 Found 4014 hits with Last Name = 'xue' and Initial = 'x'
Found 4014 hits with Last Name = 'xue' and Initial = 'x' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Rho-associated protein kinase 2
(Homo sapiens (Human)) | BDBM50546246
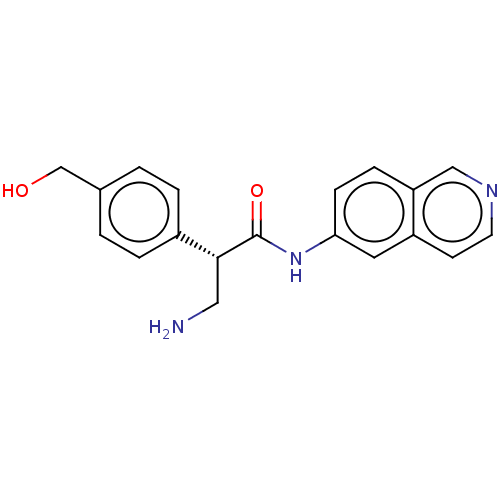
(CHEMBL4753043 | US11608319, Compound AR-13503)Show SMILES NC[C@@H](C(=O)Nc1ccc2cnccc2c1)c1ccc(CO)cc1 |r| | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Rho-associated protein kinase 1
(Homo sapiens (Human)) | BDBM50546246

(CHEMBL4753043 | US11608319, Compound AR-13503)Show SMILES NC[C@@H](C(=O)Nc1ccc2cnccc2c1)c1ccc(CO)cc1 |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Rho-associated protein kinase 1
(Homo sapiens (Human)) | BDBM50546247

(AR-11324 FREE BASE | AR-13324 | Netarsudil | US114...)Show SMILES Cc1ccc(C(=O)OCc2ccc(cc2)[C@@H](CN)C(=O)Nc2ccc3cnccc3c2)c(C)c1 |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| MCE
PC cid
PC sid
UniChem
| | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Rho-associated protein kinase 2
(Homo sapiens (Human)) | BDBM50546247

(AR-11324 FREE BASE | AR-13324 | Netarsudil | US114...)Show SMILES Cc1ccc(C(=O)OCc2ccc(cc2)[C@@H](CN)C(=O)Nc2ccc3cnccc3c2)c(C)c1 |r| | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| MCE
PC cid
PC sid
UniChem
| | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP4 subtype
(Homo sapiens (Human)) | BDBM50610937
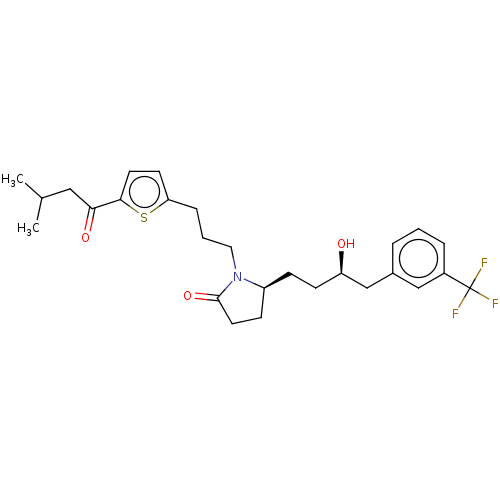
(CHEMBL5272661) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| UniChem
| | <2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Rho-associated protein kinase 2
(Homo sapiens (Human)) | BDBM50546247

(AR-11324 FREE BASE | AR-13324 | Netarsudil | US114...)Show SMILES Cc1ccc(C(=O)OCc2ccc(cc2)[C@@H](CN)C(=O)Nc2ccc3cnccc3c2)c(C)c1 |r| | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| MCE
PC cid
PC sid
UniChem
| | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP4 subtype
(Homo sapiens (Human)) | BDBM50181298
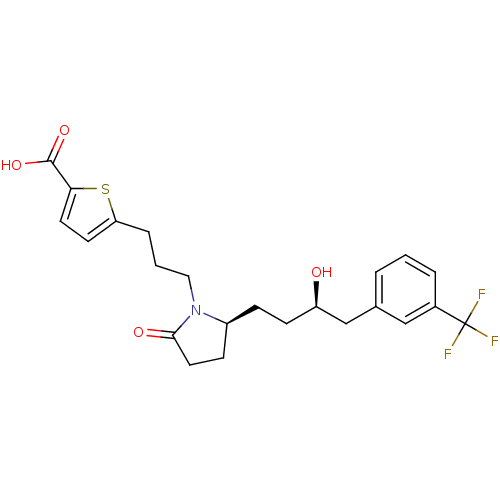
(5-(3-((S)-2-((R)-3-hydroxy-4-(3-(trifluoromethyl)p...)Show SMILES O[C@H](CC[C@H]1CCC(=O)N1CCCc1ccc(s1)C(O)=O)Cc1cccc(c1)C(F)(F)F Show InChI InChI=1S/C23H26F3NO4S/c24-23(25,26)16-4-1-3-15(13-16)14-18(28)8-6-17-7-11-21(29)27(17)12-2-5-19-9-10-20(32-19)22(30)31/h1,3-4,9-10,13,17-18,28H,2,5-8,11-12,14H2,(H,30,31)/t17-,18+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP2 subtype
(Homo sapiens (Human)) | BDBM50506546
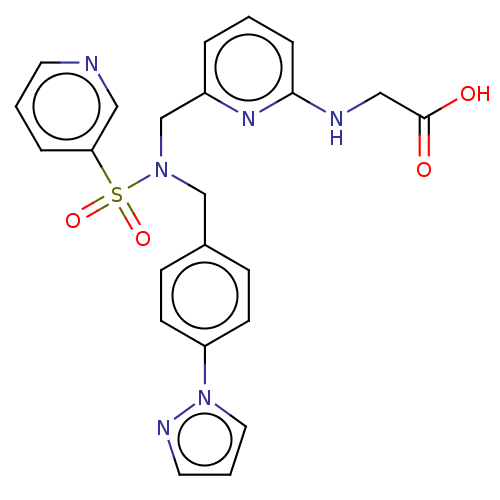
(Omidenepag | UR-7276)Show SMILES OC(=O)CNc1cccc(CN(Cc2ccc(cc2)-n2cccn2)S(=O)(=O)c2cccnc2)n1 Show InChI InChI=1S/C23H22N6O4S/c30-23(31)15-25-22-6-1-4-19(27-22)17-28(34(32,33)21-5-2-11-24-14-21)16-18-7-9-20(10-8-18)29-13-3-12-26-29/h1-14H,15-17H2,(H,25,27)(H,30,31) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| | 3.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Small conductance calcium-activated potassium channel protein 1/2/3
(Rattus norvegicus-RAT-Rattus norvegicus (Rat)) | BDBM392076

(US10301272, Example 7/9)Show SMILES CC(C)(C)NS(=O)(=O)c1ccc(-c2sc(nc2CC2CCCCC2)C(=O)N[C@H]2C[C@@H](C2)C(O)=O)c2ccccc12 |r,wU:27.28,wD:29.33,(8.76,1.15,;7.67,.06,;6.19,.46,;7.28,1.55,;8.07,-1.42,;6.98,-2.51,;6.59,-4,;8.07,-3.6,;5.5,-2.11,;5.1,-.63,;3.61,-.23,;2.52,-1.32,;1.03,-.92,;.56,.55,;-.98,.55,;-1.46,-.92,;-.21,-1.82,;-.21,-3.36,;-1.55,-4.13,;-2.88,-3.36,;-4.21,-4.13,;-4.21,-5.67,;-2.88,-6.44,;-1.55,-5.67,;-2.07,1.64,;-1.67,3.12,;-3.56,1.24,;-4.65,2.33,;-6.19,2.33,;-6.19,3.87,;-4.65,3.87,;-7.28,4.96,;-6.88,6.44,;-8.76,4.56,;2.92,-2.8,;1.83,-3.89,;2.23,-5.38,;3.72,-5.78,;4.81,-4.69,;4.41,-3.2,)| Show InChI InChI=1S/C30H37N3O5S2/c1-30(2,3)33-40(37,38)25-14-13-23(21-11-7-8-12-22(21)25)26-24(15-18-9-5-4-6-10-18)32-28(39-26)27(34)31-20-16-19(17-20)29(35)36/h7-8,11-14,18-20,33H,4-6,9-10,15-17H2,1-3H3,(H,31,34)(H,35,36)/t19-,20- | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.0120 | n/a | n/a | n/a | n/a | n/a | n/a |
Phenex Pharmaceuticals AG
Curated by ChEMBL
| Assay Description
Displacement of [125I]apamin from rat brain SkCa channel after 60 mins by scintillation counting analysis |
Bioorg Med Chem Lett 28: 1446-1455 (2018)
Article DOI: 10.1016/j.bmcl.2018.03.093
BindingDB Entry DOI: 10.7270/Q27W6FPC |
More data for this
Ligand-Target Pair | |
Endothelin-1 receptor
(Homo sapiens (Human)) | BDBM392076

