270/Q26976WC |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Glycogen synthase kinase-3 beta
(Homo sapiens (Human)) | BDBM50510152
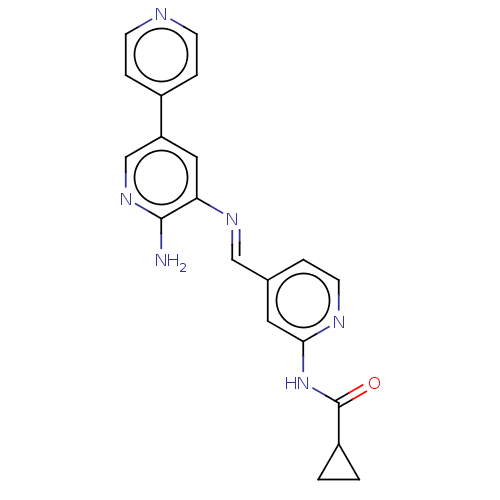
(CHEMBL4454448)Show SMILES Nc1ncc(cc1\N=C\c1ccnc(NC(=O)C2CC2)c1)-c1ccncc1 Show InChI InChI=1S/C20H18N6O/c21-19-17(10-16(12-25-19)14-4-6-22-7-5-14)24-11-13-3-8-23-18(9-13)26-20(27)15-1-2-15/h3-12,15H,1-2H2,(H2,21,25)(H,23,26,27)/b24-11+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 38 | n/a | n/a | n/a | n/a | n/a | n/a |
Shandong University
Curated by ChEMBL
| Assay Description
Inhibition of GSK-3beta (unknown origin) using FAM-labeled peptide as substrate preincubated for 10 mins followed by substrate addition by caliper as... |
Eur J Med Chem 167: 211-225 (2019)
Article DOI: 10.1016/j.ejmech.2019.02.001
BindingDB Entry DOI: 10.7270/Q26976WC |
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3 beta
(Homo sapiens (Human)) | BDBM50510144
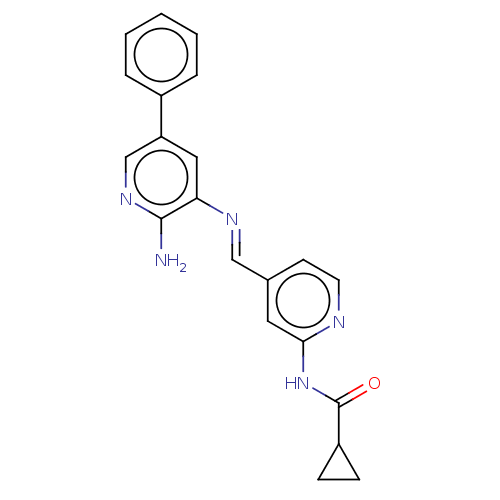
(CHEMBL4559169)Show SMILES Nc1ncc(cc1\N=C\c1ccnc(NC(=O)C2CC2)c1)-c1ccccc1 Show InChI InChI=1S/C21H19N5O/c22-20-18(11-17(13-25-20)15-4-2-1-3-5-15)24-12-14-8-9-23-19(10-14)26-21(27)16-6-7-16/h1-5,8-13,16H,6-7H2,(H2,22,25)(H,23,26,27)/b24-12+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 49 | n/a | n/a | n/a | n/a | n/a | n/a |
Shandong University
Curated by ChEMBL
| Assay Description
Inhibition of GSK-3beta (unknown origin) using FAM-labeled peptide as substrate preincubated for 10 mins followed by substrate addition by caliper as... |
Eur J Med Chem 167: 211-225 (2019)
Article DOI: 10.1016/j.ejmech.2019.02.001
BindingDB Entry DOI: 10.7270/Q26976WC |
More data for this
Ligand-Target Pair | |
Histone deacetylase 6
(Homo sapiens (Human)) | BDBM50579387
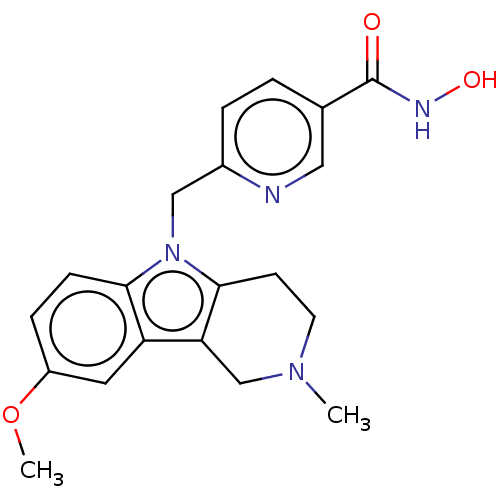
(CHEMBL4857648)Show SMILES COc1ccc2n(Cc3ccc(cn3)C(=O)NO)c3CCN(C)Cc3c2c1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 53 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human HDAC6 using fluorogenic substrate incubated for 1 hrs by fluorescence plate reader assay |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113821
BindingDB Entry DOI: 10.7270/Q2HX1HHW |
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3 beta
(Homo sapiens (Human)) | BDBM50510145
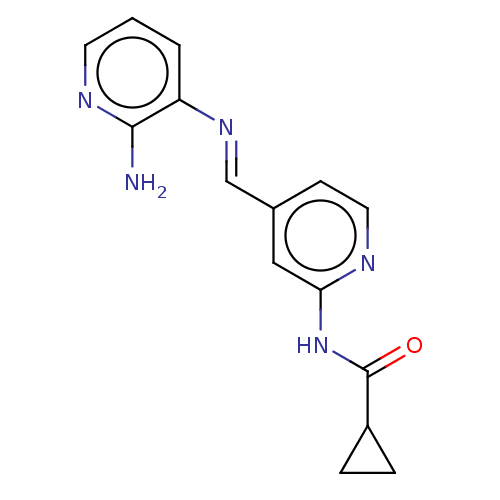
(CHEMBL4467668)Show InChI InChI=1S/C15H15N5O/c16-14-12(2-1-6-18-14)19-9-10-5-7-17-13(8-10)20-15(21)11-3-4-11/h1-2,5-9,11H,3-4H2,(H2,16,18)(H,17,20,21)/b19-9+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 71 | n/a | n/a | n/a | n/a | n/a | n/a |
Shandong University
Curated by ChEMBL
| Assay Description
Inhibition of GSK-3beta (unknown origin) using FAM-labeled peptide as substrate preincubated for 10 mins followed by substrate addition by caliper as... |
Eur J Med Chem 167: 211-225 (2019)
Article DOI: 10.1016/j.ejmech.2019.02.001
BindingDB Entry DOI: 10.7270/Q26976WC |
More data for this
Ligand-Target Pair | |
Histone deacetylase 6
(Homo sapiens (Human)) | BDBM50579386
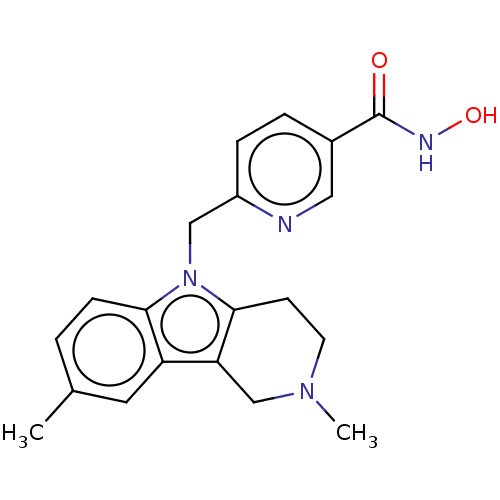
(CHEMBL4850396)Show SMILES CN1CCc2c(C1)c1cc(C)ccc1n2Cc1ccc(cn1)C(=O)NO | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 83 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human HDAC6 using fluorogenic substrate incubated for 1 hrs by fluorescence plate reader assay |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113821
BindingDB Entry DOI: 10.7270/Q2HX1HHW |
More data for this
Ligand-Target Pair | |
Histone deacetylase 6
(Homo sapiens (Human)) | BDBM50579383

