 Found 3338 hits with Last Name = 'xie' and Initial = 'z'
Found 3338 hits with Last Name = 'xie' and Initial = 'z' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Prothrombin
(Homo sapiens (Human)) | BDBM50147824
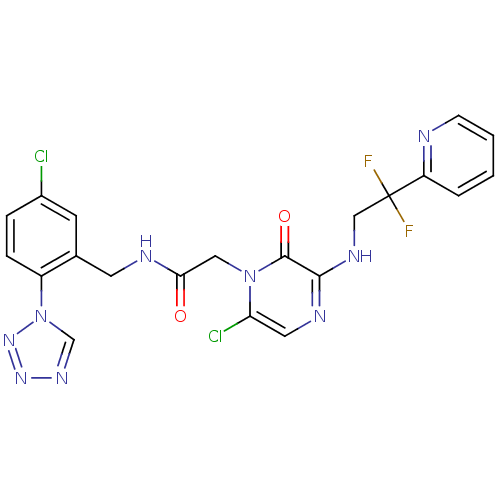
(2-[6-Chloro-3-(2,2-difluoro-2-pyridin-2-yl-ethylam...)Show SMILES FC(F)(CNc1ncc(Cl)n(CC(=O)NCc2cc(Cl)ccc2-n2cnnn2)c1=O)c1ccccn1 Show InChI InChI=1S/C21H17Cl2F2N9O2/c22-14-4-5-15(34-12-30-31-32-34)13(7-14)8-27-18(35)10-33-17(23)9-28-19(20(33)36)29-11-21(24,25)16-3-1-2-6-26-16/h1-7,9,12H,8,10-11H2,(H,27,35)(H,28,29) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.00140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hefei University of Technology
Curated by ChEMBL
| Assay Description
Binding affinity to thrombin (unknown origin) |
Eur J Med Chem 146: 299-317 (2018)
Article DOI: 10.1016/j.ejmech.2018.01.067
BindingDB Entry DOI: 10.7270/Q2251MTJ |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50147818
((2-[6-CHLORO-3-{[2,2-DIFLUORO-2-(1-OXIDOPYRIDIN-2-...)Show SMILES [O-][n+]1ccccc1C(F)(F)CNc1ncc(Cl)n(CC(=O)NCc2cc(Cl)ccc2-n2cnnn2)c1=O Show InChI InChI=1S/C21H17Cl2F2N9O3/c22-14-4-5-15(33-12-29-30-31-33)13(7-14)8-26-18(35)10-32-17(23)9-27-19(20(32)36)28-11-21(24,25)16-3-1-2-6-34(16)37/h1-7,9,12H,8,10-11H2,(H,26,35)(H,27,28) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 0.00140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hefei University of Technology
Curated by ChEMBL
| Assay Description
Binding affinity to thrombin (unknown origin) |
Eur J Med Chem 146: 299-317 (2018)
Article DOI: 10.1016/j.ejmech.2018.01.067
BindingDB Entry DOI: 10.7270/Q2251MTJ |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Prothrombin
(Homo sapiens (Human)) | BDBM50147818

((2-[6-CHLORO-3-{[2,2-DIFLUORO-2-(1-OXIDOPYRIDIN-2-...)Show SMILES [O-][n+]1ccccc1C(F)(F)CNc1ncc(Cl)n(CC(=O)NCc2cc(Cl)ccc2-n2cnnn2)c1=O Show InChI InChI=1S/C21H17Cl2F2N9O3/c22-14-4-5-15(33-12-29-30-31-33)13(7-14)8-26-18(35)10-32-17(23)9-27-19(20(32)36)28-11-21(24,25)16-3-1-2-6-34(16)37/h1-7,9,12H,8,10-11H2,(H,26,35)(H,27,28) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 0.00140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hefei University of Technology
Curated by ChEMBL
| Assay Description
Inhibition of thrombin (unknown origin) |
Eur J Med Chem 146: 299-317 (2018)
Article DOI: 10.1016/j.ejmech.2018.01.067
BindingDB Entry DOI: 10.7270/Q2251MTJ |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Prothrombin
(Homo sapiens (Human)) | BDBM50147824

(2-[6-Chloro-3-(2,2-difluoro-2-pyridin-2-yl-ethylam...)Show SMILES FC(F)(CNc1ncc(Cl)n(CC(=O)NCc2cc(Cl)ccc2-n2cnnn2)c1=O)c1ccccn1 Show InChI InChI=1S/C21H17Cl2F2N9O2/c22-14-4-5-15(34-12-30-31-32-34)13(7-14)8-27-18(35)10-33-17(23)9-28-19(20(33)36)29-11-21(24,25)16-3-1-2-6-26-16/h1-7,9,12H,8,10-11H2,(H,27,35)(H,28,29) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.00150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hefei University of Technology
Curated by ChEMBL
| Assay Description
Inhibition of thrombin (unknown origin) |
Eur J Med Chem 146: 299-317 (2018)
Article DOI: 10.1016/j.ejmech.2018.01.067
BindingDB Entry DOI: 10.7270/Q2251MTJ |
More data for this
Ligand-Target Pair | |
Adenosine deaminase
(Homo sapiens (Human)) | BDBM22925

((8R)-3-[(2R,4S,5R)-4-hydroxy-5-(hydroxymethyl)oxol...)Show SMILES OC[C@H]1O[C@H](C[C@@H]1O)n1cnc2[C@H](O)CNC=Nc12 |c:17| Show InChI InChI=1S/C11H16N4O4/c16-3-8-6(17)1-9(19-8)15-5-14-10-7(18)2-12-4-13-11(10)15/h4-9,16-18H,1-3H2,(H,12,13)/t6-,7+,8+,9+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| 0.00300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113877
BindingDB Entry DOI: 10.7270/Q2PK0M78 |
More data for this
Ligand-Target Pair | |
Adenosine deaminase
(Homo sapiens (Human)) | BDBM50367032

(COFORMYCIN)Show SMILES OC[C@H]1O[C@H]([C@H](O)[C@@H]1O)n1cnc2[C@H](O)CNC=Nc12 |r,c:18| Show InChI InChI=1S/C11H16N4O5/c16-2-6-8(18)9(19)11(20-6)15-4-14-7-5(17)1-12-3-13-10(7)15/h3-6,8-9,11,16-19H,1-2H2,(H,12,13)/t5-,6-,8-,9-,11-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113877
BindingDB Entry DOI: 10.7270/Q2PK0M78 |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50457929
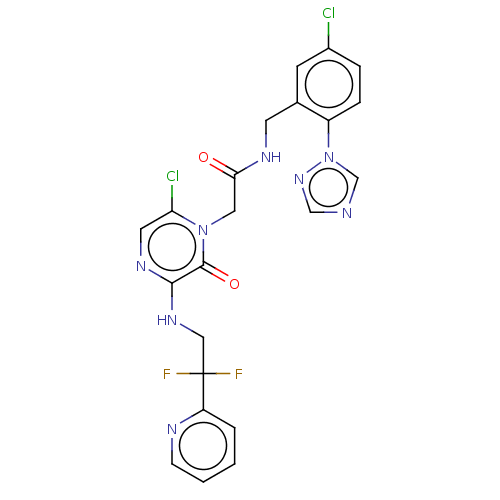
(CHEMBL104951)Show SMILES FC(F)(CNc1ncc(Cl)n(CC(=O)NCc2cc(Cl)ccc2-n2cncn2)c1=O)c1ccccn1 Show InChI InChI=1S/C22H18Cl2F2N8O2/c23-15-4-5-16(34-13-27-12-32-34)14(7-15)8-29-19(35)10-33-18(24)9-30-20(21(33)36)31-11-22(25,26)17-3-1-2-6-28-17/h1-7,9,12-13H,8,10-11H2,(H,29,35)(H,30,31) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| <0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hefei University of Technology
Curated by ChEMBL
| Assay Description
Binding affinity to thrombin (unknown origin) |
Eur J Med Chem 146: 299-317 (2018)
Article DOI: 10.1016/j.ejmech.2018.01.067
BindingDB Entry DOI: 10.7270/Q2251MTJ |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50457929