(US10301272, Example 7/9)Show SMILES CC(C)(C)NS(=O)(=O)c1ccc(-c2sc(nc2CC2CCCCC2)C(=O)N[C@H]2C[C@@H](C2)C(O)=O)c2ccccc12 |r,wU:27.28,wD:29.33,(8.76,1.15,;7.67,.06,;6.19,.46,;7.28,1.55,;8.07,-1.42,;6.98,-2.51,;6.59,-4,;8.07,-3.6,;5.5,-2.11,;5.1,-.63,;3.61,-.23,;2.52,-1.32,;1.03,-.92,;.56,.55,;-.98,.55,;-1.46,-.92,;-.21,-1.82,;-.21,-3.36,;-1.55,-4.13,;-2.88,-3.36,;-4.21,-4.13,;-4.21,-5.67,;-2.88,-6.44,;-1.55,-5.67,;-2.07,1.64,;-1.67,3.12,;-3.56,1.24,;-4.65,2.33,;-6.19,2.33,;-6.19,3.87,;-4.65,3.87,;-7.28,4.96,;-6.88,6.44,;-8.76,4.56,;2.92,-2.8,;1.83,-3.89,;2.23,-5.38,;3.72,-5.78,;4.81,-4.69,;4.41,-3.2,)| Show InChI InChI=1S/C30H37N3O5S2/c1-30(2,3)33-40(37,38)25-14-13-23(21-11-7-8-12-22(21)25)26-24(15-18-9-5-4-6-10-18)32-28(39-26)27(34)31-20-16-19(17-20)29(35)36/h7-8,11-14,18-20,33H,4-6,9-10,15-17H2,1-3H3,(H,31,34)(H,35,36)/t19-,20- | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.0230 | n/a | n/a | n/a | n/a | n/a | n/a |
Phenex Pharmaceuticals AG
Curated by ChEMBL
| Assay Description
Displacement of [125I]-endothelin-1 from human recombinant ETA receptor after 120 mins by scintillation counting analysis |
Bioorg Med Chem Lett 28: 1446-1455 (2018)
Article DOI: 10.1016/j.bmcl.2018.03.093
BindingDB Entry DOI: 10.7270/Q27W6FPC |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 1
(Homo sapiens (Human)) | BDBM392076

(US10301272, Example 7/9)Show SMILES CC(C)(C)NS(=O)(=O)c1ccc(-c2sc(nc2CC2CCCCC2)C(=O)N[C@H]2C[C@@H](C2)C(O)=O)c2ccccc12 |r,wU:27.28,wD:29.33,(8.76,1.15,;7.67,.06,;6.19,.46,;7.28,1.55,;8.07,-1.42,;6.98,-2.51,;6.59,-4,;8.07,-3.6,;5.5,-2.11,;5.1,-.63,;3.61,-.23,;2.52,-1.32,;1.03,-.92,;.56,.55,;-.98,.55,;-1.46,-.92,;-.21,-1.82,;-.21,-3.36,;-1.55,-4.13,;-2.88,-3.36,;-4.21,-4.13,;-4.21,-5.67,;-2.88,-6.44,;-1.55,-5.67,;-2.07,1.64,;-1.67,3.12,;-3.56,1.24,;-4.65,2.33,;-6.19,2.33,;-6.19,3.87,;-4.65,3.87,;-7.28,4.96,;-6.88,6.44,;-8.76,4.56,;2.92,-2.8,;1.83,-3.89,;2.23,-5.38,;3.72,-5.78,;4.81,-4.69,;4.41,-3.2,)| Show InChI InChI=1S/C30H37N3O5S2/c1-30(2,3)33-40(37,38)25-14-13-23(21-11-7-8-12-22(21)25)26-24(15-18-9-5-4-6-10-18)32-28(39-26)27(34)31-20-16-19(17-20)29(35)36/h7-8,11-14,18-20,33H,4-6,9-10,15-17H2,1-3H3,(H,31,34)(H,35,36)/t19-,20- | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.0810 | n/a | n/a | n/a | n/a | n/a | n/a |
Phenex Pharmaceuticals AG
Curated by ChEMBL
| Assay Description
Displacement of [125I]MIP-1alpha from human recombinant CCR1 receptor after 120 mins by scintillation counting analysis |
Bioorg Med Chem Lett 28: 1446-1455 (2018)
Article DOI: 10.1016/j.bmcl.2018.03.093
BindingDB Entry DOI: 10.7270/Q27W6FPC |
More data for this
Ligand-Target Pair | |
Nuclear receptor ROR-gamma
(Homo sapiens (Human)) | BDBM50543677

(CHEMBL4635823)Show SMILES C[C@H](NS(=O)(=O)c1ccc(-c2sc(nc2C(=O)N2CCCC[C@@H]2C)-c2nnc(CC(C)(C)C(O)=O)o2)c(C(F)F)c1F)C(F)(F)F |r| Show InChI InChI=1S/C27H29F6N5O6S2/c1-12-7-5-6-10-38(12)24(39)19-20(45-23(34-19)22-36-35-16(44-22)11-26(3,4)25(40)41)14-8-9-15(18(28)17(14)21(29)30)46(42,43)37-13(2)27(31,32)33/h8-9,12-13,21,37H,5-7,10-11H2,1-4H3,(H,40,41)/t12-,13-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
Phenex Pharmaceuticals AG
Curated by ChEMBL
| Assay Description
Binding affinity to recombinant human RORgammat transfected in human HEK293T cells incubated for 24 hrs by dual-glo luciferase reporter gene assay |
Bioorg Med Chem Lett 30: (2020)
Article DOI: 10.1016/j.bmcl.2020.127174
BindingDB Entry DOI: 10.7270/Q2862M1T |
More data for this
Ligand-Target Pair | |
Cholecystokinin receptor type A
(Homo sapiens (Human)) | BDBM392076

(US10301272, Example 7/9)Show SMILES CC(C)(C)NS(=O)(=O)c1ccc(-c2sc(nc2CC2CCCCC2)C(=O)N[C@H]2C[C@@H](C2)C(O)=O)c2ccccc12 |r,wU:27.28,wD:29.33,(8.76,1.15,;7.67,.06,;6.19,.46,;7.28,1.55,;8.07,-1.42,;6.98,-2.51,;6.59,-4,;8.07,-3.6,;5.5,-2.11,;5.1,-.63,;3.61,-.23,;2.52,-1.32,;1.03,-.92,;.56,.55,;-.98,.55,;-1.46,-.92,;-.21,-1.82,;-.21,-3.36,;-1.55,-4.13,;-2.88,-3.36,;-4.21,-4.13,;-4.21,-5.67,;-2.88,-6.44,;-1.55,-5.67,;-2.07,1.64,;-1.67,3.12,;-3.56,1.24,;-4.65,2.33,;-6.19,2.33,;-6.19,3.87,;-4.65,3.87,;-7.28,4.96,;-6.88,6.44,;-8.76,4.56,;2.92,-2.8,;1.83,-3.89,;2.23,-5.38,;3.72,-5.78,;4.81,-4.69,;4.41,-3.2,)| Show InChI InChI=1S/C30H37N3O5S2/c1-30(2,3)33-40(37,38)25-14-13-23(21-11-7-8-12-22(21)25)26-24(15-18-9-5-4-6-10-18)32-28(39-26)27(34)31-20-16-19(17-20)29(35)36/h7-8,11-14,18-20,33H,4-6,9-10,15-17H2,1-3H3,(H,31,34)(H,35,36)/t19-,20- | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.120 | n/a | n/a | n/a | n/a | n/a | n/a |
Phenex Pharmaceuticals AG
Curated by ChEMBL
| Assay Description
Displacement of [125I]CCK-8s from human recombinant CCK1 receptor after 60 mins by scintillation counting analysis |
Bioorg Med Chem Lett 28: 1446-1455 (2018)
Article DOI: 10.1016/j.bmcl.2018.03.093
BindingDB Entry DOI: 10.7270/Q27W6FPC |
More data for this
Ligand-Target Pair | |
Neuropeptide Y receptor type 1
(Homo sapiens (Human)) | BDBM392076