(CHEMBL4849709)Show SMILES CCCN(Cc1ccc(cn1)C(=O)NO)C12CC3C[C@](C)(C[C@](C)(C3)C1)C2 |r,TLB:24:17:26:21.22.25,24:22:18.17.16:26,23:22:18:16.15.26,3:15:18:21.24.22,THB:25:22:18:16.15.26,25:15:18:21.24.22| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 102 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human HDAC6 using fluorogenic substrate incubated for 1 hrs by fluorescence plate reader assay |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113821
BindingDB Entry DOI: 10.7270/Q2HX1HHW |
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3 beta
(Homo sapiens (Human)) | BDBM50510150
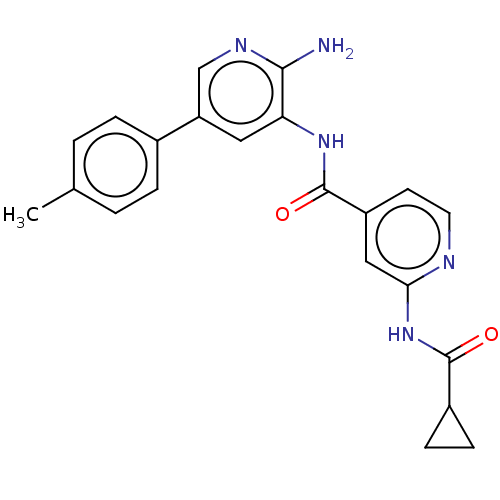
(CHEMBL4472042)Show SMILES Cc1ccc(cc1)-c1cnc(N)c(NC(=O)c2ccnc(NC(=O)C3CC3)c2)c1 Show InChI InChI=1S/C22H21N5O2/c1-13-2-4-14(5-3-13)17-10-18(20(23)25-12-17)26-22(29)16-8-9-24-19(11-16)27-21(28)15-6-7-15/h2-5,8-12,15H,6-7H2,1H3,(H2,23,25)(H,26,29)(H,24,27,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 127 | n/a | n/a | n/a | n/a | n/a | n/a |
Shandong University
Curated by ChEMBL
| Assay Description
Inhibition of GSK-3beta (unknown origin) using FAM-labeled peptide as substrate preincubated for 10 mins followed by substrate addition by caliper as... |
Eur J Med Chem 167: 211-225 (2019)
Article DOI: 10.1016/j.ejmech.2019.02.001
BindingDB Entry DOI: 10.7270/Q26976WC |
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3 beta
(Homo sapiens (Human)) | BDBM50510149
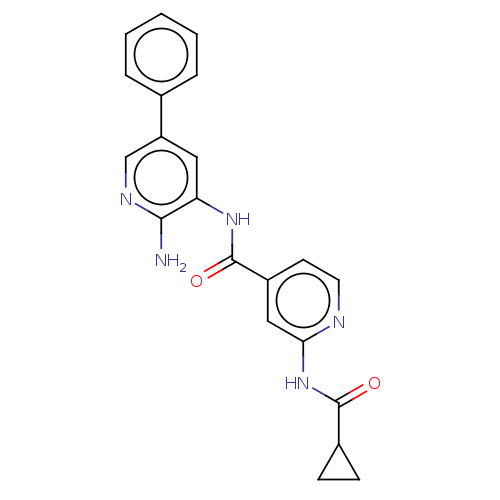
(CHEMBL4515584)Show SMILES Nc1ncc(cc1NC(=O)c1ccnc(NC(=O)C2CC2)c1)-c1ccccc1 Show InChI InChI=1S/C21H19N5O2/c22-19-17(10-16(12-24-19)13-4-2-1-3-5-13)25-21(28)15-8-9-23-18(11-15)26-20(27)14-6-7-14/h1-5,8-12,14H,6-7H2,(H2,22,24)(H,25,28)(H,23,26,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 148 | n/a | n/a | n/a | n/a | n/a | n/a |
Shandong University
Curated by ChEMBL
| Assay Description
Inhibition of GSK-3beta (unknown origin) using FAM-labeled peptide as substrate preincubated for 10 mins followed by substrate addition by caliper as... |
Eur J Med Chem 167: 211-225 (2019)
Article DOI: 10.1016/j.ejmech.2019.02.001
BindingDB Entry DOI: 10.7270/Q26976WC |
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3 beta
(Homo sapiens (Human)) | BDBM50510138
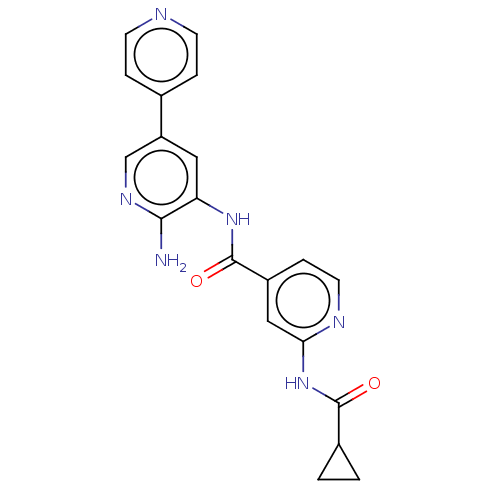
(CHEMBL4535643)Show SMILES Nc1ncc(cc1NC(=O)c1ccnc(NC(=O)C2CC2)c1)-c1ccncc1 Show InChI InChI=1S/C20H18N6O2/c21-18-16(9-15(11-24-18)12-3-6-22-7-4-12)25-20(28)14-5-8-23-17(10-14)26-19(27)13-1-2-13/h3-11,13H,1-2H2,(H2,21,24)(H,25,28)(H,23,26,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 199 | n/a | n/a | n/a | n/a | n/a | n/a |
Shandong University
Curated by ChEMBL
| Assay Description
Inhibition of GSK-3beta (unknown origin) using FAM-labeled peptide as substrate preincubated for 10 mins followed by substrate addition by caliper as... |
Eur J Med Chem 167: 211-225 (2019)
Article DOI: 10.1016/j.ejmech.2019.02.001
BindingDB Entry DOI: 10.7270/Q26976WC |
More data for this
Ligand-Target Pair | |
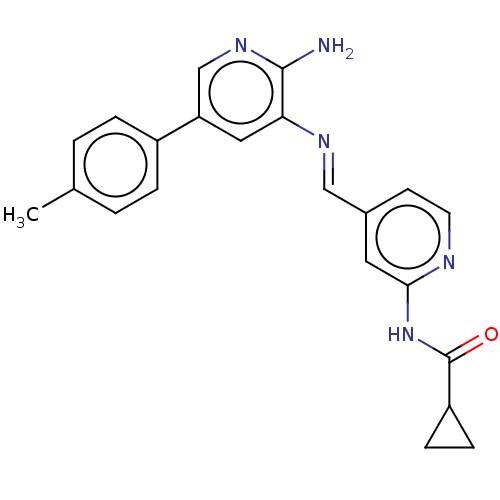
Glycogen synthase kinase-3 beta
(Homo sapiens (Human)) | BDBM50510146
(CHEMBL4578996)Show SMILES Cc1csc(c1)-c1cnc(N)c(NC(=O)c2ccnc(NC(=O)C3CC3)c2)c1 Show InChI InChI=1S/C20H19N5O2S/c1-11-6-16(28-10-11)14-7-15(18(21)23-9-14)24-20(27)13-4-5-22-17(8-13)25-19(26)12-2-3-12/h4-10,12H,2-3H2,1H3,(H2,21,23)(H,24,27)(H,22,25,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 219 | n/a | n/a | n/a | n/a | n/a | n/a |
Shandong University
Curated by ChEMBL
| Assay Description