(CHEMBL104951)Show SMILES FC(F)(CNc1ncc(Cl)n(CC(=O)NCc2cc(Cl)ccc2-n2cncn2)c1=O)c1ccccn1 Show InChI InChI=1S/C22H18Cl2F2N8O2/c23-15-4-5-16(34-13-27-12-32-34)14(7-15)8-29-19(35)10-33-18(24)9-30-20(21(33)36)31-11-22(25,26)17-3-1-2-6-28-17/h1-7,9,12-13H,8,10-11H2,(H,29,35)(H,30,31) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| <0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hefei University of Technology
Curated by ChEMBL
| Assay Description
Inhibition of thrombin (unknown origin) |
Eur J Med Chem 146: 299-317 (2018)
Article DOI: 10.1016/j.ejmech.2018.01.067
BindingDB Entry DOI: 10.7270/Q2251MTJ |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50457933

(CHEMBL327265)Show SMILES [O-][n+]1ccccc1C(F)(F)CNc1ncc(Cl)n(CC(=O)NCc2cc(Cl)ccc2-n2cncn2)c1=O Show InChI InChI=1S/C22H18Cl2F2N8O3/c23-15-4-5-16(33-13-27-12-31-33)14(7-15)8-28-19(35)10-32-18(24)9-29-20(21(32)36)30-11-22(25,26)17-3-1-2-6-34(17)37/h1-7,9,12-13H,8,10-11H2,(H,28,35)(H,29,30) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| <0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hefei University of Technology
Curated by ChEMBL
| Assay Description
Binding affinity to thrombin (unknown origin) |
Eur J Med Chem 146: 299-317 (2018)
Article DOI: 10.1016/j.ejmech.2018.01.067
BindingDB Entry DOI: 10.7270/Q2251MTJ |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50457933

(CHEMBL327265)Show SMILES [O-][n+]1ccccc1C(F)(F)CNc1ncc(Cl)n(CC(=O)NCc2cc(Cl)ccc2-n2cncn2)c1=O Show InChI InChI=1S/C22H18Cl2F2N8O3/c23-15-4-5-16(33-13-27-12-31-33)14(7-15)8-28-19(35)10-32-18(24)9-29-20(21(32)36)30-11-22(25,26)17-3-1-2-6-34(17)37/h1-7,9,12-13H,8,10-11H2,(H,28,35)(H,29,30) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| <0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hefei University of Technology
Curated by ChEMBL
| Assay Description
Inhibition of thrombin (unknown origin) |
Eur J Med Chem 146: 299-317 (2018)
Article DOI: 10.1016/j.ejmech.2018.01.067
BindingDB Entry DOI: 10.7270/Q2251MTJ |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM12751

(1-(3-carbamimidoylphenyl)-3-methyl-N-[4-(2-sulfamo...)Show SMILES Cc1cc(C(=O)Nc2ccc(cc2)-c2ccccc2S(N)(=O)=O)n(n1)-c1cccc(c1)C(N)=N Show InChI InChI=1S/C24H22N6O3S/c1-15-13-21(30(29-15)19-6-4-5-17(14-19)23(25)26)24(31)28-18-11-9-16(10-12-18)20-7-2-3-8-22(20)34(27,32)33/h2-14H,1H3,(H3,25,26)(H,28,31)(H2,27,32,33) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.0130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hefei University of Technology
Curated by ChEMBL
| Assay Description
Inhibition of human factor 10a using S-2765 as substrate after 30 mins by spectrophotometric method |
Eur J Med Chem 146: 299-317 (2018)
Article DOI: 10.1016/j.ejmech.2018.01.067
BindingDB Entry DOI: 10.7270/Q2251MTJ |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50123490
(CHEMBL143418 | N-(6-Amino-2-methyl-pyridin-3-ylmet...)Show SMILES Cc1cnc(NCC(F)(F)c2ccccn2)c(=O)n1CC(=O)NCc1ccc(N)nc1C Show InChI InChI=1S/C21H23F2N7O2/c1-13-9-27-19(28-12-21(22,23)16-5-3-4-8-25-16)20(32)30(13)11-18(31)26-10-15-6-7-17(24)29-14(15)2/h3-9H,10-12H2,1-2H3,(H2,24,29)(H,26,31)(H,27,28) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0420 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hefei University of Technology
Curated by ChEMBL
| Assay Description
Inhibition of thrombin (unknown origin) |
Eur J Med Chem 146: 299-317 (2018)
Article DOI: 10.1016/j.ejmech.2018.01.067
BindingDB Entry DOI: 10.7270/Q2251MTJ |
More data for this
Ligand-Target Pair | |
Plasma kallikrein
(Homo sapiens (Human)) | BDBM416775

(1-(3-(aminomethyl)phenyl)-N-(3-((cyclopropylmethox...)Show SMILES NCc1cccc(c1)-n1nc(cc1C(=O)Nc1cccc(c1)C(OCC1CC1)c1ccccc1)C(F)(F)F Show InChI InChI=1S/C29H27F3N4O2/c30-29(31,32)26-16-25(36(35-26)24-11-4-6-20(14-24)17-33)28(37)34-23-10-5-9-22(15-23)27(38-18-19-12-13-19)21-7-2-1-3-8-21/h1-11,14-16,19,27H,12-13,17-18,33H2,(H,34,37) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Plasma kallikrein
(Homo sapiens (Human)) | BDBM417026
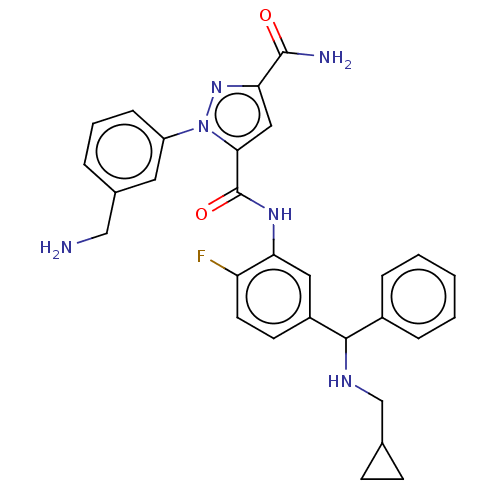
((+-)-1-(3-(aminomethyl)phenyl)-N5-(5-((cyclopropyl...)Show SMILES NCc1cccc(c1)-n1nc(cc1C(=O)Nc1cc(ccc1F)C(NCC1CC1)c1ccccc1)C(N)=O Show InChI InChI=1S/C29H29FN6O2/c30-23-12-11-21(27(33-17-18-9-10-18)20-6-2-1-3-7-20)14-24(23)34-29(38)26-15-25(28(32)37)35-36(26)22-8-4-5-19(13-22)16-31/h1-8,11-15,18,27,33H,9-10,16-17,31H2,(H2,32,37)(H,34,38) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK3
(Homo sapiens (Human)) | BDBM50193995

(3-((3R,4R)-4-methyl-3-(methyl(7H-pyrrolo[2,3-d]pyr...)Show SMILES C[C@@H]1CCN(C[C@@H]1N(C)c1ncnc2[nH]ccc12)C(=O)CC#N |r| Show InChI InChI=1S/C16H20N6O/c1-11-5-8-22(14(23)3-6-17)9-13(11)21(2)16-12-4-7-18-15(12)19-10-20-16/h4,7,10-11,13H,3,5,8-9H2,1-2H3,(H,18,19,20)/t11-,13+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01511
BindingDB Entry DOI: 10.7270/Q22Z19M2 |
More data for this
Ligand-Target Pair | |
Lethal factor
(Bacillus anthracis) | BDBM50601786

(CHEMBL5180162)Show SMILES CCN(CCCC[C@@H](C[C@@H](OC)c1ccc(F)cc1)C(=O)NO)c1ccc(F)cc1 |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.270 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113877
BindingDB Entry DOI: 10.7270/Q2PK0M78 |
More data for this
Ligand-Target Pair | |
Delta-type/Kappa-type/Mu-type opioid receptor
(MOUSE-Mus musculus (Mouse)) | BDBM50474629