(US10301272, Example 7/9)Show SMILES CC(C)(C)NS(=O)(=O)c1ccc(-c2sc(nc2CC2CCCCC2)C(=O)N[C@H]2C[C@@H](C2)C(O)=O)c2ccccc12 |r,wU:27.28,wD:29.33,(8.76,1.15,;7.67,.06,;6.19,.46,;7.28,1.55,;8.07,-1.42,;6.98,-2.51,;6.59,-4,;8.07,-3.6,;5.5,-2.11,;5.1,-.63,;3.61,-.23,;2.52,-1.32,;1.03,-.92,;.56,.55,;-.98,.55,;-1.46,-.92,;-.21,-1.82,;-.21,-3.36,;-1.55,-4.13,;-2.88,-3.36,;-4.21,-4.13,;-4.21,-5.67,;-2.88,-6.44,;-1.55,-5.67,;-2.07,1.64,;-1.67,3.12,;-3.56,1.24,;-4.65,2.33,;-6.19,2.33,;-6.19,3.87,;-4.65,3.87,;-7.28,4.96,;-6.88,6.44,;-8.76,4.56,;2.92,-2.8,;1.83,-3.89,;2.23,-5.38,;3.72,-5.78,;4.81,-4.69,;4.41,-3.2,)| Show InChI InChI=1S/C30H37N3O5S2/c1-30(2,3)33-40(37,38)25-14-13-23(21-11-7-8-12-22(21)25)26-24(15-18-9-5-4-6-10-18)32-28(39-26)27(34)31-20-16-19(17-20)29(35)36/h7-8,11-14,18-20,33H,4-6,9-10,15-17H2,1-3H3,(H,31,34)(H,35,36)/t19-,20- | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.130 | n/a | n/a | n/a | n/a | n/a | n/a |
Phenex Pharmaceuticals AG
Curated by ChEMBL
| Assay Description
Displacement of [125I]peptide YY from human Y1 receptor after 120 mins by scintillation counting analysis |
Bioorg Med Chem Lett 28: 1446-1455 (2018)
Article DOI: 10.1016/j.bmcl.2018.03.093
BindingDB Entry DOI: 10.7270/Q27W6FPC |
More data for this
Ligand-Target Pair | |
Melatonin receptor type 1A
(Homo sapiens (Human)) | BDBM392076

(US10301272, Example 7/9)Show SMILES CC(C)(C)NS(=O)(=O)c1ccc(-c2sc(nc2CC2CCCCC2)C(=O)N[C@H]2C[C@@H](C2)C(O)=O)c2ccccc12 |r,wU:27.28,wD:29.33,(8.76,1.15,;7.67,.06,;6.19,.46,;7.28,1.55,;8.07,-1.42,;6.98,-2.51,;6.59,-4,;8.07,-3.6,;5.5,-2.11,;5.1,-.63,;3.61,-.23,;2.52,-1.32,;1.03,-.92,;.56,.55,;-.98,.55,;-1.46,-.92,;-.21,-1.82,;-.21,-3.36,;-1.55,-4.13,;-2.88,-3.36,;-4.21,-4.13,;-4.21,-5.67,;-2.88,-6.44,;-1.55,-5.67,;-2.07,1.64,;-1.67,3.12,;-3.56,1.24,;-4.65,2.33,;-6.19,2.33,;-6.19,3.87,;-4.65,3.87,;-7.28,4.96,;-6.88,6.44,;-8.76,4.56,;2.92,-2.8,;1.83,-3.89,;2.23,-5.38,;3.72,-5.78,;4.81,-4.69,;4.41,-3.2,)| Show InChI InChI=1S/C30H37N3O5S2/c1-30(2,3)33-40(37,38)25-14-13-23(21-11-7-8-12-22(21)25)26-24(15-18-9-5-4-6-10-18)32-28(39-26)27(34)31-20-16-19(17-20)29(35)36/h7-8,11-14,18-20,33H,4-6,9-10,15-17H2,1-3H3,(H,31,34)(H,35,36)/t19-,20- | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.170 | n/a | n/a | n/a | n/a | n/a | n/a |
Phenex Pharmaceuticals AG
Curated by ChEMBL
| Assay Description
Displacement of [125I]NDP-alpha -MSH from human recombinant MT1 receptor after 240 mins by scintillation counting analysis |
Bioorg Med Chem Lett 28: 1446-1455 (2018)
Article DOI: 10.1016/j.bmcl.2018.03.093
BindingDB Entry DOI: 10.7270/Q27W6FPC |
More data for this
Ligand-Target Pair | |
Adenosine receptor A3
(Homo sapiens (Human)) | BDBM392076

(US10301272, Example 7/9)Show SMILES CC(C)(C)NS(=O)(=O)c1ccc(-c2sc(nc2CC2CCCCC2)C(=O)N[C@H]2C[C@@H](C2)C(O)=O)c2ccccc12 |r,wU:27.28,wD:29.33,(8.76,1.15,;7.67,.06,;6.19,.46,;7.28,1.55,;8.07,-1.42,;6.98,-2.51,;6.59,-4,;8.07,-3.6,;5.5,-2.11,;5.1,-.63,;3.61,-.23,;2.52,-1.32,;1.03,-.92,;.56,.55,;-.98,.55,;-1.46,-.92,;-.21,-1.82,;-.21,-3.36,;-1.55,-4.13,;-2.88,-3.36,;-4.21,-4.13,;-4.21,-5.67,;-2.88,-6.44,;-1.55,-5.67,;-2.07,1.64,;-1.67,3.12,;-3.56,1.24,;-4.65,2.33,;-6.19,2.33,;-6.19,3.87,;-4.65,3.87,;-7.28,4.96,;-6.88,6.44,;-8.76,4.56,;2.92,-2.8,;1.83,-3.89,;2.23,-5.38,;3.72,-5.78,;4.81,-4.69,;4.41,-3.2,)| Show InChI InChI=1S/C30H37N3O5S2/c1-30(2,3)33-40(37,38)25-14-13-23(21-11-7-8-12-22(21)25)26-24(15-18-9-5-4-6-10-18)32-28(39-26)27(34)31-20-16-19(17-20)29(35)36/h7-8,11-14,18-20,33H,4-6,9-10,15-17H2,1-3H3,(H,31,34)(H,35,36)/t19-,20- | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.170 | n/a | n/a | n/a | n/a | n/a | n/a |
Phenex Pharmaceuticals AG
Curated by ChEMBL
| Assay Description
Displacement of [125I]AB-MECA from human recombinant adenosine A3 receptor after 120 mins by scintillation counting analysis |
Bioorg Med Chem Lett 28: 1446-1455 (2018)
Article DOI: 10.1016/j.bmcl.2018.03.093
BindingDB Entry DOI: 10.7270/Q27W6FPC |
More data for this
Ligand-Target Pair | |
Galanin receptor type 2
(Homo sapiens (Human)) | BDBM392076

(US10301272, Example 7/9)Show SMILES CC(C)(C)NS(=O)(=O)c1ccc(-c2sc(nc2CC2CCCCC2)C(=O)N[C@H]2C[C@@H](C2)C(O)=O)c2ccccc12 |r,wU:27.28,wD:29.33,(8.76,1.15,;7.67,.06,;6.19,.46,;7.28,1.55,;8.07,-1.42,;6.98,-2.51,;6.59,-4,;8.07,-3.6,;5.5,-2.11,;5.1,-.63,;3.61,-.23,;2.52,-1.32,;1.03,-.92,;.56,.55,;-.98,.55,;-1.46,-.92,;-.21,-1.82,;-.21,-3.36,;-1.55,-4.13,;-2.88,-3.36,;-4.21,-4.13,;-4.21,-5.67,;-2.88,-6.44,;-1.55,-5.67,;-2.07,1.64,;-1.67,3.12,;-3.56,1.24,;-4.65,2.33,;-6.19,2.33,;-6.19,3.87,;-4.65,3.87,;-7.28,4.96,;-6.88,6.44,;-8.76,4.56,;2.92,-2.8,;1.83,-3.89,;2.23,-5.38,;3.72,-5.78,;4.81,-4.69,;4.41,-3.2,)| Show InChI InChI=1S/C30H37N3O5S2/c1-30(2,3)33-40(37,38)25-14-13-23(21-11-7-8-12-22(21)25)26-24(15-18-9-5-4-6-10-18)32-28(39-26)27(34)31-20-16-19(17-20)29(35)36/h7-8,11-14,18-20,33H,4-6,9-10,15-17H2,1-3H3,(H,31,34)(H,35,36)/t19-,20- | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.190 | n/a | n/a | n/a | n/a | n/a | n/a |
Phenex Pharmaceuticals AG
Curated by ChEMBL
| Assay Description
Displacement of [125I]-endothelin-1 from human recombinant GAL2 receptor after 120 mins by scintillation counting analysis |
Bioorg Med Chem Lett 28: 1446-1455 (2018)
Article DOI: 10.1016/j.bmcl.2018.03.093
BindingDB Entry DOI: 10.7270/Q27W6FPC |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM255355
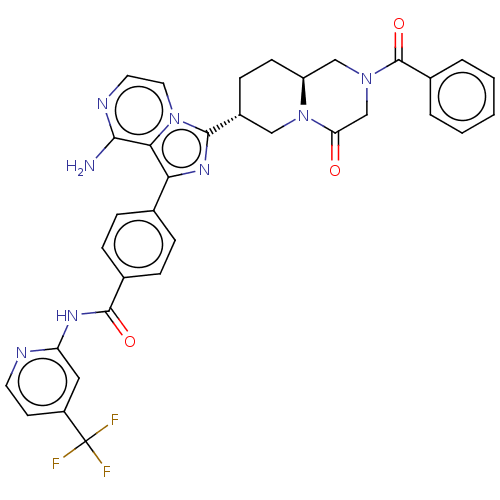
(US9481682, 105)Show SMILES Nc1nccn2c(nc(-c3ccc(cc3)C(=O)Nc3cc(ccn3)C(F)(F)F)c12)[C@@H]1CC[C@H]2CN(CC(=O)N2C1)C(=O)c1ccccc1 |r| Show InChI InChI=1S/C34H29F3N8O3/c35-34(36,37)24-12-13-39-26(16-24)41-32(47)21-8-6-20(7-9-21)28-29-30(38)40-14-15-44(29)31(42-28)23-10-11-25-18-43(19-27(46)45(25)17-23)33(48)22-4-2-1-3-5-22/h1-9,12-16,23,25H,10-11,17-19H2,(H2,38,40)(H,39,41,47)/t23-,25+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00030
BindingDB Entry DOI: 10.7270/Q2FX7FHQ |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM255335