Inhibition of GSK-3beta (unknown origin) using FAM-labeled peptide as substrate preincubated for 10 mins followed by substrate addition by caliper as... |
Eur J Med Chem 167: 211-225 (2019)
Article DOI: 10.1016/j.ejmech.2019.02.001
BindingDB Entry DOI: 10.7270/Q26976WC |
More data for this
Ligand-Target Pair | |
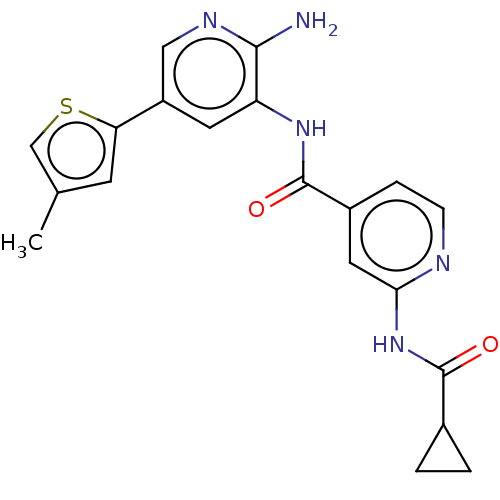
Glycogen synthase kinase-3 beta
(Homo sapiens (Human)) | BDBM50510153
(CHEMBL4550261)Show SMILES Nc1ncc(cc1NC(=O)c1ccnc(NC(=O)C2CC2)c1)-c1ccc(F)cc1 Show InChI InChI=1S/C21H18FN5O2/c22-16-5-3-12(4-6-16)15-9-17(19(23)25-11-15)26-21(29)14-7-8-24-18(10-14)27-20(28)13-1-2-13/h3-11,13H,1-2H2,(H2,23,25)(H,26,29)(H,24,27,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 240 | n/a | n/a | n/a | n/a | n/a | n/a |
Shandong University
Curated by ChEMBL
| Assay Description
Inhibition of GSK-3beta (unknown origin) using FAM-labeled peptide as substrate preincubated for 10 mins followed by substrate addition by caliper as... |
Eur J Med Chem 167: 211-225 (2019)
Article DOI: 10.1016/j.ejmech.2019.02.001
BindingDB Entry DOI: 10.7270/Q26976WC |
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3 beta
(Homo sapiens (Human)) | BDBM50510143

(CHEMBL4474810)Show SMILES Cc1csc(c1)-c1cnc(N)c(c1)\N=C\c1ccnc(NC(=O)C2CC2)c1 Show InChI InChI=1S/C20H19N5OS/c1-12-6-17(27-11-12)15-8-16(19(21)24-10-15)23-9-13-4-5-22-18(7-13)25-20(26)14-2-3-14/h4-11,14H,2-3H2,1H3,(H2,21,24)(H,22,25,26)/b23-9+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 315 | n/a | n/a | n/a | n/a | n/a | n/a |
Shandong University
Curated by ChEMBL
| Assay Description
Inhibition of GSK-3beta (unknown origin) using FAM-labeled peptide as substrate preincubated for 10 mins followed by substrate addition by caliper as... |
Eur J Med Chem 167: 211-225 (2019)
Article DOI: 10.1016/j.ejmech.2019.02.001
BindingDB Entry DOI: 10.7270/Q26976WC |
More data for this
Ligand-Target Pair | |
Histone deacetylase 6
(Homo sapiens (Human)) | BDBM50579373
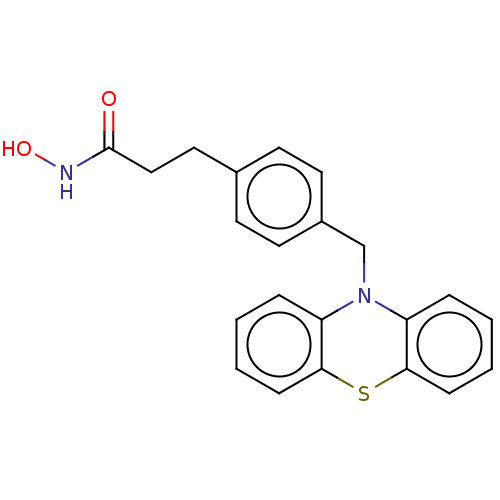
(CHEMBL4860339) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 343 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human HDAC6 using fluorogenic substrate incubated for 1 hrs by fluorescence plate reader assay |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113821
BindingDB Entry DOI: 10.7270/Q2HX1HHW |
More data for this
Ligand-Target Pair | |
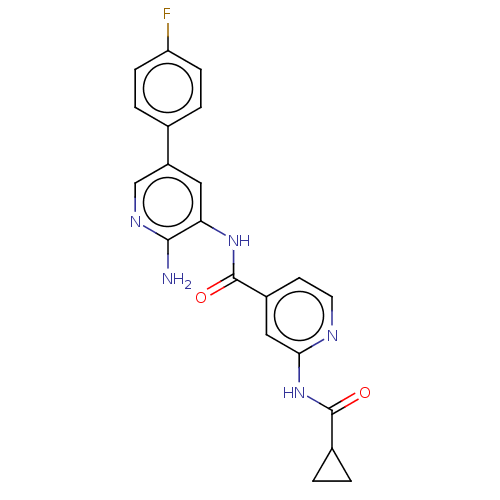
Glycogen synthase kinase-3 beta
(Homo sapiens (Human)) | BDBM50510148
(CHEMBL4458206)Show SMILES Cc1ccc(cc1)-c1cnc(N)c(c1)\N=C\c1ccnc(NC(=O)C2CC2)c1 Show InChI InChI=1S/C22H21N5O/c1-14-2-4-16(5-3-14)18-11-19(21(23)26-13-18)25-12-15-8-9-24-20(10-15)27-22(28)17-6-7-17/h2-5,8-13,17H,6-7H2,1H3,(H2,23,26)(H,24,27,28)/b25-12+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 372 | n/a | n/a | n/a | n/a | n/a | n/a |
Shandong University
Curated by ChEMBL
| Assay Description
Inhibition of GSK-3beta (unknown origin) using FAM-labeled peptide as substrate preincubated for 10 mins followed by substrate addition by caliper as... |
Eur J Med Chem 167: 211-225 (2019)
Article DOI: 10.1016/j.ejmech.2019.02.001
BindingDB Entry DOI: 10.7270/Q26976WC |
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3 beta
(Homo sapiens (Human)) | BDBM50510142
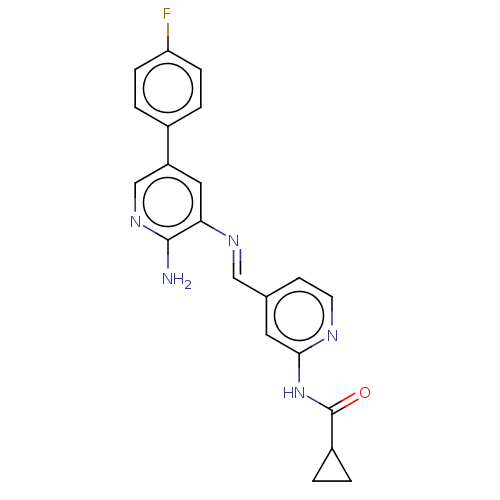
(CHEMBL4455516)Show SMILES Nc1ncc(cc1\N=C\c1ccnc(NC(=O)C2CC2)c1)-c1ccc(F)cc1 Show InChI InChI=1S/C21H18FN5O/c22-17-5-3-14(4-6-17)16-10-18(20(23)26-12-16)25-11-13-7-8-24-19(9-13)27-21(28)15-1-2-15/h3-12,15H,1-2H2,(H2,23,26)(H,24,27,28)/b25-11+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 418 | n/a | n/a | n/a | n/a | n/a | n/a |