(CHEMBL415006)Show SMILES [H][C@@]12Oc3c4c(CC5N(CC6CC6)CCC14[C@@]5(O)Cc1c2[nH]c2ccc(N\C(N)=N\CCCNC(=O)CNC(=O)CNC(=O)CCC(=O)NCC(=O)NCC(=O)Nc4cccc5c6C[C@@]7(O)C8Cc9ccc(O)c%10O[C@@]([H])(c6[nH]c45)C7(CCN8CC4CC4)c9%10)cc12)ccc3O |TLB:65:64:88.68.67:83.81.82,84:83:64:88.68.67,73:88:64:83.81.82,THB:17:16:4.5.6:8.14.13,9:8:16:4.5.6,3:4:16:8.14.13| Show InChI InChI=1S/C68H77N13O12/c69-64(76-38-11-12-43-40(25-38)42-27-68(91)48-24-36-9-13-45(82)60-55(36)65(68,62(92-60)58(42)78-43)17-21-81(48)33-35-7-8-35)71-20-2-19-70-51(86)28-74-52(87)29-72-49(84)15-16-50(85)73-30-53(88)75-31-54(89)77-44-4-1-3-39-41-26-67(90)47-23-37-10-14-46(83)61-56(37)66(67,18-22-80(47)32-34-5-6-34)63(93-61)59(41)79-57(39)44/h1,3-4,9-14,25,34-35,47-48,62-63,78-79,82-83,90-91H,2,5-8,15-24,26-33H2,(H,70,86)(H,72,84)(H,73,85)(H,74,87)(H,75,88)(H,77,89)(H3,69,71,76)/t47?,48?,62-,63-,65?,66?,67+,68+/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Minnesota
Curated by ChEMBL
| Assay Description
Compound was tested for binding affinity on intact HEK cells using [3H]diprenorphine as radioligand co-expressed with delta and kappa opioid receptor... |
J Med Chem 47: 2969-72 (2004)
Article DOI: 10.1021/jm0342358
BindingDB Entry DOI: 10.7270/Q2N58Q39 |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase kinase kinase kinase 4
(Homo sapiens (Human)) | BDBM50541592
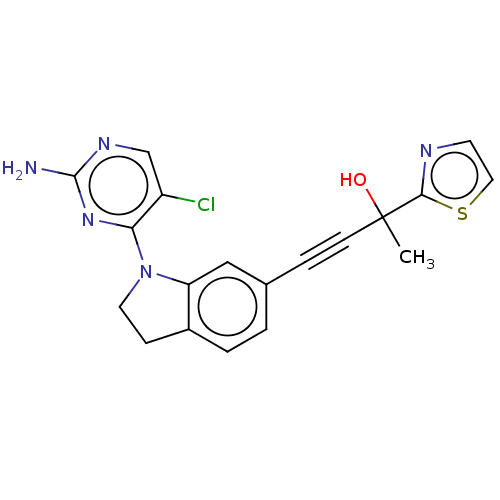
(CHEMBL3187788)Show SMILES CC(O)(C#Cc1ccc2CCN(c2c1)c1nc(N)ncc1Cl)c1nccs1 Show InChI InChI=1S/C19H16ClN5OS/c1-19(26,17-22-7-9-27-17)6-4-12-2-3-13-5-8-25(15(13)10-12)16-14(20)11-23-18(21)24-16/h2-3,7,9-11,26H,5,8H2,1H3,(H2,21,23,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01511
BindingDB Entry DOI: 10.7270/Q22Z19M2 |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50067796
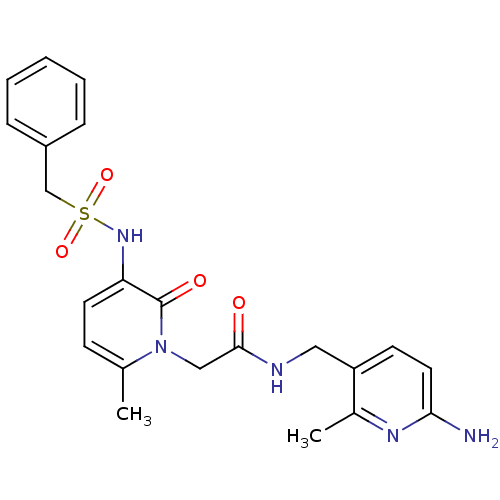
(CHEMBL11157 | L-374087 | N-((6-amino-2-methylpyrid...)Show SMILES Cc1nc(N)ccc1CNC(=O)Cn1c(C)ccc(NS(=O)(=O)Cc2ccccc2)c1=O Show InChI InChI=1S/C22H25N5O4S/c1-15-8-10-19(26-32(30,31)14-17-6-4-3-5-7-17)22(29)27(15)13-21(28)24-12-18-9-11-20(23)25-16(18)2/h3-11,26H,12-14H2,1-2H3,(H2,23,25)(H,24,28) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hefei University of Technology
Curated by ChEMBL
| Assay Description
Inhibition of human thrombin assessed as release of p-nitroanilide from chromogenic substrate |
Eur J Med Chem 146: 299-317 (2018)
Article DOI: 10.1016/j.ejmech.2018.01.067
BindingDB Entry DOI: 10.7270/Q2251MTJ |
More data for this
Ligand-Target Pair | |
Delta-type/Kappa-type/Mu-type opioid receptor
(MOUSE-Mus musculus (Mouse)) | BDBM50474628
(CHEMBL386810)Show SMILES [H][C@@]12Oc3c4c(CC5N(CC6CC6)CCC14[C@@]5(O)Cc1c2[nH]c2ccc(NC(N)=NCCNC(=O)CNC(=O)CNC(=O)CCC(=O)NCC(=O)NCC(=O)Nc4cccc5c6C[C@@]7(O)C8Cc9ccc(O)c%10O[C@@]([H])(c6[nH]c45)C7(CCN8CC4CC4)c9%10)cc12)ccc3O |TLB:64:63:87.67.66:82.80.81,83:82:63:87.67.66,72:87:63:82.80.81,THB:17:16:4.5.6:8.14.13,9:8:16:4.5.6,3:4:16:8.14.13| Show InChI InChI=1S/C67H75N13O12/c68-63(75-37-10-11-42-39(24-37)41-26-67(90)47-23-35-8-12-44(81)59-54(35)64(67,61(91-59)57(41)77-42)16-20-80(47)32-34-6-7-34)70-19-18-69-50(85)27-73-51(86)28-71-48(83)14-15-49(84)72-29-52(87)74-30-53(88)76-43-3-1-2-38-40-25-66(89)46-22-36-9-13-45(82)60-55(36)65(66,17-21-79(46)31-33-4-5-33)62(92-60)58(40)78-56(38)43/h1-3,8-13,24,33-34,46-47,61-62,77-78,81-82,89-90H,4-7,14-23,25-32H2,(H,69,85)(H,71,83)(H,72,84)(H,73,86)(H,74,87)(H,76,88)(H3,68,70,75)/t46?,47?,61-,62-,64?,65?,66+,67+/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Minnesota
Curated by ChEMBL
| Assay Description
Compound was tested for binding affinity on intact HEK cells using [3H]diprenorphine as radioligand co-expressed with delta and kappa opioid receptor... |
J Med Chem 47: 2969-72 (2004)
Article DOI: 10.1021/jm0342358
BindingDB Entry DOI: 10.7270/Q2N58Q39 |
More data for this
Ligand-Target Pair | |
Disintegrin and metalloproteinase domain-containing protein 17
(Sus scrofa (pig)) | BDBM26526
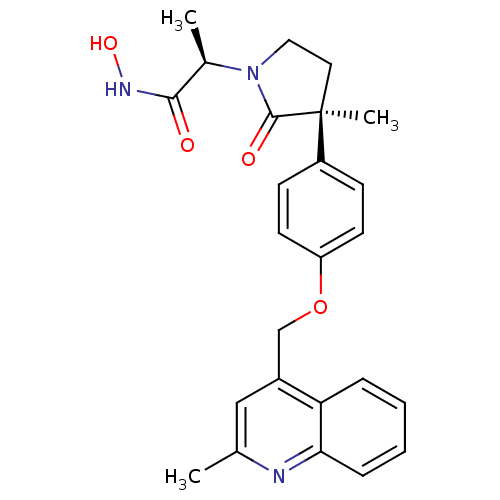
((2R)-N-hydroxy-2-[(3S)-3-methyl-3-{4-[(2-methylqui...)Show SMILES C[C@@H](N1CC[C@](C)(C1=O)c1ccc(OCc2cc(C)nc3ccccc23)cc1)C(=O)NO |r| Show InChI InChI=1S/C25H27N3O4/c1-16-14-18(21-6-4-5-7-22(21)26-16)15-32-20-10-8-19(9-11-20)25(3)12-13-28(24(25)30)17(2)23(29)27-31/h4-11,14,17,31H,12-13,15H2,1-3H3,(H,27,29)/t17-,25+/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
Article
PubMed
| 0.560 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113877
BindingDB Entry DOI: 10.7270/Q2PK0M78 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
3-phosphoinositide-dependent protein kinase 1
(Homo sapiens (Human)) | BDBM50361648
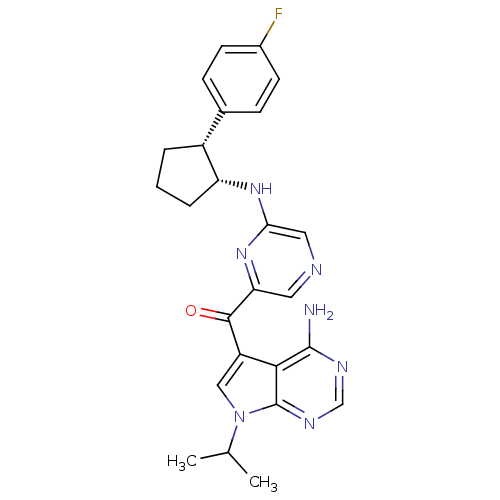
(CHEMBL1940246)Show SMILES CC(C)n1cc(C(=O)c2cncc(N[C@@H]3CCC[C@@H]3c3ccc(F)cc3)n2)c2c(N)ncnc12 |r| Show InChI InChI=1S/C25H26FN7O/c1-14(2)33-12-18(22-24(27)29-13-30-25(22)33)23(34)20-10-28-11-21(32-20)31-19-5-3-4-17(19)15-6-8-16(26)9-7-15/h6-14,17,19H,3-5H2,1-2H3,(H,31,32)(H2,27,29,30)/t17-,19-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of PDK1-mediated AKT1 phosphorylation at T308 in human H460 cells after 2 hrs by ELISA |
J Med Chem 54: 8490-500 (2011)
Article DOI: 10.1021/jm201019k
BindingDB Entry DOI: 10.7270/Q23N23TV |
More data for this
Ligand-Target Pair | |
3-phosphoinositide-dependent protein kinase 1
(Homo sapiens (Human)) | BDBM50361649
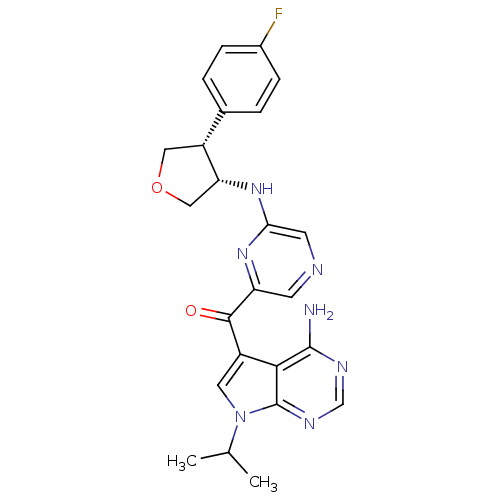
(CHEMBL1938415)Show SMILES CC(C)n1cc(C(=O)c2cncc(N[C@@H]3COC[C@@H]3c3ccc(F)cc3)n2)c2c(N)ncnc12 |r| Show InChI InChI=1S/C24H24FN7O2/c1-13(2)32-9-16(21-23(26)28-12-29-24(21)32)22(33)18-7-27-8-20(30-18)31-19-11-34-10-17(19)14-3-5-15(25)6-4-14/h3-9,12-13,17,19H,10-11H2,1-2H3,(H,30,31)(H2,26,28,29)/t17-,19-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of PDK1-mediated AKT1 phosphorylation at T308 in human H460 cells after 2 hrs by ELISA |
J Med Chem 54: 8490-500 (2011)
Article DOI: 10.1021/jm201019k
BindingDB Entry DOI: 10.7270/Q23N23TV |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Tyrosine-protein kinase JAK1
(Homo sapiens (Human)) | BDBM50193995