(US9481682, 85)Show SMILES COc1cc(ccc1-c1nc([C@@H]2CC[C@H]3CN(CC(=O)N3C2)C(=O)c2ccncc2)n2ccnc(N)c12)C(=O)Nc1cc(ccn1)C(F)(F)F |r| Show InChI InChI=1S/C34H30F3N9O4/c1-50-25-14-20(32(48)42-26-15-22(8-11-40-26)34(35,36)37)3-5-24(25)28-29-30(38)41-12-13-45(29)31(43-28)21-2-4-23-17-44(18-27(47)46(23)16-21)33(49)19-6-9-39-10-7-19/h3,5-15,21,23H,2,4,16-18H2,1H3,(H2,38,41)(H,40,42,48)/t21-,23+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00030
BindingDB Entry DOI: 10.7270/Q2FX7FHQ |
More data for this
Ligand-Target Pair | |
Melanocortin receptor 4
(Homo sapiens (Human)) | BDBM392076

(US10301272, Example 7/9)Show SMILES CC(C)(C)NS(=O)(=O)c1ccc(-c2sc(nc2CC2CCCCC2)C(=O)N[C@H]2C[C@@H](C2)C(O)=O)c2ccccc12 |r,wU:27.28,wD:29.33,(8.76,1.15,;7.67,.06,;6.19,.46,;7.28,1.55,;8.07,-1.42,;6.98,-2.51,;6.59,-4,;8.07,-3.6,;5.5,-2.11,;5.1,-.63,;3.61,-.23,;2.52,-1.32,;1.03,-.92,;.56,.55,;-.98,.55,;-1.46,-.92,;-.21,-1.82,;-.21,-3.36,;-1.55,-4.13,;-2.88,-3.36,;-4.21,-4.13,;-4.21,-5.67,;-2.88,-6.44,;-1.55,-5.67,;-2.07,1.64,;-1.67,3.12,;-3.56,1.24,;-4.65,2.33,;-6.19,2.33,;-6.19,3.87,;-4.65,3.87,;-7.28,4.96,;-6.88,6.44,;-8.76,4.56,;2.92,-2.8,;1.83,-3.89,;2.23,-5.38,;3.72,-5.78,;4.81,-4.69,;4.41,-3.2,)| Show InChI InChI=1S/C30H37N3O5S2/c1-30(2,3)33-40(37,38)25-14-13-23(21-11-7-8-12-22(21)25)26-24(15-18-9-5-4-6-10-18)32-28(39-26)27(34)31-20-16-19(17-20)29(35)36/h7-8,11-14,18-20,33H,4-6,9-10,15-17H2,1-3H3,(H,31,34)(H,35,36)/t19-,20- | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.210 | n/a | n/a | n/a | n/a | n/a | n/a |
Phenex Pharmaceuticals AG
Curated by ChEMBL
| Assay Description
Displacement of [125I]NDP-alpha -MSH from human recombinant MC4 receptor after 120 mins by scintillation counting analysis |
Bioorg Med Chem Lett 28: 1446-1455 (2018)
Article DOI: 10.1016/j.bmcl.2018.03.093
BindingDB Entry DOI: 10.7270/Q27W6FPC |
More data for this
Ligand-Target Pair | |
D(1A) dopamine receptor
(Homo sapiens (Human)) | BDBM392076

(US10301272, Example 7/9)Show SMILES CC(C)(C)NS(=O)(=O)c1ccc(-c2sc(nc2CC2CCCCC2)C(=O)N[C@H]2C[C@@H](C2)C(O)=O)c2ccccc12 |r,wU:27.28,wD:29.33,(8.76,1.15,;7.67,.06,;6.19,.46,;7.28,1.55,;8.07,-1.42,;6.98,-2.51,;6.59,-4,;8.07,-3.6,;5.5,-2.11,;5.1,-.63,;3.61,-.23,;2.52,-1.32,;1.03,-.92,;.56,.55,;-.98,.55,;-1.46,-.92,;-.21,-1.82,;-.21,-3.36,;-1.55,-4.13,;-2.88,-3.36,;-4.21,-4.13,;-4.21,-5.67,;-2.88,-6.44,;-1.55,-5.67,;-2.07,1.64,;-1.67,3.12,;-3.56,1.24,;-4.65,2.33,;-6.19,2.33,;-6.19,3.87,;-4.65,3.87,;-7.28,4.96,;-6.88,6.44,;-8.76,4.56,;2.92,-2.8,;1.83,-3.89,;2.23,-5.38,;3.72,-5.78,;4.81,-4.69,;4.41,-3.2,)| Show InChI InChI=1S/C30H37N3O5S2/c1-30(2,3)33-40(37,38)25-14-13-23(21-11-7-8-12-22(21)25)26-24(15-18-9-5-4-6-10-18)32-28(39-26)27(34)31-20-16-19(17-20)29(35)36/h7-8,11-14,18-20,33H,4-6,9-10,15-17H2,1-3H3,(H,31,34)(H,35,36)/t19-,20- | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.210 | n/a | n/a | n/a | n/a | n/a | n/a |
Phenex Pharmaceuticals AG
Curated by ChEMBL
| Assay Description
Displacement of [3H]-SCH 23390 from human recombinant dopamine D1 receptor after 60 mins by scintillation counting analysis |
Bioorg Med Chem Lett 28: 1446-1455 (2018)
Article DOI: 10.1016/j.bmcl.2018.03.093
BindingDB Entry DOI: 10.7270/Q27W6FPC |
More data for this
Ligand-Target Pair | |
C-X-C chemokine receptor type 2
(Homo sapiens (Human)) | BDBM392076

(US10301272, Example 7/9)Show SMILES CC(C)(C)NS(=O)(=O)c1ccc(-c2sc(nc2CC2CCCCC2)C(=O)N[C@H]2C[C@@H](C2)C(O)=O)c2ccccc12 |r,wU:27.28,wD:29.33,(8.76,1.15,;7.67,.06,;6.19,.46,;7.28,1.55,;8.07,-1.42,;6.98,-2.51,;6.59,-4,;8.07,-3.6,;5.5,-2.11,;5.1,-.63,;3.61,-.23,;2.52,-1.32,;1.03,-.92,;.56,.55,;-.98,.55,;-1.46,-.92,;-.21,-1.82,;-.21,-3.36,;-1.55,-4.13,;-2.88,-3.36,;-4.21,-4.13,;-4.21,-5.67,;-2.88,-6.44,;-1.55,-5.67,;-2.07,1.64,;-1.67,3.12,;-3.56,1.24,;-4.65,2.33,;-6.19,2.33,;-6.19,3.87,;-4.65,3.87,;-7.28,4.96,;-6.88,6.44,;-8.76,4.56,;2.92,-2.8,;1.83,-3.89,;2.23,-5.38,;3.72,-5.78,;4.81,-4.69,;4.41,-3.2,)| Show InChI InChI=1S/C30H37N3O5S2/c1-30(2,3)33-40(37,38)25-14-13-23(21-11-7-8-12-22(21)25)26-24(15-18-9-5-4-6-10-18)32-28(39-26)27(34)31-20-16-19(17-20)29(35)36/h7-8,11-14,18-20,33H,4-6,9-10,15-17H2,1-3H3,(H,31,34)(H,35,36)/t19-,20- | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.240 | n/a | n/a | n/a | n/a | n/a | n/a |
Phenex Pharmaceuticals AG
Curated by ChEMBL
| Assay Description
Displacement of [125I]IL-8 from human recombinant CXCR2 receptor after 60 mins by scintillation counting analysis |
Bioorg Med Chem Lett 28: 1446-1455 (2018)
Article DOI: 10.1016/j.bmcl.2018.03.093
BindingDB Entry DOI: 10.7270/Q27W6FPC |
More data for this
Ligand-Target Pair | |
Alpha-1A/Alpha-1B/Alpha-1D adrenergic receptor
(Rattus norvegicus (rat)-Rattus norvegicus (Rat)) | BDBM392076