Shandong University
Curated by ChEMBL
| Assay Description
Inhibition of GSK-3beta (unknown origin) using FAM-labeled peptide as substrate preincubated for 10 mins followed by substrate addition by caliper as... |
Eur J Med Chem 167: 211-225 (2019)
Article DOI: 10.1016/j.ejmech.2019.02.001
BindingDB Entry DOI: 10.7270/Q26976WC |
More data for this
Ligand-Target Pair | |
Histone deacetylase 6
(Homo sapiens (Human)) | BDBM50579372
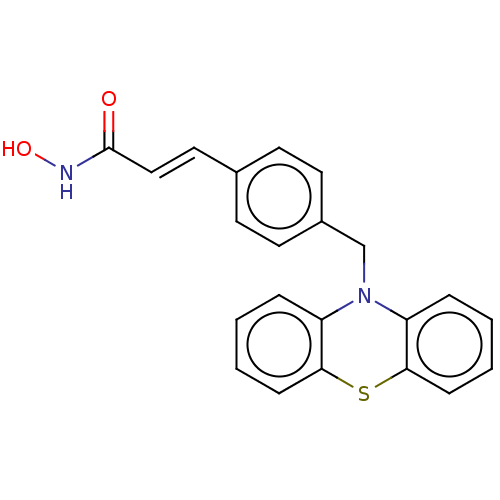
(CHEMBL4867268)Show SMILES ONC(=O)\C=C\c1ccc(CN2c3ccccc3Sc3ccccc23)cc1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 463 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human HDAC6 using fluorogenic substrate incubated for 1 hrs by fluorescence plate reader assay |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113821
BindingDB Entry DOI: 10.7270/Q2HX1HHW |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50285416
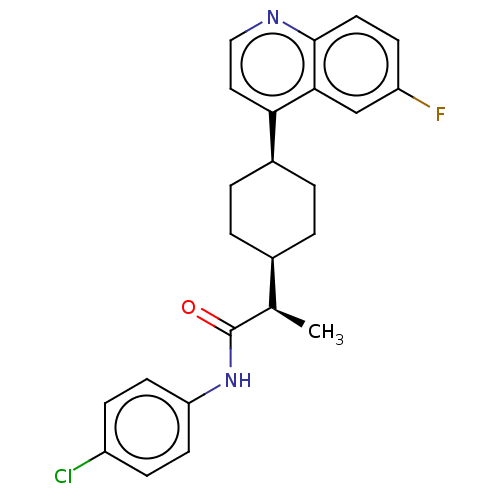
(CHEMBL4161733)Show SMILES [H][C@]1(CC[C@@H](CC1)c1ccnc2ccc(F)cc12)[C@@H](C)C(=O)Nc1ccc(Cl)cc1 |r,wU:18.21,1.0,wD:4.7,(66.16,-9.22,;66.17,-7.68,;66.16,-6.15,;67.5,-5.38,;68.84,-6.15,;68.83,-7.69,;67.5,-8.46,;70.18,-5.39,;71.5,-6.16,;72.84,-5.4,;72.84,-3.85,;71.5,-3.08,;71.5,-1.55,;70.17,-.79,;68.84,-1.56,;67.5,-.8,;68.85,-3.09,;70.18,-3.85,;64.83,-8.46,;64.84,-10,;63.5,-7.69,;63.49,-6.14,;62.16,-8.46,;60.83,-7.69,;59.49,-8.47,;58.15,-7.69,;58.16,-6.14,;56.82,-5.38,;59.49,-5.38,;60.83,-6.14,)| Show InChI InChI=1S/C24H24ClFN2O/c1-15(24(29)28-20-9-6-18(25)7-10-20)16-2-4-17(5-3-16)21-12-13-27-23-11-8-19(26)14-22(21)23/h6-17H,2-5H2,1H3,(H,28,29)/t15-,16-,17+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 500 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00771
BindingDB Entry DOI: 10.7270/Q2T43Z5D |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Histone deacetylase 6
(Homo sapiens (Human)) | BDBM50579381
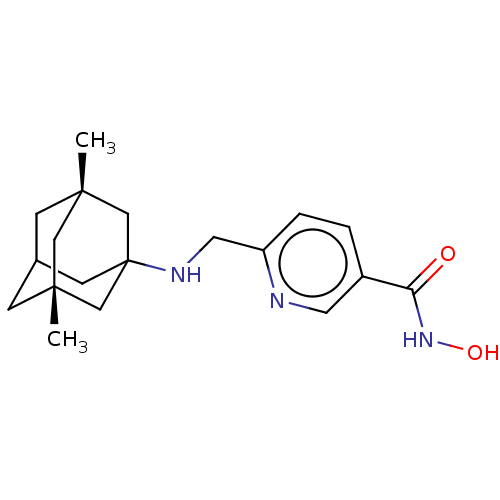
(CHEMBL4852393)Show SMILES C[C@@]12CC3C[C@@](C)(C1)CC(C3)(C2)NCc1ccc(cn1)C(=O)NO |r,TLB:4:3:11:7.5.8,4:5:2.3.10:11,6:5:2:10.9.11,12:9:2:7.4.5,THB:8:5:2:10.9.11,8:9:2:7.4.5| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 537 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human HDAC6 using fluorogenic substrate incubated for 1 hrs by fluorescence plate reader assay |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113821
BindingDB Entry DOI: 10.7270/Q2HX1HHW |
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3 beta
(Homo sapiens (Human)) | BDBM50510147
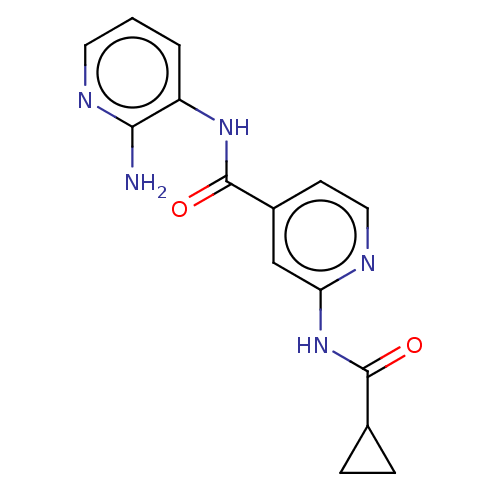
(CHEMBL4442834)Show InChI InChI=1S/C15H15N5O2/c16-13-11(2-1-6-18-13)19-15(22)10-5-7-17-12(8-10)20-14(21)9-3-4-9/h1-2,5-9H,3-4H2,(H2,16,18)(H,19,22)(H,17,20,21) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 629 | n/a | n/a | n/a | n/a | n/a | n/a |
Shandong University
Curated by ChEMBL
| Assay Description
Inhibition of GSK-3beta (unknown origin) using FAM-labeled peptide as substrate preincubated for 10 mins followed by substrate addition by caliper as... |
Eur J Med Chem 167: 211-225 (2019)
Article DOI: 10.1016/j.ejmech.2019.02.001
BindingDB Entry DOI: 10.7270/Q26976WC |
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3 beta
(Homo sapiens (Human)) | BDBM50510141
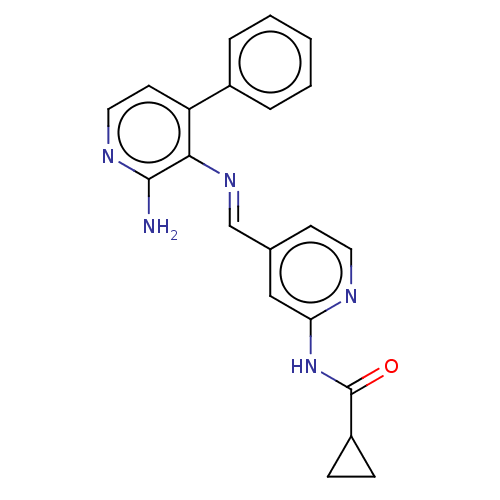
(CHEMBL4580131)Show SMILES Nc1nccc(-c2ccccc2)c1\N=C\c1ccnc(NC(=O)C2CC2)c1 Show InChI InChI=1S/C21H19N5O/c22-20-19(17(9-11-24-20)15-4-2-1-3-5-15)25-13-14-8-10-23-18(12-14)26-21(27)16-6-7-16/h1-5,8-13,16H,6-7H2,(H2,22,24)(H,23,26,27)/b25-13+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 633 | n/a | n/a | n/a | n/a | n/a | n/a |