(3-((3R,4R)-4-methyl-3-(methyl(7H-pyrrolo[2,3-d]pyr...)Show SMILES C[C@@H]1CCN(C[C@@H]1N(C)c1ncnc2[nH]ccc12)C(=O)CC#N |r| Show InChI InChI=1S/C16H20N6O/c1-11-5-8-22(14(23)3-6-17)9-13(11)21(2)16-12-4-7-18-15(12)19-10-20-16/h4,7,10-11,13H,3,5,8-9H2,1-2H3,(H,18,19,20)/t11-,13+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01511
BindingDB Entry DOI: 10.7270/Q22Z19M2 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50088373
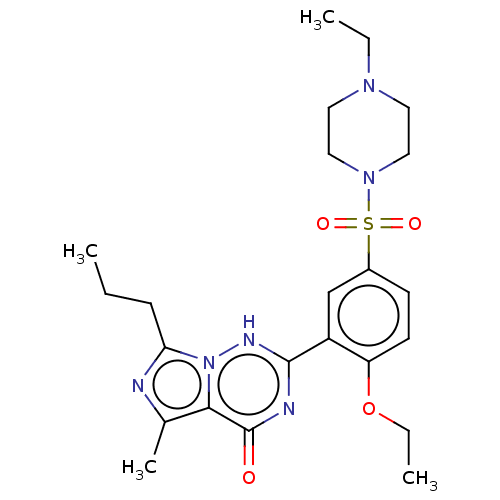
(CHEBI:46295 | Vardenafil | cid_110634)Show SMILES CCCc1nc(C)c2n1[nH]c(nc2=O)-c1cc(ccc1OCC)S(=O)(=O)N1CCN(CC)CC1 Show InChI InChI=1S/C23H32N6O4S/c1-5-8-20-24-16(4)21-23(30)25-22(26-29(20)21)18-15-17(9-10-19(18)33-7-3)34(31,32)28-13-11-27(6-2)12-14-28/h9-10,15H,5-8,11-14H2,1-4H3,(H,25,26,30) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Similars
| DrugBank
PDB
Article
PubMed
| 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Shandong University
Curated by ChEMBL
| Assay Description
Inhibition of PDE5 (unknown origin) |
Eur J Med Chem 158: 767-780 (2018)
Article DOI: 10.1016/j.ejmech.2018.09.028
BindingDB Entry DOI: 10.7270/Q2JS9T4N |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
3-phosphoinositide-dependent protein kinase 1
(Homo sapiens (Human)) | BDBM50361642
(CHEMBL1940251)Show SMILES CC(C)n1cc(C(=O)c2cncc(N[C@@H]3CCN(C=O)[C@@H]3c3ccc(F)cc3)n2)c2c(N)ncnc12 |r| Show InChI InChI=1S/C25H25FN8O2/c1-14(2)34-11-17(21-24(27)29-12-30-25(21)34)23(36)19-9-28-10-20(32-19)31-18-7-8-33(13-35)22(18)15-3-5-16(26)6-4-15/h3-6,9-14,18,22H,7-8H2,1-2H3,(H,31,32)(H2,27,29,30)/t18-,22-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human His-tagged PDK1 catalytic domain using Ac-Sox-PKTFCGTPEYLAPEVRREPRILSEEEQEMFRDFDYIAD-NH2 as substrate by fluorescence... |
J Med Chem 54: 8490-500 (2011)
Article DOI: 10.1021/jm201019k
BindingDB Entry DOI: 10.7270/Q23N23TV |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50067797
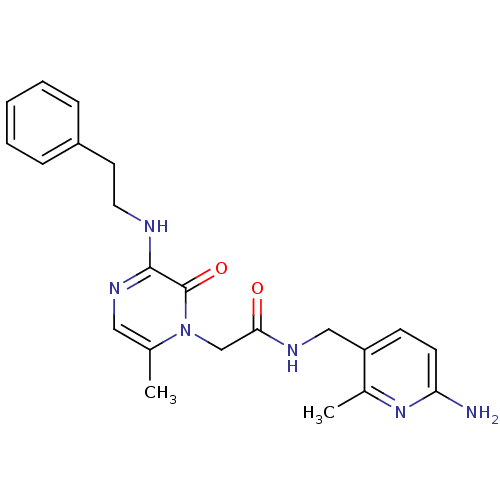
(CHEMBL19080 | L-37378 | N-(6-Amino-2-methyl-pyridi...)Show SMILES Cc1nc(N)ccc1CNC(=O)Cn1c(C)cnc(NCCc2ccccc2)c1=O Show InChI InChI=1S/C22H26N6O2/c1-15-12-26-21(24-11-10-17-6-4-3-5-7-17)22(30)28(15)14-20(29)25-13-18-8-9-19(23)27-16(18)2/h3-9,12H,10-11,13-14H2,1-2H3,(H2,23,27)(H,24,26)(H,25,29) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hefei University of Technology
Curated by ChEMBL
| Assay Description
Inhibition of human thrombin assessed as release of p-nitroanilide from chromogenic substrate |
Eur J Med Chem 146: 299-317 (2018)
Article DOI: 10.1016/j.ejmech.2018.01.067
BindingDB Entry DOI: 10.7270/Q2251MTJ |
More data for this
Ligand-Target Pair | |
3-phosphoinositide-dependent protein kinase 1
(Homo sapiens (Human)) | BDBM50361641
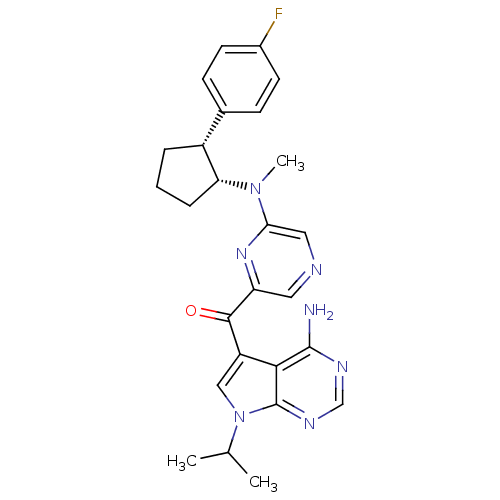
(CHEMBL1940247)Show SMILES CC(C)n1cc(C(=O)c2cncc(n2)N(C)[C@@H]2CCC[C@@H]2c2ccc(F)cc2)c2c(N)ncnc12 |r| Show InChI InChI=1S/C26H28FN7O/c1-15(2)34-13-19(23-25(28)30-14-31-26(23)34)24(35)20-11-29-12-22(32-20)33(3)21-6-4-5-18(21)16-7-9-17(27)10-8-16/h7-15,18,21H,4-6H2,1-3H3,(H2,28,30,31)/t18-,21-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of PDK1-mediated AKT1 phosphorylation at T308 in human H460 cells after 2 hrs by ELISA |
J Med Chem 54: 8490-500 (2011)
Article DOI: 10.1021/jm201019k
BindingDB Entry DOI: 10.7270/Q23N23TV |
More data for this
Ligand-Target Pair | |
3-phosphoinositide-dependent protein kinase 1
(Homo sapiens (Human)) | BDBM50361652
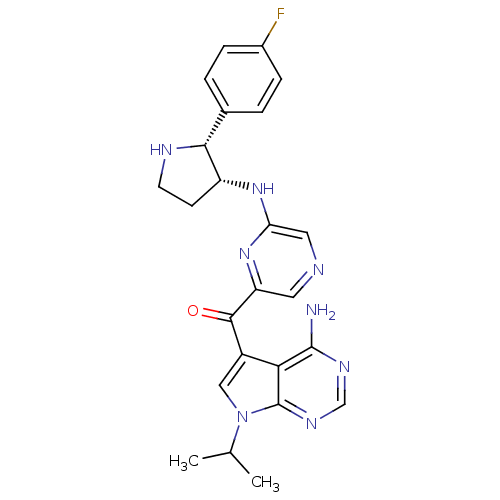
(CHEMBL1940250)Show SMILES CC(C)n1cc(C(=O)c2cncc(N[C@@H]3CCN[C@@H]3c3ccc(F)cc3)n2)c2c(N)ncnc12 |r| Show InChI InChI=1S/C24H25FN8O/c1-13(2)33-11-16(20-23(26)29-12-30-24(20)33)22(34)18-9-27-10-19(32-18)31-17-7-8-28-21(17)14-3-5-15(25)6-4-14/h3-6,9-13,17,21,28H,7-8H2,1-2H3,(H,31,32)(H2,26,29,30)/t17-,21-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of PDK1-mediated AKT1 phosphorylation at T308 in human H460 cells after 2 hrs by ELISA |
J Med Chem 54: 8490-500 (2011)
Article DOI: 10.1021/jm201019k
BindingDB Entry DOI: 10.7270/Q23N23TV |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 4
(Homo sapiens (Human)) | BDBM12621