(US10301272, Example 7/9)Show SMILES CC(C)(C)NS(=O)(=O)c1ccc(-c2sc(nc2CC2CCCCC2)C(=O)N[C@H]2C[C@@H](C2)C(O)=O)c2ccccc12 |r,wU:27.28,wD:29.33,(8.76,1.15,;7.67,.06,;6.19,.46,;7.28,1.55,;8.07,-1.42,;6.98,-2.51,;6.59,-4,;8.07,-3.6,;5.5,-2.11,;5.1,-.63,;3.61,-.23,;2.52,-1.32,;1.03,-.92,;.56,.55,;-.98,.55,;-1.46,-.92,;-.21,-1.82,;-.21,-3.36,;-1.55,-4.13,;-2.88,-3.36,;-4.21,-4.13,;-4.21,-5.67,;-2.88,-6.44,;-1.55,-5.67,;-2.07,1.64,;-1.67,3.12,;-3.56,1.24,;-4.65,2.33,;-6.19,2.33,;-6.19,3.87,;-4.65,3.87,;-7.28,4.96,;-6.88,6.44,;-8.76,4.56,;2.92,-2.8,;1.83,-3.89,;2.23,-5.38,;3.72,-5.78,;4.81,-4.69,;4.41,-3.2,)| Show InChI InChI=1S/C30H37N3O5S2/c1-30(2,3)33-40(37,38)25-14-13-23(21-11-7-8-12-22(21)25)26-24(15-18-9-5-4-6-10-18)32-28(39-26)27(34)31-20-16-19(17-20)29(35)36/h7-8,11-14,18-20,33H,4-6,9-10,15-17H2,1-3H3,(H,31,34)(H,35,36)/t19-,20- | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.25 | n/a | n/a | n/a | n/a | n/a | n/a |
Phenex Pharmaceuticals AG
Curated by ChEMBL
| Assay Description
Displacement of [3H]-prazosin from rat alpha1 adrenoceptor after 60 mins by scintillation counting analysis |
Bioorg Med Chem Lett 28: 1446-1455 (2018)
Article DOI: 10.1016/j.bmcl.2018.03.093
BindingDB Entry DOI: 10.7270/Q27W6FPC |
More data for this
Ligand-Target Pair | |
Neurotensin receptor type 1
(Homo sapiens (Human)) | BDBM392076

(US10301272, Example 7/9)Show SMILES CC(C)(C)NS(=O)(=O)c1ccc(-c2sc(nc2CC2CCCCC2)C(=O)N[C@H]2C[C@@H](C2)C(O)=O)c2ccccc12 |r,wU:27.28,wD:29.33,(8.76,1.15,;7.67,.06,;6.19,.46,;7.28,1.55,;8.07,-1.42,;6.98,-2.51,;6.59,-4,;8.07,-3.6,;5.5,-2.11,;5.1,-.63,;3.61,-.23,;2.52,-1.32,;1.03,-.92,;.56,.55,;-.98,.55,;-1.46,-.92,;-.21,-1.82,;-.21,-3.36,;-1.55,-4.13,;-2.88,-3.36,;-4.21,-4.13,;-4.21,-5.67,;-2.88,-6.44,;-1.55,-5.67,;-2.07,1.64,;-1.67,3.12,;-3.56,1.24,;-4.65,2.33,;-6.19,2.33,;-6.19,3.87,;-4.65,3.87,;-7.28,4.96,;-6.88,6.44,;-8.76,4.56,;2.92,-2.8,;1.83,-3.89,;2.23,-5.38,;3.72,-5.78,;4.81,-4.69,;4.41,-3.2,)| Show InChI InChI=1S/C30H37N3O5S2/c1-30(2,3)33-40(37,38)25-14-13-23(21-11-7-8-12-22(21)25)26-24(15-18-9-5-4-6-10-18)32-28(39-26)27(34)31-20-16-19(17-20)29(35)36/h7-8,11-14,18-20,33H,4-6,9-10,15-17H2,1-3H3,(H,31,34)(H,35,36)/t19-,20- | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.25 | n/a | n/a | n/a | n/a | n/a | n/a |
Phenex Pharmaceuticals AG
Curated by ChEMBL
| Assay Description
Displacement of [125I]Tyr3-neurotensin from human recombinant NTS1 receptor after 120 mins by scintillation counting analysis |
Bioorg Med Chem Lett 28: 1446-1455 (2018)
Article DOI: 10.1016/j.bmcl.2018.03.093
BindingDB Entry DOI: 10.7270/Q27W6FPC |
More data for this
Ligand-Target Pair | |
Neuropeptide Y receptor type 2
(Homo sapiens (Human)) | BDBM392076

(US10301272, Example 7/9)Show SMILES CC(C)(C)NS(=O)(=O)c1ccc(-c2sc(nc2CC2CCCCC2)C(=O)N[C@H]2C[C@@H](C2)C(O)=O)c2ccccc12 |r,wU:27.28,wD:29.33,(8.76,1.15,;7.67,.06,;6.19,.46,;7.28,1.55,;8.07,-1.42,;6.98,-2.51,;6.59,-4,;8.07,-3.6,;5.5,-2.11,;5.1,-.63,;3.61,-.23,;2.52,-1.32,;1.03,-.92,;.56,.55,;-.98,.55,;-1.46,-.92,;-.21,-1.82,;-.21,-3.36,;-1.55,-4.13,;-2.88,-3.36,;-4.21,-4.13,;-4.21,-5.67,;-2.88,-6.44,;-1.55,-5.67,;-2.07,1.64,;-1.67,3.12,;-3.56,1.24,;-4.65,2.33,;-6.19,2.33,;-6.19,3.87,;-4.65,3.87,;-7.28,4.96,;-6.88,6.44,;-8.76,4.56,;2.92,-2.8,;1.83,-3.89,;2.23,-5.38,;3.72,-5.78,;4.81,-4.69,;4.41,-3.2,)| Show InChI InChI=1S/C30H37N3O5S2/c1-30(2,3)33-40(37,38)25-14-13-23(21-11-7-8-12-22(21)25)26-24(15-18-9-5-4-6-10-18)32-28(39-26)27(34)31-20-16-19(17-20)29(35)36/h7-8,11-14,18-20,33H,4-6,9-10,15-17H2,1-3H3,(H,31,34)(H,35,36)/t19-,20- | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.280 | n/a | n/a | n/a | n/a | n/a | n/a |
Phenex Pharmaceuticals AG
Curated by ChEMBL
| Assay Description
Displacement of [125I]peptide YY from human Y2 receptor after 120 mins by scintillation counting analysis |
Bioorg Med Chem Lett 28: 1446-1455 (2018)
Article DOI: 10.1016/j.bmcl.2018.03.093
BindingDB Entry DOI: 10.7270/Q27W6FPC |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM50594234

(CHEMBL5200336)Show SMILES Cc1cc(C(N)=O)c2[nH]c3cc(ccc3c2c1-c1cccc(c1O)-n1c(=O)[nH]c2c(F)cccc2c1=O)C(C)(C)O |(3.12,1.54,;1.79,2.31,;1.79,3.85,;.47,4.61,;.47,6.15,;1.8,6.92,;-.87,6.92,;-.86,3.85,;-2.33,4.32,;-3.23,3.08,;-4.77,2.91,;-5.39,1.5,;-4.49,.27,;-2.95,.43,;-2.33,1.83,;-.86,2.31,;.47,1.55,;.47,.01,;-.87,-.77,;-.86,-2.31,;.47,-3.07,;1.8,-2.3,;1.8,-.76,;3.14,.01,;3.13,-3.07,;3.13,-4.61,;1.8,-5.38,;4.47,-5.38,;5.8,-4.61,;7.14,-5.38,;7.14,-6.92,;8.47,-4.6,;8.47,-3.07,;7.13,-2.3,;5.8,-3.07,;4.47,-2.3,;4.47,-.76,;-6.93,1.5,;-7.7,.17,;-7.7,2.84,;-8.47,1.5,)| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00030
BindingDB Entry DOI: 10.7270/Q2FX7FHQ |
More data for this
Ligand-Target Pair | |
Leukotriene A-4 hydrolase
(Homo sapiens (Human)) | BDBM50402386
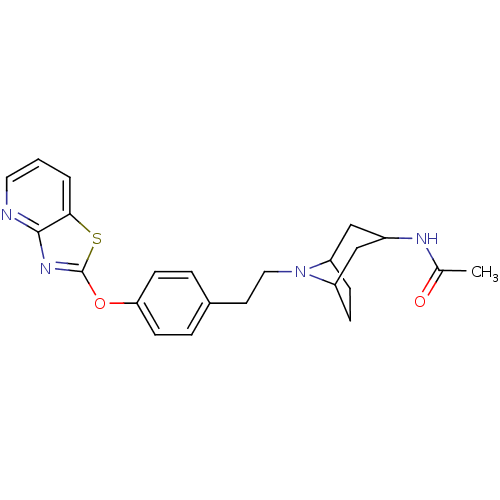
(CHEMBL2207747)Show SMILES CC(=O)NC1CC2CCC(C1)N2CCc1ccc(Oc2nc3ncccc3s2)cc1 |TLB:3:4:11:7.8,THB:12:11:4.5.10:7.8| Show InChI InChI=1S/C23H26N4O2S/c1-15(28)25-17-13-18-6-7-19(14-17)27(18)12-10-16-4-8-20(9-5-16)29-23-26-22-21(30-23)3-2-11-24-22/h2-5,8-9,11,17-19H,6-7,10,12-14H2,1H3,(H,25,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Research& Development LLC
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant LTA4 hydrolase expressed in SF9 cells using LTA4 as substrate assessed as LTB4 production incubated for 10 mins prior... |
Bioorg Med Chem Lett 22: 7504-11 (2012)
Article DOI: 10.1016/j.bmcl.2012.10.036
BindingDB Entry DOI: 10.7270/Q2ZG6TDQ |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 1/2/3/4/5
(Mus musculus-MOUSE) | BDBM392076