Shandong University
Curated by ChEMBL
| Assay Description
Inhibition of GSK-3beta (unknown origin) using FAM-labeled peptide as substrate preincubated for 10 mins followed by substrate addition by caliper as... |
Eur J Med Chem 167: 211-225 (2019)
Article DOI: 10.1016/j.ejmech.2019.02.001
BindingDB Entry DOI: 10.7270/Q26976WC |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50579375

(CHEMBL4858133) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 743 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human HDAC1 using fluorogenic substrate incubated for 1 hrs by fluorescence plate reader assay |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113821
BindingDB Entry DOI: 10.7270/Q2HX1HHW |
More data for this
Ligand-Target Pair | |
Histone deacetylase 6
(Homo sapiens (Human)) | BDBM50579382
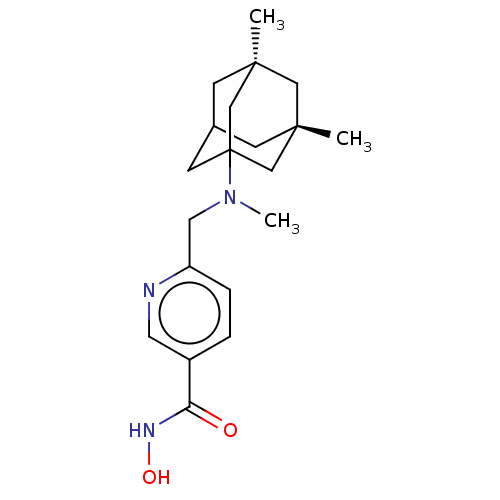
(CHEMBL4848432)Show SMILES CN(Cc1ccc(cn1)C(=O)NO)C12CC3C[C@](C)(C[C@](C)(C3)C1)C2 |r,TLB:22:15:24:19.20.23,22:20:16.15.14:24,21:20:16:14.13.24,1:13:16:19.22.20,THB:23:20:16:14.13.24,23:13:16:19.22.20| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 835 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human HDAC6 using fluorogenic substrate incubated for 1 hrs by fluorescence plate reader assay |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113821
BindingDB Entry DOI: 10.7270/Q2HX1HHW |
More data for this
Ligand-Target Pair | |
Histone deacetylase 6
(Homo sapiens (Human)) | BDBM50579374
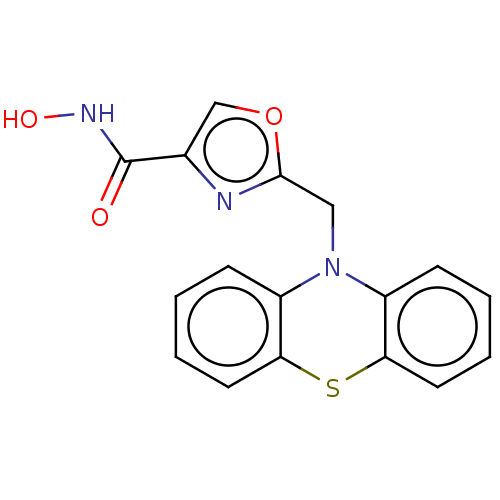
(CHEMBL4874713) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 884 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human HDAC6 using fluorogenic substrate incubated for 1 hrs by fluorescence plate reader assay |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113821
BindingDB Entry DOI: 10.7270/Q2HX1HHW |
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3 beta
(Homo sapiens (Human)) | BDBM50510154
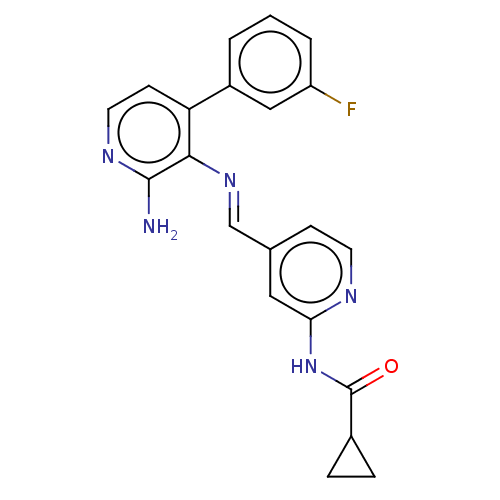
(CHEMBL4462480)Show SMILES Nc1nccc(-c2cccc(F)c2)c1\N=C\c1ccnc(NC(=O)C2CC2)c1 Show InChI InChI=1S/C21H18FN5O/c22-16-3-1-2-15(11-16)17-7-9-25-20(23)19(17)26-12-13-6-8-24-18(10-13)27-21(28)14-4-5-14/h1-3,6-12,14H,4-5H2,(H2,23,25)(H,24,27,28)/b26-12+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 891 | n/a | n/a | n/a | n/a | n/a | n/a |
Shandong University
Curated by ChEMBL
| Assay Description
Inhibition of GSK-3beta (unknown origin) using FAM-labeled peptide as substrate preincubated for 10 mins followed by substrate addition by caliper as... |
Eur J Med Chem 167: 211-225 (2019)
Article DOI: 10.1016/j.ejmech.2019.02.001
BindingDB Entry DOI: 10.7270/Q26976WC |
More data for this
Ligand-Target Pair | |
Histone deacetylase 6
(Homo sapiens (Human)) | BDBM50579380
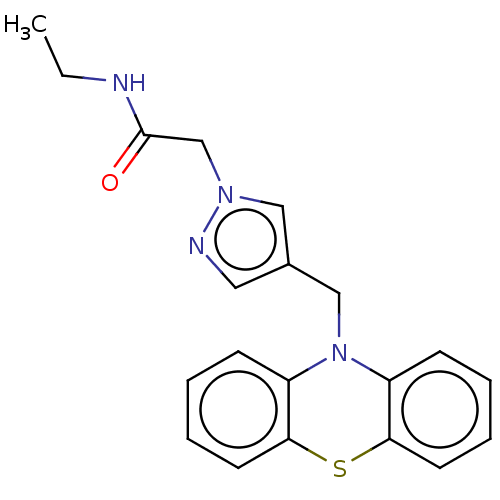
(CHEMBL4876901) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human HDAC6 using fluorogenic substrate incubated for 1 hrs by fluorescence plate reader assay |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113821
BindingDB Entry DOI: 10.7270/Q2HX1HHW |
More data for this
Ligand-Target Pair | |
Histone deacetylase 6
(Homo sapiens (Human)) | BDBM50579378
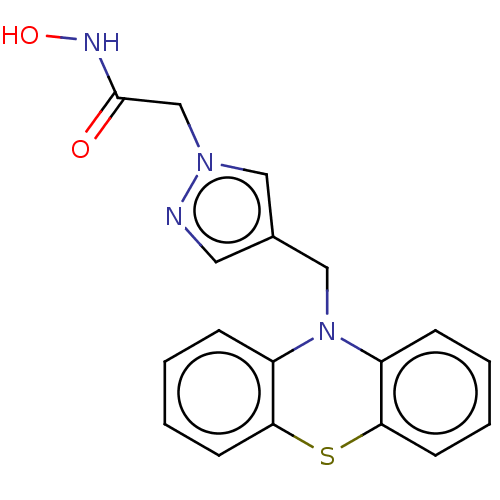
(CHEMBL4863449) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human HDAC6 using fluorogenic substrate incubated for 1 hrs by fluorescence plate reader assay |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113821