(2,4-Diamino-5-ketopyrimidine 39 | 5-[(2,3-difluoro...)Show SMILES COc1ccc(F)c(F)c1C(=O)c1cnc(NC2CCN(CC2)S(C)(=O)=O)nc1N Show InChI InChI=1S/C18H21F2N5O4S/c1-29-13-4-3-12(19)15(20)14(13)16(26)11-9-22-18(24-17(11)21)23-10-5-7-25(8-6-10)30(2,27)28/h3-4,9-10H,5-8H2,1-2H3,(H3,21,22,23,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c02190
BindingDB Entry DOI: 10.7270/Q2BV7MPD |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK2
(Homo sapiens (Human)) | BDBM50193995

(3-((3R,4R)-4-methyl-3-(methyl(7H-pyrrolo[2,3-d]pyr...)Show SMILES C[C@@H]1CCN(C[C@@H]1N(C)c1ncnc2[nH]ccc12)C(=O)CC#N |r| Show InChI InChI=1S/C16H20N6O/c1-11-5-8-22(14(23)3-6-17)9-13(11)21(2)16-12-4-7-18-15(12)19-10-20-16/h4,7,10-11,13H,3,5,8-9H2,1-2H3,(H,18,19,20)/t11-,13+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01511
BindingDB Entry DOI: 10.7270/Q22Z19M2 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Rho-associated protein kinase 1
(Homo sapiens (Human)) | BDBM50515430
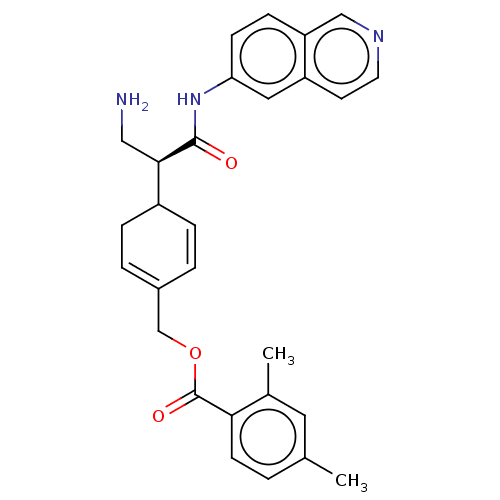
(NETARSUDIL | US11608319, Compound AR-13324)Show SMILES Cc1ccc(C(=O)OCC2=CCC(C=C2)[C@@H](CN)C(=O)Nc2ccc3cnccc3c2)c(C)c1 |r,c:13,t:9| Show InChI InChI=1S/C28H29N3O3/c1-18-3-10-25(19(2)13-18)28(33)34-17-20-4-6-21(7-5-20)26(15-29)27(32)31-24-9-8-23-16-30-12-11-22(23)14-24/h3-6,8-14,16,21,26H,7,15,17,29H2,1-2H3,(H,31,32)/t21?,26-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01511
BindingDB Entry DOI: 10.7270/Q22Z19M2 |
More data for this
Ligand-Target Pair | |
Adenosine deaminase
(Homo sapiens (Human)) | BDBM28393
((+)-EHNA | (2S,3R)-3-(6-amino-9H-purin-9-yl)nonan-...)Show InChI InChI=1S/C14H23N5O/c1-3-4-5-6-7-11(10(2)20)19-9-18-12-13(15)16-8-17-14(12)19/h8-11,20H,3-7H2,1-2H3,(H2,15,16,17)/t10-,11+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| MMDB
PDB
Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113877
BindingDB Entry DOI: 10.7270/Q2PK0M78 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Rho-associated protein kinase 2
(Homo sapiens (Human)) | BDBM50515430