(US10301272, Example 7/9)Show SMILES CC(C)(C)NS(=O)(=O)c1ccc(-c2sc(nc2CC2CCCCC2)C(=O)N[C@H]2C[C@@H](C2)C(O)=O)c2ccccc12 |r,wU:27.28,wD:29.33,(8.76,1.15,;7.67,.06,;6.19,.46,;7.28,1.55,;8.07,-1.42,;6.98,-2.51,;6.59,-4,;8.07,-3.6,;5.5,-2.11,;5.1,-.63,;3.61,-.23,;2.52,-1.32,;1.03,-.92,;.56,.55,;-.98,.55,;-1.46,-.92,;-.21,-1.82,;-.21,-3.36,;-1.55,-4.13,;-2.88,-3.36,;-4.21,-4.13,;-4.21,-5.67,;-2.88,-6.44,;-1.55,-5.67,;-2.07,1.64,;-1.67,3.12,;-3.56,1.24,;-4.65,2.33,;-6.19,2.33,;-6.19,3.87,;-4.65,3.87,;-7.28,4.96,;-6.88,6.44,;-8.76,4.56,;2.92,-2.8,;1.83,-3.89,;2.23,-5.38,;3.72,-5.78,;4.81,-4.69,;4.41,-3.2,)| Show InChI InChI=1S/C30H37N3O5S2/c1-30(2,3)33-40(37,38)25-14-13-23(21-11-7-8-12-22(21)25)26-24(15-18-9-5-4-6-10-18)32-28(39-26)27(34)31-20-16-19(17-20)29(35)36/h7-8,11-14,18-20,33H,4-6,9-10,15-17H2,1-3H3,(H,31,34)(H,35,36)/t19-,20- | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.310 | n/a | n/a | n/a | n/a | n/a | n/a |
Phenex Pharmaceuticals AG
Curated by ChEMBL
| Assay Description
Displacement of [125I]Tyr11-somatostatin-14 from mouse SST receptor after 60 mins by scintillation counting analysis |
Bioorg Med Chem Lett 28: 1446-1455 (2018)
Article DOI: 10.1016/j.bmcl.2018.03.093
BindingDB Entry DOI: 10.7270/Q27W6FPC |
More data for this
Ligand-Target Pair | |
Nuclear receptor ROR-gamma
(Homo sapiens (Human)) | BDBM392066

(US10301272, Example 6/64)Show SMILES CC(C)(C)NS(=O)(=O)c1ccc(-c2sc(nc2CC2CCCCC2)C(=O)NCC(C)(C)C(N)=O)c2ccccc12 Show InChI InChI=1S/C30H40N4O4S2/c1-29(2,3)34-40(37,38)24-16-15-22(20-13-9-10-14-21(20)24)25-23(17-19-11-7-6-8-12-19)33-27(39-25)26(35)32-18-30(4,5)28(31)36/h9-10,13-16,19,34H,6-8,11-12,17-18H2,1-5H3,(H2,31,36)(H,32,35) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.370 | n/a | n/a | n/a | n/a | n/a | n/a |
Phenex Pharmaceuticals AG
Curated by ChEMBL
| Assay Description
Inverse agonist activity at human GAL4 DBD-fused RORgammat LBD (237 to 497 residues) expressed in HEK293T cells assessed as reduction in ROR mediated... |
Bioorg Med Chem Lett 28: 1446-1455 (2018)
Article DOI: 10.1016/j.bmcl.2018.03.093
BindingDB Entry DOI: 10.7270/Q27W6FPC |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 7
(Homo sapiens (Human)) | BDBM392076

(US10301272, Example 7/9)Show SMILES CC(C)(C)NS(=O)(=O)c1ccc(-c2sc(nc2CC2CCCCC2)C(=O)N[C@H]2C[C@@H](C2)C(O)=O)c2ccccc12 |r,wU:27.28,wD:29.33,(8.76,1.15,;7.67,.06,;6.19,.46,;7.28,1.55,;8.07,-1.42,;6.98,-2.51,;6.59,-4,;8.07,-3.6,;5.5,-2.11,;5.1,-.63,;3.61,-.23,;2.52,-1.32,;1.03,-.92,;.56,.55,;-.98,.55,;-1.46,-.92,;-.21,-1.82,;-.21,-3.36,;-1.55,-4.13,;-2.88,-3.36,;-4.21,-4.13,;-4.21,-5.67,;-2.88,-6.44,;-1.55,-5.67,;-2.07,1.64,;-1.67,3.12,;-3.56,1.24,;-4.65,2.33,;-6.19,2.33,;-6.19,3.87,;-4.65,3.87,;-7.28,4.96,;-6.88,6.44,;-8.76,4.56,;2.92,-2.8,;1.83,-3.89,;2.23,-5.38,;3.72,-5.78,;4.81,-4.69,;4.41,-3.2,)| Show InChI InChI=1S/C30H37N3O5S2/c1-30(2,3)33-40(37,38)25-14-13-23(21-11-7-8-12-22(21)25)26-24(15-18-9-5-4-6-10-18)32-28(39-26)27(34)31-20-16-19(17-20)29(35)36/h7-8,11-14,18-20,33H,4-6,9-10,15-17H2,1-3H3,(H,31,34)(H,35,36)/t19-,20- | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.390 | n/a | n/a | n/a | n/a | n/a | n/a |
Phenex Pharmaceuticals AG
Curated by ChEMBL
| Assay Description
Displacement of [3H]LSD from human recombinant 5-HT7 receptor after 120 mins by scintillation counting analysis |
Bioorg Med Chem Lett 28: 1446-1455 (2018)
Article DOI: 10.1016/j.bmcl.2018.03.093
BindingDB Entry DOI: 10.7270/Q27W6FPC |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM50594232

(CHEMBL5171706)Show SMILES OC[C@H]1CCC[C@H](Nc2ncnc3[nH]cc(C(=O)c4ccc(Oc5ccccc5)cc4Cl)c23)O1 |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.390 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00030
BindingDB Entry DOI: 10.7270/Q2FX7FHQ |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1A
(Homo sapiens (Human)) | BDBM392076

(US10301272, Example 7/9)Show SMILES CC(C)(C)NS(=O)(=O)c1ccc(-c2sc(nc2CC2CCCCC2)C(=O)N[C@H]2C[C@@H](C2)C(O)=O)c2ccccc12 |r,wU:27.28,wD:29.33,(8.76,1.15,;7.67,.06,;6.19,.46,;7.28,1.55,;8.07,-1.42,;6.98,-2.51,;6.59,-4,;8.07,-3.6,;5.5,-2.11,;5.1,-.63,;3.61,-.23,;2.52,-1.32,;1.03,-.92,;.56,.55,;-.98,.55,;-1.46,-.92,;-.21,-1.82,;-.21,-3.36,;-1.55,-4.13,;-2.88,-3.36,;-4.21,-4.13,;-4.21,-5.67,;-2.88,-6.44,;-1.55,-5.67,;-2.07,1.64,;-1.67,3.12,;-3.56,1.24,;-4.65,2.33,;-6.19,2.33,;-6.19,3.87,;-4.65,3.87,;-7.28,4.96,;-6.88,6.44,;-8.76,4.56,;2.92,-2.8,;1.83,-3.89,;2.23,-5.38,;3.72,-5.78,;4.81,-4.69,;4.41,-3.2,)| Show InChI InChI=1S/C30H37N3O5S2/c1-30(2,3)33-40(37,38)25-14-13-23(21-11-7-8-12-22(21)25)26-24(15-18-9-5-4-6-10-18)32-28(39-26)27(34)31-20-16-19(17-20)29(35)36/h7-8,11-14,18-20,33H,4-6,9-10,15-17H2,1-3H3,(H,31,34)(H,35,36)/t19-,20- | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.430 | n/a | n/a | n/a | n/a | n/a | n/a |
Phenex Pharmaceuticals AG
Curated by ChEMBL
| Assay Description
Displacement of [3H]8-OH-DPAT from recombinant human 5-HT1A receptor after 60 mins by scintillation counting analysis |
Bioorg Med Chem Lett 28: 1446-1455 (2018)
Article DOI: 10.1016/j.bmcl.2018.03.093
BindingDB Entry DOI: 10.7270/Q27W6FPC |
More data for this
Ligand-Target Pair | |
Vasoactive intestinal polypeptide receptor 1
(Homo sapiens (Human)) | BDBM392076