BindingDB Entry DOI: 10.7270/Q2HX1HHW |
More data for this
Ligand-Target Pair | |
Histone deacetylase 6
(Homo sapiens (Human)) | BDBM50579377
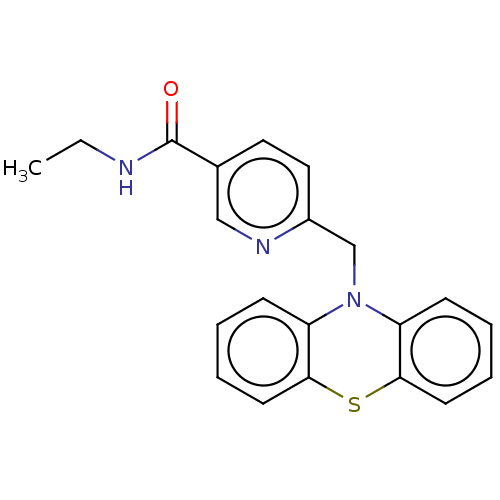
(CHEMBL4874166) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human HDAC6 using fluorogenic substrate incubated for 1 hrs by fluorescence plate reader assay |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113821
BindingDB Entry DOI: 10.7270/Q2HX1HHW |
More data for this
Ligand-Target Pair | |
Histone deacetylase 6
(Homo sapiens (Human)) | BDBM50579376
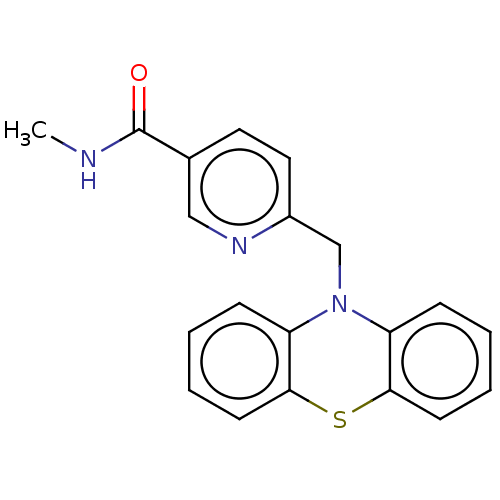
(CHEMBL4865749) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human HDAC6 using fluorogenic substrate incubated for 1 hrs by fluorescence plate reader assay |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113821
BindingDB Entry DOI: 10.7270/Q2HX1HHW |
More data for this
Ligand-Target Pair | |
Histone deacetylase 6
(Homo sapiens (Human)) | BDBM50579384
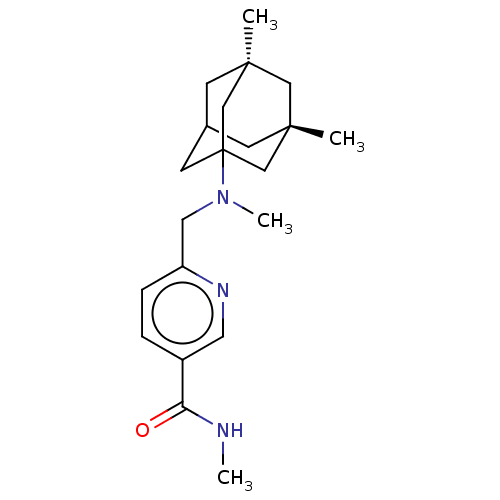
(CHEMBL4872652)Show SMILES CNC(=O)c1ccc(CN(C)C23CC4C[C@](C)(C[C@](C)(C4)C2)C3)nc1 |r,TLB:20:13:22:17.18.21,20:18:14.13.12:22,19:18:14:12.11.22,9:11:14:17.20.18,THB:21:18:14:12.11.22,21:11:14:17.20.18| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human HDAC6 using fluorogenic substrate incubated for 1 hrs by fluorescence plate reader assay |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113821
BindingDB Entry DOI: 10.7270/Q2HX1HHW |
More data for this
Ligand-Target Pair | |
Histone deacetylase 6
(Homo sapiens (Human)) | BDBM50579385
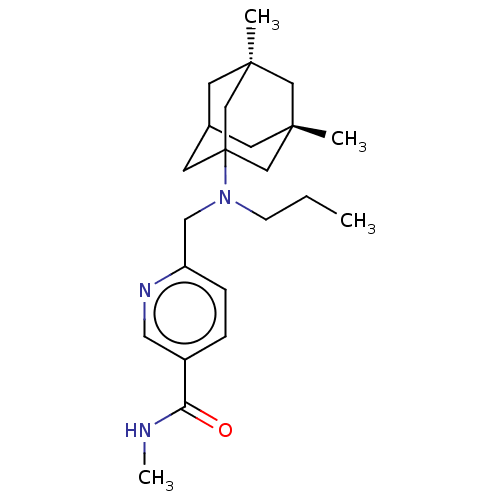
(CHEMBL4865223)Show SMILES CCCN(Cc1ccc(cn1)C(=O)NC)C12CC3C[C@](C)(C[C@](C)(C3)C1)C2 |r,TLB:24:17:26:21.22.25,24:22:18.17.16:26,23:22:18:16.15.26,3:15:18:21.24.22,THB:25:22:18:16.15.26,25:15:18:21.24.22| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human HDAC6 using fluorogenic substrate incubated for 1 hrs by fluorescence plate reader assay |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113821
BindingDB Entry DOI: 10.7270/Q2HX1HHW |
More data for this
Ligand-Target Pair | |
Histone deacetylase 6
(Homo sapiens (Human)) | BDBM50579379
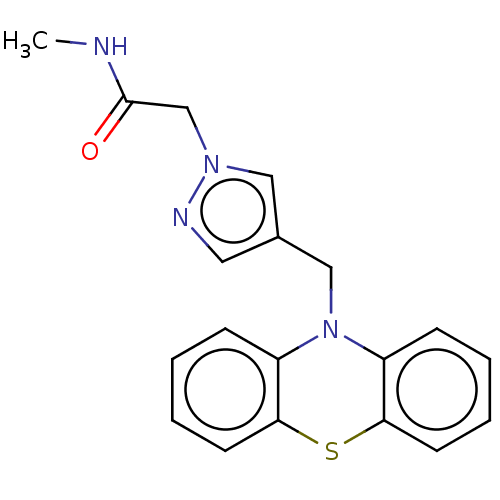
(CHEMBL4854355) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human HDAC6 using fluorogenic substrate incubated for 1 hrs by fluorescence plate reader assay |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113821
BindingDB Entry DOI: 10.7270/Q2HX1HHW |
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3 beta
(Homo sapiens (Human)) | BDBM50510139
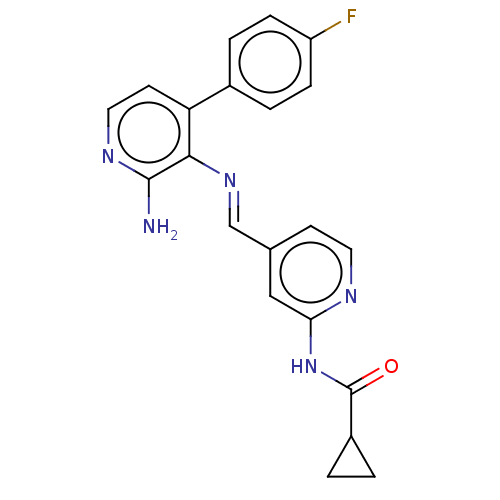
(CHEMBL4539700)Show SMILES Nc1nccc(-c2ccc(F)cc2)c1\N=C\c1ccnc(NC(=O)C2CC2)c1 Show InChI InChI=1S/C21H18FN5O/c22-16-5-3-14(4-6-16)17-8-10-25-20(23)19(17)26-12-13-7-9-24-18(11-13)27-21(28)15-1-2-15/h3-12,15H,1-2H2,(H2,23,25)(H,24,27,28)/b26-12+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Shandong University
Curated by ChEMBL
| Assay Description
Inhibition of GSK-3beta (unknown origin) using FAM-labeled peptide as substrate preincubated for 10 mins followed by substrate addition by caliper as... |
Eur J Med Chem 167: 211-225 (2019)
Article DOI: 10.1016/j.ejmech.2019.02.001
BindingDB Entry DOI: 10.7270/Q26976WC |
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3 beta
(Homo sapiens (Human)) | BDBM50510140

(CHEMBL4460479)Show SMILES Cc1ccc(cc1)-c1ccnc(N)c1\N=C\c1ccnc(NC(=O)C2CC2)c1 Show InChI InChI=1S/C22H21N5O/c1-14-2-4-16(5-3-14)18-9-11-25-21(23)20(18)26-13-15-8-10-24-19(12-15)27-22(28)17-6-7-17/h2-5,8-13,17H,6-7H2,1H3,(H2,23,25)(H,24,27,28)/b26-13+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.25E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Shandong University