(NETARSUDIL | US11608319, Compound AR-13324)Show SMILES Cc1ccc(C(=O)OCC2=CCC(C=C2)[C@@H](CN)C(=O)Nc2ccc3cnccc3c2)c(C)c1 |r,c:13,t:9| Show InChI InChI=1S/C28H29N3O3/c1-18-3-10-25(19(2)13-18)28(33)34-17-20-4-6-21(7-5-20)26(15-29)27(32)31-24-9-8-23-16-30-12-11-22(23)14-24/h3-6,8-14,16,21,26H,7,15,17,29H2,1-2H3,(H,31,32)/t21?,26-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01511
BindingDB Entry DOI: 10.7270/Q22Z19M2 |
More data for this
Ligand-Target Pair | |
Delta-type/Kappa-type/Mu-type opioid receptor
(MOUSE-Mus musculus (Mouse)) | BDBM50474624
(CHEMBL409172)Show SMILES [H][C@@]12Oc3c4c(CC5N(CC6CC6)CCC14[C@@]5(O)Cc1c2[nH]c2ccc(NC(N)=NCCCCNC(=O)CNC(=O)CNC(=O)CCC(=O)NCC(=O)NCC(=O)Nc4cccc5c6C[C@@]7(O)C8Cc9ccc(O)c%10O[C@@]([H])(c6[nH]c45)C7(CCN8CC4CC4)c9%10)cc12)ccc3O |TLB:66:65:89.69.68:84.82.83,85:84:65:89.69.68,74:89:65:84.82.83,THB:17:16:4.5.6:8.14.13,9:8:16:4.5.6,3:4:16:8.14.13| Show InChI InChI=1S/C69H79N13O12/c70-65(77-39-12-13-44-41(26-39)43-28-69(92)49-25-37-10-14-46(83)61-56(37)66(69,63(93-61)59(43)79-44)18-22-82(49)34-36-8-9-36)72-21-2-1-20-71-52(87)29-75-53(88)30-73-50(85)16-17-51(86)74-31-54(89)76-32-55(90)78-45-5-3-4-40-42-27-68(91)48-24-38-11-15-47(84)62-57(38)67(68,19-23-81(48)33-35-6-7-35)64(94-62)60(42)80-58(40)45/h3-5,10-15,26,35-36,48-49,63-64,79-80,83-84,91-92H,1-2,6-9,16-25,27-34H2,(H,71,87)(H,73,85)(H,74,86)(H,75,88)(H,76,89)(H,78,90)(H3,70,72,77)/t48?,49?,63-,64-,66?,67?,68+,69+/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Minnesota
Curated by ChEMBL
| Assay Description
Compound was tested for binding affinity on intact HEK cells using [3H]diprenorphine as radioligand singly expressed with delta or kappa receptor |
J Med Chem 47: 2969-72 (2004)
Article DOI: 10.1021/jm0342358
BindingDB Entry DOI: 10.7270/Q2N58Q39 |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50124086

(2-[6-Methyl-2-oxo-3-(2-pyridin-2-yl-ethylamino)-2H...)Show SMILES Cc1cnc(NCCc2ccccn2)c(=O)n1CC(=O)NCc1cc2cc[nH]c2cn1 Show InChI InChI=1S/C22H23N7O2/c1-15-11-28-21(25-9-6-17-4-2-3-7-23-17)22(31)29(15)14-20(30)27-12-18-10-16-5-8-24-19(16)13-26-18/h2-5,7-8,10-11,13,24H,6,9,12,14H2,1H3,(H,25,28)(H,27,30) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hefei University of Technology
Curated by ChEMBL
| Assay Description
Inhibition of human thrombin assessed as release of p-nitroanilide from chromogenic substrate |
Eur J Med Chem 146: 299-317 (2018)
Article DOI: 10.1016/j.ejmech.2018.01.067
BindingDB Entry DOI: 10.7270/Q2251MTJ |
More data for this
Ligand-Target Pair | |
3-phosphoinositide-dependent protein kinase 1
(Homo sapiens (Human)) | BDBM50361650
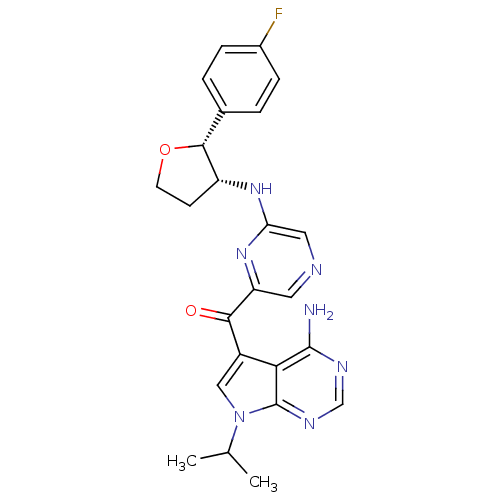
(CHEMBL1940248)Show SMILES CC(C)n1cc(C(=O)c2cncc(N[C@@H]3CCO[C@@H]3c3ccc(F)cc3)n2)c2c(N)ncnc12 |r| Show InChI InChI=1S/C24H24FN7O2/c1-13(2)32-11-16(20-23(26)28-12-29-24(20)32)21(33)18-9-27-10-19(31-18)30-17-7-8-34-22(17)14-3-5-15(25)6-4-14/h3-6,9-13,17,22H,7-8H2,1-2H3,(H,30,31)(H2,26,28,29)/t17-,22-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of PDK1-mediated AKT1 phosphorylation at T308 in human H460 cells after 2 hrs by ELISA |
J Med Chem 54: 8490-500 (2011)
Article DOI: 10.1021/jm201019k
BindingDB Entry DOI: 10.7270/Q23N23TV |
More data for this
Ligand-Target Pair | |
Delta-type/Kappa-type/Mu-type opioid receptor
(MOUSE-Mus musculus (Mouse)) | BDBM50474624