(US10301272, Example 7/9)Show SMILES CC(C)(C)NS(=O)(=O)c1ccc(-c2sc(nc2CC2CCCCC2)C(=O)N[C@H]2C[C@@H](C2)C(O)=O)c2ccccc12 |r,wU:27.28,wD:29.33,(8.76,1.15,;7.67,.06,;6.19,.46,;7.28,1.55,;8.07,-1.42,;6.98,-2.51,;6.59,-4,;8.07,-3.6,;5.5,-2.11,;5.1,-.63,;3.61,-.23,;2.52,-1.32,;1.03,-.92,;.56,.55,;-.98,.55,;-1.46,-.92,;-.21,-1.82,;-.21,-3.36,;-1.55,-4.13,;-2.88,-3.36,;-4.21,-4.13,;-4.21,-5.67,;-2.88,-6.44,;-1.55,-5.67,;-2.07,1.64,;-1.67,3.12,;-3.56,1.24,;-4.65,2.33,;-6.19,2.33,;-6.19,3.87,;-4.65,3.87,;-7.28,4.96,;-6.88,6.44,;-8.76,4.56,;2.92,-2.8,;1.83,-3.89,;2.23,-5.38,;3.72,-5.78,;4.81,-4.69,;4.41,-3.2,)| Show InChI InChI=1S/C30H37N3O5S2/c1-30(2,3)33-40(37,38)25-14-13-23(21-11-7-8-12-22(21)25)26-24(15-18-9-5-4-6-10-18)32-28(39-26)27(34)31-20-16-19(17-20)29(35)36/h7-8,11-14,18-20,33H,4-6,9-10,15-17H2,1-3H3,(H,31,34)(H,35,36)/t19-,20- | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.470 | n/a | n/a | n/a | n/a | n/a | n/a |
Phenex Pharmaceuticals AG
Curated by ChEMBL
| Assay Description
Displacement of [125I]VIP from human recombinant VPAC1 receptor after 60 mins by scintillation counting analysis |
Bioorg Med Chem Lett 28: 1446-1455 (2018)
Article DOI: 10.1016/j.bmcl.2018.03.093
BindingDB Entry DOI: 10.7270/Q27W6FPC |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor
(Homo sapiens (Human)) | BDBM392076

(US10301272, Example 7/9)Show SMILES CC(C)(C)NS(=O)(=O)c1ccc(-c2sc(nc2CC2CCCCC2)C(=O)N[C@H]2C[C@@H](C2)C(O)=O)c2ccccc12 |r,wU:27.28,wD:29.33,(8.76,1.15,;7.67,.06,;6.19,.46,;7.28,1.55,;8.07,-1.42,;6.98,-2.51,;6.59,-4,;8.07,-3.6,;5.5,-2.11,;5.1,-.63,;3.61,-.23,;2.52,-1.32,;1.03,-.92,;.56,.55,;-.98,.55,;-1.46,-.92,;-.21,-1.82,;-.21,-3.36,;-1.55,-4.13,;-2.88,-3.36,;-4.21,-4.13,;-4.21,-5.67,;-2.88,-6.44,;-1.55,-5.67,;-2.07,1.64,;-1.67,3.12,;-3.56,1.24,;-4.65,2.33,;-6.19,2.33,;-6.19,3.87,;-4.65,3.87,;-7.28,4.96,;-6.88,6.44,;-8.76,4.56,;2.92,-2.8,;1.83,-3.89,;2.23,-5.38,;3.72,-5.78,;4.81,-4.69,;4.41,-3.2,)| Show InChI InChI=1S/C30H37N3O5S2/c1-30(2,3)33-40(37,38)25-14-13-23(21-11-7-8-12-22(21)25)26-24(15-18-9-5-4-6-10-18)32-28(39-26)27(34)31-20-16-19(17-20)29(35)36/h7-8,11-14,18-20,33H,4-6,9-10,15-17H2,1-3H3,(H,31,34)(H,35,36)/t19-,20- | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.470 | n/a | n/a | n/a | n/a | n/a | n/a |
Phenex Pharmaceuticals AG
Curated by ChEMBL
| Assay Description
Displacement of [125I][Sar1,Ile8]-AT-II from human recombinant AT1 receptor after 120 mins by scintillation counting analysis |
Bioorg Med Chem Lett 28: 1446-1455 (2018)
Article DOI: 10.1016/j.bmcl.2018.03.093
BindingDB Entry DOI: 10.7270/Q27W6FPC |
More data for this
Ligand-Target Pair | |
Adenosine receptor A1
(Homo sapiens (Human)) | BDBM392076

(US10301272, Example 7/9)Show SMILES CC(C)(C)NS(=O)(=O)c1ccc(-c2sc(nc2CC2CCCCC2)C(=O)N[C@H]2C[C@@H](C2)C(O)=O)c2ccccc12 |r,wU:27.28,wD:29.33,(8.76,1.15,;7.67,.06,;6.19,.46,;7.28,1.55,;8.07,-1.42,;6.98,-2.51,;6.59,-4,;8.07,-3.6,;5.5,-2.11,;5.1,-.63,;3.61,-.23,;2.52,-1.32,;1.03,-.92,;.56,.55,;-.98,.55,;-1.46,-.92,;-.21,-1.82,;-.21,-3.36,;-1.55,-4.13,;-2.88,-3.36,;-4.21,-4.13,;-4.21,-5.67,;-2.88,-6.44,;-1.55,-5.67,;-2.07,1.64,;-1.67,3.12,;-3.56,1.24,;-4.65,2.33,;-6.19,2.33,;-6.19,3.87,;-4.65,3.87,;-7.28,4.96,;-6.88,6.44,;-8.76,4.56,;2.92,-2.8,;1.83,-3.89,;2.23,-5.38,;3.72,-5.78,;4.81,-4.69,;4.41,-3.2,)| Show InChI InChI=1S/C30H37N3O5S2/c1-30(2,3)33-40(37,38)25-14-13-23(21-11-7-8-12-22(21)25)26-24(15-18-9-5-4-6-10-18)32-28(39-26)27(34)31-20-16-19(17-20)29(35)36/h7-8,11-14,18-20,33H,4-6,9-10,15-17H2,1-3H3,(H,31,34)(H,35,36)/t19-,20- | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.610 | n/a | n/a | n/a | n/a | n/a | n/a |
Phenex Pharmaceuticals AG
Curated by ChEMBL
| Assay Description
Displacement of [3H]-DPCPX from human recombinant adenosine A1 receptor after 60 mins by scintillation counting analysis |
Bioorg Med Chem Lett 28: 1446-1455 (2018)
Article DOI: 10.1016/j.bmcl.2018.03.093
BindingDB Entry DOI: 10.7270/Q27W6FPC |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Homo sapiens (Human)) | BDBM392076

(US10301272, Example 7/9)Show SMILES CC(C)(C)NS(=O)(=O)c1ccc(-c2sc(nc2CC2CCCCC2)C(=O)N[C@H]2C[C@@H](C2)C(O)=O)c2ccccc12 |r,wU:27.28,wD:29.33,(8.76,1.15,;7.67,.06,;6.19,.46,;7.28,1.55,;8.07,-1.42,;6.98,-2.51,;6.59,-4,;8.07,-3.6,;5.5,-2.11,;5.1,-.63,;3.61,-.23,;2.52,-1.32,;1.03,-.92,;.56,.55,;-.98,.55,;-1.46,-.92,;-.21,-1.82,;-.21,-3.36,;-1.55,-4.13,;-2.88,-3.36,;-4.21,-4.13,;-4.21,-5.67,;-2.88,-6.44,;-1.55,-5.67,;-2.07,1.64,;-1.67,3.12,;-3.56,1.24,;-4.65,2.33,;-6.19,2.33,;-6.19,3.87,;-4.65,3.87,;-7.28,4.96,;-6.88,6.44,;-8.76,4.56,;2.92,-2.8,;1.83,-3.89,;2.23,-5.38,;3.72,-5.78,;4.81,-4.69,;4.41,-3.2,)| Show InChI InChI=1S/C30H37N3O5S2/c1-30(2,3)33-40(37,38)25-14-13-23(21-11-7-8-12-22(21)25)26-24(15-18-9-5-4-6-10-18)32-28(39-26)27(34)31-20-16-19(17-20)29(35)36/h7-8,11-14,18-20,33H,4-6,9-10,15-17H2,1-3H3,(H,31,34)(H,35,36)/t19-,20- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.640 | n/a | n/a | n/a | n/a | n/a | n/a |
Phenex Pharmaceuticals AG
Curated by ChEMBL
| Assay Description
Displacement of [3H]ketanserin human recombinant 5-HT2A receptor after 60 mins by scintillation counting analysis |
Bioorg Med Chem Lett 28: 1446-1455 (2018)
Article DOI: 10.1016/j.bmcl.2018.03.093
BindingDB Entry DOI: 10.7270/Q27W6FPC |
More data for this
Ligand-Target Pair | |
Nuclear receptor ROR-gamma
(Homo sapiens (Human)) | BDBM392013
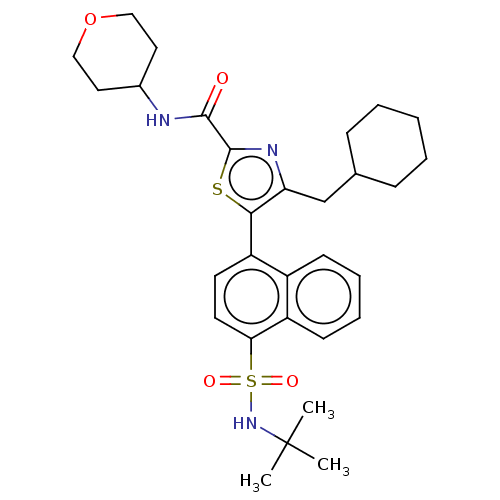
(US10301272, Example 6/11)Show SMILES CC(C)(C)NS(=O)(=O)c1ccc(-c2sc(nc2CC2CCCCC2)C(=O)NC2CCOCC2)c2ccccc12 Show InChI InChI=1S/C30H39N3O4S2/c1-30(2,3)33-39(35,36)26-14-13-24(22-11-7-8-12-23(22)26)27-25(19-20-9-5-4-6-10-20)32-29(38-27)28(34)31-21-15-17-37-18-16-21/h7-8,11-14,20-21,33H,4-6,9-10,15-19H2,1-3H3,(H,31,34) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.690 | n/a | n/a | n/a | n/a | n/a | n/a |
Phenex Pharmaceuticals AG
Curated by ChEMBL
| Assay Description
Inverse agonist activity at human GAL4 DBD-fused RORgammat LBD (237 to 497 residues) expressed in HEK293T cells assessed as reduction in ROR mediated... |
Bioorg Med Chem Lett 28: 1446-1455 (2018)
Article DOI: 10.1016/j.bmcl.2018.03.093
BindingDB Entry DOI: 10.7270/Q27W6FPC |
More data for this
Ligand-Target Pair | |
Nuclear receptor ROR-gamma
(Homo sapiens (Human)) | BDBM392136