Curated by ChEMBL
| Assay Description
Inhibition of GSK-3beta (unknown origin) using FAM-labeled peptide as substrate preincubated for 10 mins followed by substrate addition by caliper as... |
Eur J Med Chem 167: 211-225 (2019)
Article DOI: 10.1016/j.ejmech.2019.02.001
BindingDB Entry DOI: 10.7270/Q26976WC |
More data for this
Ligand-Target Pair | |
Histone deacetylase 2
(Homo sapiens (Human)) | BDBM50579375

(CHEMBL4858133) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.96E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human HDAC2 using fluorogenic substrate incubated for 1 hrs by fluorescence plate reader assay |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113821
BindingDB Entry DOI: 10.7270/Q2HX1HHW |
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3 beta
(Homo sapiens (Human)) | BDBM50510151
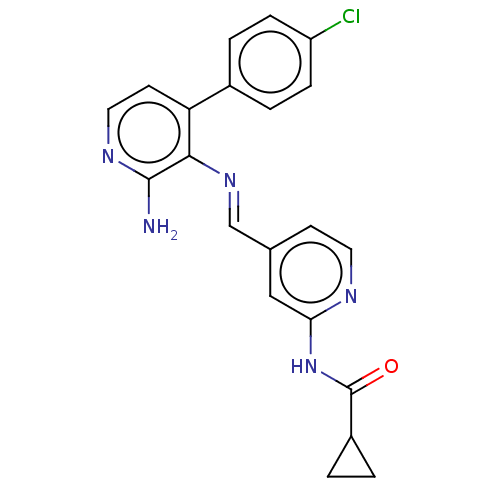
(CHEMBL4453426)Show SMILES Nc1nccc(-c2ccc(Cl)cc2)c1\N=C\c1ccnc(NC(=O)C2CC2)c1 Show InChI InChI=1S/C21H18ClN5O/c22-16-5-3-14(4-6-16)17-8-10-25-20(23)19(17)26-12-13-7-9-24-18(11-13)27-21(28)15-1-2-15/h3-12,15H,1-2H2,(H2,23,25)(H,24,27,28)/b26-12+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Shandong University
Curated by ChEMBL
| Assay Description
Inhibition of GSK-3beta (unknown origin) using FAM-labeled peptide as substrate preincubated for 10 mins followed by substrate addition by caliper as... |
Eur J Med Chem 167: 211-225 (2019)
Article DOI: 10.1016/j.ejmech.2019.02.001
BindingDB Entry DOI: 10.7270/Q26976WC |
More data for this
Ligand-Target Pair | |
Histone deacetylase 11
(Homo sapiens (Human)) | BDBM50579375

(CHEMBL4858133) | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human HDAC11 using fluorogenic substrate incubated for 1 hrs by fluorescence plate reader assay |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113821
BindingDB Entry DOI: 10.7270/Q2HX1HHW |
More data for this
Ligand-Target Pair | |
Histone deacetylase 4
(Homo sapiens (Human)) | BDBM50579375

(CHEMBL4858133) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 5.63E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human HDAC4 using fluorogenic substrate incubated for 1 hrs by fluorescence plate reader assay |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113821
BindingDB Entry DOI: 10.7270/Q2HX1HHW |
More data for this
Ligand-Target Pair | |
Histone deacetylase 8
(Homo sapiens (Human)) | BDBM50579375

(CHEMBL4858133) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 6.12E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human HDAC8 using fluorogenic substrate incubated for 1 hrs by fluorescence plate reader assay |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113821
BindingDB Entry DOI: 10.7270/Q2HX1HHW |
More data for this
Ligand-Target Pair | |
Polyamine deacetylase HDAC10
(Homo sapiens (Human)) | BDBM50579375

(CHEMBL4858133) | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 8.63E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human HDAC10 using fluorogenic substrate incubated for 1 hrs by fluorescence plate reader assay |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113821
BindingDB Entry DOI: 10.7270/Q2HX1HHW |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50602173