(CHEMBL409172)Show SMILES [H][C@@]12Oc3c4c(CC5N(CC6CC6)CCC14[C@@]5(O)Cc1c2[nH]c2ccc(NC(N)=NCCCCNC(=O)CNC(=O)CNC(=O)CCC(=O)NCC(=O)NCC(=O)Nc4cccc5c6C[C@@]7(O)C8Cc9ccc(O)c%10O[C@@]([H])(c6[nH]c45)C7(CCN8CC4CC4)c9%10)cc12)ccc3O |TLB:66:65:89.69.68:84.82.83,85:84:65:89.69.68,74:89:65:84.82.83,THB:17:16:4.5.6:8.14.13,9:8:16:4.5.6,3:4:16:8.14.13| Show InChI InChI=1S/C69H79N13O12/c70-65(77-39-12-13-44-41(26-39)43-28-69(92)49-25-37-10-14-46(83)61-56(37)66(69,63(93-61)59(43)79-44)18-22-82(49)34-36-8-9-36)72-21-2-1-20-71-52(87)29-75-53(88)30-73-50(85)16-17-51(86)74-31-54(89)76-32-55(90)78-45-5-3-4-40-42-27-68(91)48-24-38-11-15-47(84)62-57(38)67(68,19-23-81(48)33-35-6-7-35)64(94-62)60(42)80-58(40)45/h3-5,10-15,26,35-36,48-49,63-64,79-80,83-84,91-92H,1-2,6-9,16-25,27-34H2,(H,71,87)(H,73,85)(H,74,86)(H,75,88)(H,76,89)(H,78,90)(H3,70,72,77)/t48?,49?,63-,64-,66?,67?,68+,69+/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Minnesota
Curated by ChEMBL
| Assay Description
Compound was tested for binding affinity on intact HEK cells using [3H]diprenorphine as radioligand co-expressed with delta and kappa opioid receptor... |
J Med Chem 47: 2969-72 (2004)
Article DOI: 10.1021/jm0342358
BindingDB Entry DOI: 10.7270/Q2N58Q39 |
More data for this
Ligand-Target Pair | |
3-phosphoinositide-dependent protein kinase 1
(Homo sapiens (Human)) | BDBM50361644
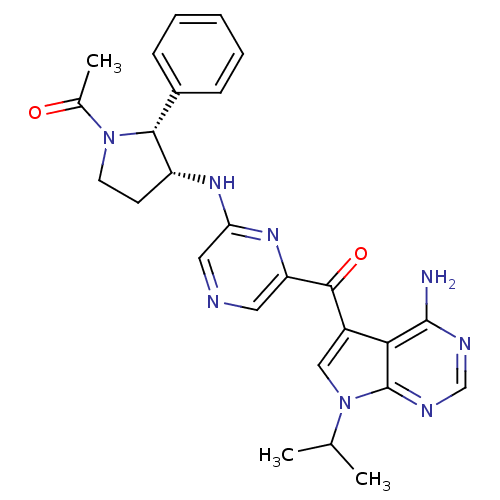
(CHEMBL1940253)Show SMILES CC(C)n1cc(C(=O)c2cncc(N[C@@H]3CCN([C@@H]3c3ccccc3)C(C)=O)n2)c2c(N)ncnc12 |r| Show InChI InChI=1S/C26H28N8O2/c1-15(2)34-13-18(22-25(27)29-14-30-26(22)34)24(36)20-11-28-12-21(32-20)31-19-9-10-33(16(3)35)23(19)17-7-5-4-6-8-17/h4-8,11-15,19,23H,9-10H2,1-3H3,(H,31,32)(H2,27,29,30)/t19-,23-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human His-tagged PDK1 catalytic domain using Ac-Sox-PKTFCGTPEYLAPEVRREPRILSEEEQEMFRDFDYIAD-NH2 as substrate by fluorescence... |
J Med Chem 54: 8490-500 (2011)
Article DOI: 10.1021/jm201019k
BindingDB Entry DOI: 10.7270/Q23N23TV |
More data for this
Ligand-Target Pair | |
3-phosphoinositide-dependent protein kinase 1
(Homo sapiens (Human)) | BDBM50361643
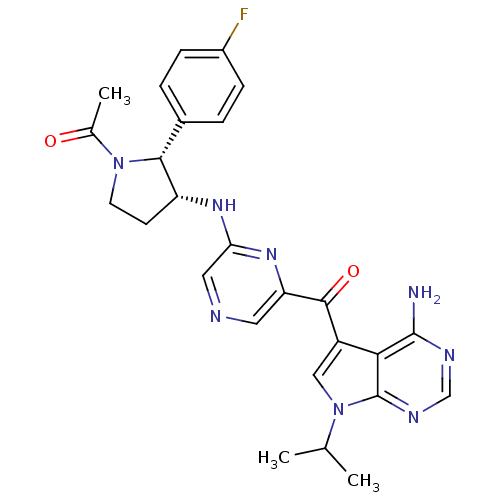
(CHEMBL1940252)Show SMILES CC(C)n1cc(C(=O)c2cncc(N[C@@H]3CCN([C@@H]3c3ccc(F)cc3)C(C)=O)n2)c2c(N)ncnc12 |r| Show InChI InChI=1S/C26H27FN8O2/c1-14(2)35-12-18(22-25(28)30-13-31-26(22)35)24(37)20-10-29-11-21(33-20)32-19-8-9-34(15(3)36)23(19)16-4-6-17(27)7-5-16/h4-7,10-14,19,23H,8-9H2,1-3H3,(H,32,33)(H2,28,30,31)/t19-,23-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human His-tagged PDK1 catalytic domain using Ac-Sox-PKTFCGTPEYLAPEVRREPRILSEEEQEMFRDFDYIAD-NH2 as substrate by fluorescence... |
J Med Chem 54: 8490-500 (2011)
Article DOI: 10.1021/jm201019k
BindingDB Entry DOI: 10.7270/Q23N23TV |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM14390

(5-[2-ethoxy-5-(4-methyl-1-piperazinylsulfonyl)phen...)Show SMILES CCCc1nn(C)c2c1nc([nH]c2=O)-c1cc(ccc1OCC)S(=O)(=O)N1CCN(C)CC1 Show InChI InChI=1S/C22H30N6O4S/c1-5-7-17-19-20(27(4)25-17)22(29)24-21(23-19)16-14-15(8-9-18(16)32-6-2)33(30,31)28-12-10-26(3)11-13-28/h8-9,14H,5-7,10-13H2,1-4H3,(H,23,24,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
Article
PubMed
| 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Shandong University
Curated by ChEMBL
| Assay Description
Inhibition of PDE5 (unknown origin) |
Eur J Med Chem 158: 767-780 (2018)
Article DOI: 10.1016/j.ejmech.2018.09.028
BindingDB Entry DOI: 10.7270/Q2JS9T4N |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Prothrombin
(Homo sapiens (Human)) | BDBM50124093

(CHEMBL355730 | N-(1H-Indol-5-ylmethyl)-2-[6-methyl...)Show SMILES Cc1cnc(NCCc2ccccn2)c(=O)n1CC(=O)NCc1ccc2[nH]ccc2c1 Show InChI InChI=1S/C23H24N6O2/c1-16-13-28-22(26-11-8-19-4-2-3-9-24-19)23(31)29(16)15-21(30)27-14-17-5-6-20-18(12-17)7-10-25-20/h2-7,9-10,12-13,25H,8,11,14-15H2,1H3,(H,26,28)(H,27,30) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hefei University of Technology
Curated by ChEMBL
| Assay Description
Inhibition of human thrombin assessed as release of p-nitroanilide from chromogenic substrate |
Eur J Med Chem 146: 299-317 (2018)
Article DOI: 10.1016/j.ejmech.2018.01.067
BindingDB Entry DOI: 10.7270/Q2251MTJ |
More data for this
Ligand-Target Pair | |
Rho-associated protein kinase 2
(Homo sapiens (Human)) | BDBM50601415
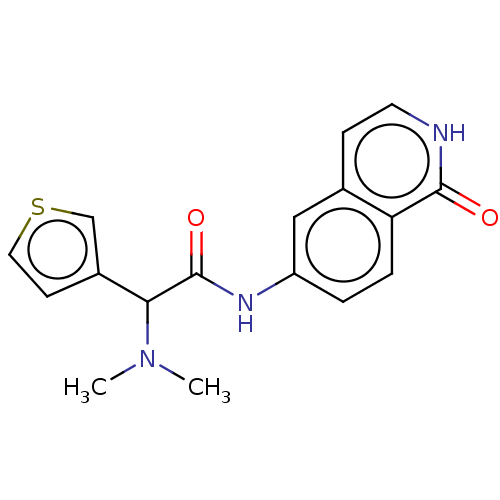
(AR-12286 | AR-12286 FREE BASE | Ar-12286 | VEROSUD...) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01511
BindingDB Entry DOI: 10.7270/Q22Z19M2 |
More data for this
Ligand-Target Pair | |
Rho-associated protein kinase 1
(Homo sapiens (Human)) | BDBM50601415

(AR-12286 | AR-12286 FREE BASE | Ar-12286 | VEROSUD...) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01511
BindingDB Entry DOI: 10.7270/Q22Z19M2 |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 1
(Homo sapiens (Human)) | BDBM12621

(2,4-Diamino-5-ketopyrimidine 39 | 5-[(2,3-difluoro...)Show SMILES COc1ccc(F)c(F)c1C(=O)c1cnc(NC2CCN(CC2)S(C)(=O)=O)nc1N Show InChI InChI=1S/C18H21F2N5O4S/c1-29-13-4-3-12(19)15(20)14(13)16(26)11-9-22-18(24-17(11)21)23-10-5-7-25(8-6-10)30(2,27)28/h3-4,9-10H,5-8H2,1-2H3,(H3,21,22,23,24) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c02190
BindingDB Entry DOI: 10.7270/Q2BV7MPD |
More data for this
Ligand-Target Pair | |
3-phosphoinositide-dependent protein kinase 1
(Homo sapiens (Human)) | BDBM50361653
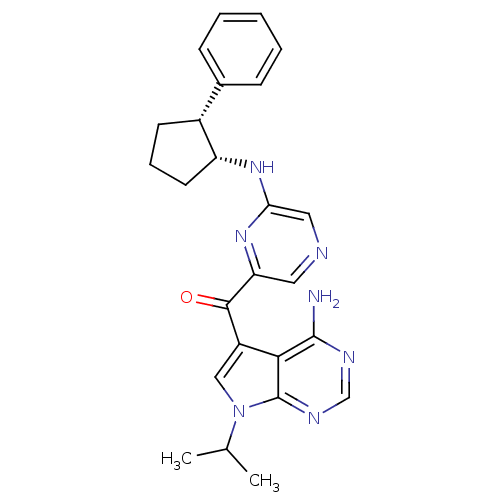
(CHEMBL1940245)Show SMILES CC(C)n1cc(C(=O)c2cncc(N[C@@H]3CCC[C@@H]3c3ccccc3)n2)c2c(N)ncnc12 |r| Show InChI InChI=1S/C25H27N7O/c1-15(2)32-13-18(22-24(26)28-14-29-25(22)32)23(33)20-11-27-12-21(31-20)30-19-10-6-9-17(19)16-7-4-3-5-8-16/h3-5,7-8,11-15,17,19H,6,9-10H2,1-2H3,(H,30,31)(H2,26,28,29)/t17-,19-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of PDK1-mediated AKT1 phosphorylation at T308 in human H460 cells after 2 hrs by ELISA |
J Med Chem 54: 8490-500 (2011)
Article DOI: 10.1021/jm201019k
BindingDB Entry DOI: 10.7270/Q23N23TV |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50126302
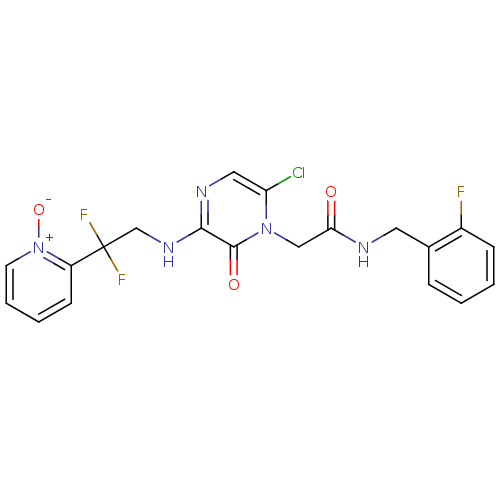
(2-(6-CHLORO-3-{[2,2-DIFLUORO-2-(1-OXIDO-2-PYRIDINY...)Show SMILES [O-][n+]1ccccc1C(F)(F)CNc1ncc(Cl)n(CC(=O)NCc2ccccc2F)c1=O Show InChI InChI=1S/C20H17ClF3N5O3/c21-16-10-26-18(27-12-20(23,24)15-7-3-4-8-29(15)32)19(31)28(16)11-17(30)25-9-13-5-1-2-6-14(13)22/h1-8,10H,9,11-12H2,(H,25,30)(H,26,27) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
DrugBank
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 2.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hefei University of Technology
Curated by ChEMBL
| Assay Description
Inhibition of thrombin (unknown origin) |
Eur J Med Chem 146: 299-317 (2018)
Article DOI: 10.1016/j.ejmech.2018.01.067
BindingDB Entry DOI: 10.7270/Q2251MTJ |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Prothrombin
(Homo sapiens (Human)) | BDBM50123481
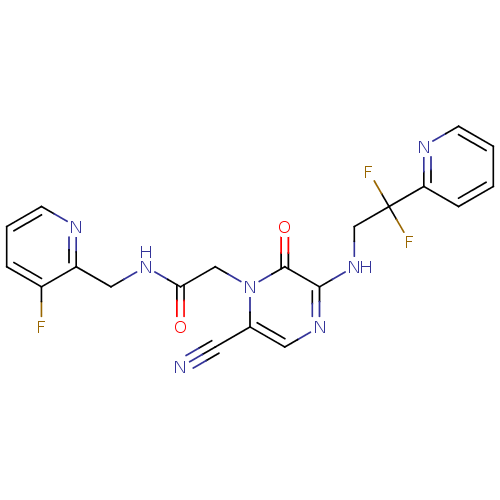
(2-(6-cyano-3-(2,2-difluoro-2-(pyridin-2-yl)ethylam...)Show SMILES Fc1cccnc1CNC(=O)Cn1c(cnc(NCC(F)(F)c2ccccn2)c1=O)C#N Show InChI InChI=1S/C20H16F3N7O2/c21-14-4-3-7-25-15(14)10-27-17(31)11-30-13(8-24)9-28-18(19(30)32)29-12-20(22,23)16-5-1-2-6-26-16/h1-7,9H,10-12H2,(H,27,31)(H,28,29) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 2.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hefei University of Technology
Curated by ChEMBL
| Assay Description
Inhibition of thrombin (unknown origin) |
Eur J Med Chem 146: 299-317 (2018)
Article DOI: 10.1016/j.ejmech.2018.01.067
BindingDB Entry DOI: 10.7270/Q2251MTJ |
More data for this
Ligand-Target Pair | |
Rho-associated protein kinase 2
(Homo sapiens (Human)) | BDBM50319005
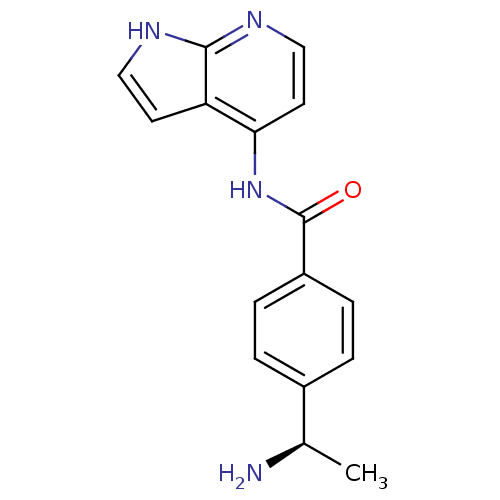
((R)-4-(1-aminoethyl)-N-(1H-pyrrolo[2,3-b]pyridin-4...)Show SMILES C[C@@H](N)c1ccc(cc1)C(=O)Nc1ccnc2[nH]ccc12 |r| Show InChI InChI=1S/C16H16N4O/c1-10(17)11-2-4-12(5-3-11)16(21)20-14-7-9-19-15-13(14)6-8-18-15/h2-10H,17H2,1H3,(H2,18,19,20,21)/t10-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| 2.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01511
BindingDB Entry DOI: 10.7270/Q22Z19M2 |
More data for this
Ligand-Target Pair | |
3-phosphoinositide-dependent protein kinase 1
(Homo sapiens (Human)) | BDBM50361651
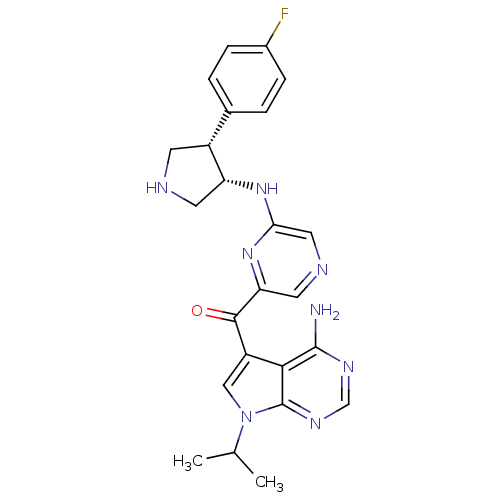
(CHEMBL1940249)Show SMILES CC(C)n1cc(C(=O)c2cncc(N[C@@H]3CNC[C@@H]3c3ccc(F)cc3)n2)c2c(N)ncnc12 |r| Show InChI InChI=1S/C24H25FN8O/c1-13(2)33-11-17(21-23(26)29-12-30-24(21)33)22(34)19-9-28-10-20(32-19)31-18-8-27-7-16(18)14-3-5-15(25)6-4-14/h3-6,9-13,16,18,27H,7-8H2,1-2H3,(H,31,32)(H2,26,29,30)/t16-,18-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of PDK1-mediated AKT1 phosphorylation at T308 in human H460 cells after 2 hrs by ELISA |
J Med Chem 54: 8490-500 (2011)
Article DOI: 10.1021/jm201019k
BindingDB Entry DOI: 10.7270/Q23N23TV |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data