(CHEMBL5192805)Show SMILES [H][C@]1(CC[C@@H](CC1)c1ccnc2ccc(F)cc12)[C@@H](C)C(=O)Nc1ccc(OC2CCN(CC2)C(=O)CCOCCOCCNc2cccc3C(=O)N(C4CCC(=O)NC4=O)C(=O)c23)cc1 |r,wU:4.4,18.21,wD:1.0,(13.83,-3.51,;13.83,-1.98,;15.16,-2.75,;16.44,-1.98,;16.44,-.44,;15.16,.33,;13.83,-.44,;17.78,.33,;19.11,-.44,;20.45,.33,;20.45,1.87,;19.11,2.64,;19.11,4.18,;17.78,4.95,;16.44,4.18,;15.16,4.95,;16.44,2.64,;17.78,1.87,;12.49,-2.75,;12.49,-4.28,;11.16,-1.98,;11.16,-.44,;9.83,-2.75,;8.49,-1.98,;7.16,-2.75,;5.82,-1.98,;5.82,-.44,;4.49,.33,;3.16,-.44,;3.16,-1.98,;1.82,-2.75,;.49,-1.98,;.49,-.44,;1.82,.33,;-.85,-2.75,;-.85,-4.28,;-2.18,-1.98,;-3.51,-2.75,;-4.85,-1.98,;-6.18,-2.75,;-7.52,-1.98,;-8.85,-2.75,;-10.18,-1.98,;-11.52,-2.75,;-12.85,-1.98,;-14.19,-2.75,;-14.19,-4.28,;-15.52,-5.05,;-16.85,-4.28,;-16.85,-2.75,;-17.98,-1.72,;-19.52,-2.03,;-17.37,-.28,;-18.14,1.05,;-19.68,1.05,;-20.45,2.39,;-19.68,3.72,;-20.45,5.05,;-18.14,3.72,;-17.37,2.39,;-15.83,2.39,;-15.83,-.44,;-14.8,.69,;-15.52,-1.98,;7.16,.33,;8.49,-.44,)| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | 290 | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00771
BindingDB Entry DOI: 10.7270/Q2T43Z5D |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50602172

(CHEMBL5189195)Show SMILES [H][C@]1(CC[C@@H](CC1)c1ccnc2ccc(F)cc12)[C@@H](C)C(=O)Nc1ccc(OC2CCN(CC2)C(=O)CCOCCOCCNc2cccc3C(=O)N(C4CCC(=O)N(C)C4=O)C(=O)c23)cc1 |r,wU:4.4,18.21,wD:1.0,(13.83,-3.51,;13.83,-1.98,;15.16,-2.75,;16.44,-1.98,;16.44,-.44,;15.16,.33,;13.83,-.44,;17.78,.33,;19.11,-.44,;20.45,.33,;20.45,1.87,;19.11,2.64,;19.11,4.18,;17.78,4.95,;16.44,4.18,;15.16,4.95,;16.44,2.64,;17.78,1.87,;12.49,-2.75,;12.49,-4.28,;11.16,-1.98,;11.16,-.44,;9.83,-2.75,;8.49,-1.98,;7.16,-2.75,;5.82,-1.98,;5.82,-.44,;4.49,.33,;3.16,-.44,;3.16,-1.98,;1.82,-2.75,;.49,-1.98,;.49,-.44,;1.82,.33,;-.85,-2.75,;-.85,-4.28,;-2.18,-1.98,;-3.51,-2.75,;-4.85,-1.98,;-6.18,-2.75,;-7.52,-1.98,;-8.85,-2.75,;-10.18,-1.98,;-11.52,-2.75,;-12.85,-1.98,;-14.19,-2.75,;-14.19,-4.28,;-15.52,-5.05,;-16.85,-4.28,;-16.85,-2.75,;-17.98,-1.72,;-19.52,-2.03,;-17.37,-.28,;-18.14,1.05,;-19.68,1.05,;-20.45,2.39,;-19.68,3.72,;-20.45,5.05,;-18.14,3.72,;-17.37,5.05,;-17.37,2.39,;-15.83,2.39,;-15.83,-.44,;-14.8,.69,;-15.52,-1.98,;7.16,.33,;8.49,-.44,)| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | 1.10E+3 | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00771
BindingDB Entry DOI: 10.7270/Q2T43Z5D |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50602173

(CHEMBL5192805)Show SMILES [H][C@]1(CC[C@@H](CC1)c1ccnc2ccc(F)cc12)[C@@H](C)C(=O)Nc1ccc(OC2CCN(CC2)C(=O)CCOCCOCCNc2cccc3C(=O)N(C4CCC(=O)NC4=O)C(=O)c23)cc1 |r,wU:4.4,18.21,wD:1.0,(13.83,-3.51,;13.83,-1.98,;15.16,-2.75,;16.44,-1.98,;16.44,-.44,;15.16,.33,;13.83,-.44,;17.78,.33,;19.11,-.44,;20.45,.33,;20.45,1.87,;19.11,2.64,;19.11,4.18,;17.78,4.95,;16.44,4.18,;15.16,4.95,;16.44,2.64,;17.78,1.87,;12.49,-2.75,;12.49,-4.28,;11.16,-1.98,;11.16,-.44,;9.83,-2.75,;8.49,-1.98,;7.16,-2.75,;5.82,-1.98,;5.82,-.44,;4.49,.33,;3.16,-.44,;3.16,-1.98,;1.82,-2.75,;.49,-1.98,;.49,-.44,;1.82,.33,;-.85,-2.75,;-.85,-4.28,;-2.18,-1.98,;-3.51,-2.75,;-4.85,-1.98,;-6.18,-2.75,;-7.52,-1.98,;-8.85,-2.75,;-10.18,-1.98,;-11.52,-2.75,;-12.85,-1.98,;-14.19,-2.75,;-14.19,-4.28,;-15.52,-5.05,;-16.85,-4.28,;-16.85,-2.75,;-17.98,-1.72,;-19.52,-2.03,;-17.37,-.28,;-18.14,1.05,;-19.68,1.05,;-20.45,2.39,;-19.68,3.72,;-20.45,5.05,;-18.14,3.72,;-17.37,2.39,;-15.83,2.39,;-15.83,-.44,;-14.8,.69,;-15.52,-1.98,;7.16,.33,;8.49,-.44,)| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | 640 | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00771
BindingDB Entry DOI: 10.7270/Q2T43Z5D |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data