(US10301272, Example 14/6)Show SMILES CCC(C)(C)NS(=O)(=O)c1ccc(-c2sc(nc2CC2CCCCC2)C(=O)N[C@H]2C[C@@H](C2)C(O)=O)c2ccccc12 |r,wU:28.29,wD:30.34,(9.88,-3.89,;9.88,-2.35,;8.54,-1.58,;8.54,-3.12,;9.88,-.81,;7.21,-.81,;7.21,.73,;8.75,.73,;7.21,2.27,;5.67,.73,;4.9,-.6,;3.36,-.6,;2.59,.73,;1.05,.73,;.14,1.98,;-1.32,1.5,;-1.32,-.04,;.14,-.52,;.54,-2,;-.55,-3.09,;-2.03,-2.69,;-3.12,-3.78,;-2.72,-5.27,;-1.24,-5.67,;-.15,-4.58,;-2.65,2.27,;-2.65,3.81,;-3.99,1.5,;-5.32,2.27,;-6.81,1.87,;-7.21,3.36,;-5.72,3.76,;-8.54,4.13,;-8.54,5.67,;-9.88,3.36,;3.36,2.06,;2.59,3.4,;3.36,4.73,;4.9,4.73,;5.67,3.4,;4.9,2.06,)| Show InChI InChI=1S/C31H39N3O5S2/c1-4-31(2,3)34-41(38,39)26-15-14-24(22-12-8-9-13-23(22)26)27-25(16-19-10-6-5-7-11-19)33-29(40-27)28(35)32-21-17-20(18-21)30(36)37/h8-9,12-15,19-21,34H,4-7,10-11,16-18H2,1-3H3,(H,32,35)(H,36,37)/t20-,21- | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.710 | n/a | n/a | n/a | n/a | n/a | n/a |
Phenex Pharmaceuticals AG
Curated by ChEMBL
| Assay Description
Inverse agonist activity at human GAL4 DBD-fused RORgammat LBD (237 to 497 residues) expressed in HEK293T cells assessed as reduction in ROR mediated... |
Bioorg Med Chem Lett 28: 1446-1455 (2018)
Article DOI: 10.1016/j.bmcl.2018.03.093
BindingDB Entry DOI: 10.7270/Q27W6FPC |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM50357312

(IBRUTINIB | PCI-32765 | US10124003, Ref. Ex. Compo...)Show SMILES Nc1ncnc2n(nc(-c3ccc(Oc4ccccc4)cc3)c12)[C@@H]1CCCN(C1)C(=O)C=C Show InChI InChI=1S/C25H24N6O2/c1-2-21(32)30-14-6-7-18(15-30)31-25-22(24(26)27-16-28-25)23(29-31)17-10-12-20(13-11-17)33-19-8-4-3-5-9-19/h2-5,8-13,16,18H,1,6-7,14-15H2,(H2,26,27,28)/t18-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| n/a | n/a | 0.720 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00030
BindingDB Entry DOI: 10.7270/Q2FX7FHQ |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Muscarinic acetylcholine receptor M3
(Homo sapiens (Human)) | BDBM392076

(US10301272, Example 7/9)Show SMILES CC(C)(C)NS(=O)(=O)c1ccc(-c2sc(nc2CC2CCCCC2)C(=O)N[C@H]2C[C@@H](C2)C(O)=O)c2ccccc12 |r,wU:27.28,wD:29.33,(8.76,1.15,;7.67,.06,;6.19,.46,;7.28,1.55,;8.07,-1.42,;6.98,-2.51,;6.59,-4,;8.07,-3.6,;5.5,-2.11,;5.1,-.63,;3.61,-.23,;2.52,-1.32,;1.03,-.92,;.56,.55,;-.98,.55,;-1.46,-.92,;-.21,-1.82,;-.21,-3.36,;-1.55,-4.13,;-2.88,-3.36,;-4.21,-4.13,;-4.21,-5.67,;-2.88,-6.44,;-1.55,-5.67,;-2.07,1.64,;-1.67,3.12,;-3.56,1.24,;-4.65,2.33,;-6.19,2.33,;-6.19,3.87,;-4.65,3.87,;-7.28,4.96,;-6.88,6.44,;-8.76,4.56,;2.92,-2.8,;1.83,-3.89,;2.23,-5.38,;3.72,-5.78,;4.81,-4.69,;4.41,-3.2,)| Show InChI InChI=1S/C30H37N3O5S2/c1-30(2,3)33-40(37,38)25-14-13-23(21-11-7-8-12-22(21)25)26-24(15-18-9-5-4-6-10-18)32-28(39-26)27(34)31-20-16-19(17-20)29(35)36/h7-8,11-14,18-20,33H,4-6,9-10,15-17H2,1-3H3,(H,31,34)(H,35,36)/t19-,20- | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.730 | n/a | n/a | n/a | n/a | n/a | n/a |
Phenex Pharmaceuticals AG
Curated by ChEMBL
| Assay Description
Displacement of [3H]4-DAMP from human recombinant M3 receptor after 60 mins by scintillation counting analysis |
Bioorg Med Chem Lett 28: 1446-1455 (2018)
Article DOI: 10.1016/j.bmcl.2018.03.093
BindingDB Entry DOI: 10.7270/Q27W6FPC |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM50594232

(CHEMBL5171706)Show SMILES OC[C@H]1CCC[C@H](Nc2ncnc3[nH]cc(C(=O)c4ccc(Oc5ccccc5)cc4Cl)c23)O1 |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.850 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00030
BindingDB Entry DOI: 10.7270/Q2FX7FHQ |
More data for this
Ligand-Target Pair | |
Nuclear receptor ROR-gamma
(Homo sapiens (Human)) | BDBM392076

(US10301272, Example 7/9)Show SMILES CC(C)(C)NS(=O)(=O)c1ccc(-c2sc(nc2CC2CCCCC2)C(=O)N[C@H]2C[C@@H](C2)C(O)=O)c2ccccc12 |r,wU:27.28,wD:29.33,(8.76,1.15,;7.67,.06,;6.19,.46,;7.28,1.55,;8.07,-1.42,;6.98,-2.51,;6.59,-4,;8.07,-3.6,;5.5,-2.11,;5.1,-.63,;3.61,-.23,;2.52,-1.32,;1.03,-.92,;.56,.55,;-.98,.55,;-1.46,-.92,;-.21,-1.82,;-.21,-3.36,;-1.55,-4.13,;-2.88,-3.36,;-4.21,-4.13,;-4.21,-5.67,;-2.88,-6.44,;-1.55,-5.67,;-2.07,1.64,;-1.67,3.12,;-3.56,1.24,;-4.65,2.33,;-6.19,2.33,;-6.19,3.87,;-4.65,3.87,;-7.28,4.96,;-6.88,6.44,;-8.76,4.56,;2.92,-2.8,;1.83,-3.89,;2.23,-5.38,;3.72,-5.78,;4.81,-4.69,;4.41,-3.2,)| Show InChI InChI=1S/C30H37N3O5S2/c1-30(2,3)33-40(37,38)25-14-13-23(21-11-7-8-12-22(21)25)26-24(15-18-9-5-4-6-10-18)32-28(39-26)27(34)31-20-16-19(17-20)29(35)36/h7-8,11-14,18-20,33H,4-6,9-10,15-17H2,1-3H3,(H,31,34)(H,35,36)/t19-,20- | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.940 | n/a | n/a | n/a | n/a | n/a | n/a |
Phenex Pharmaceuticals AG
Curated by ChEMBL
| Assay Description
Inverse agonist activity at human GAL4 DBD-fused RORgammat LBD (237 to 497 residues) expressed in HEK293T cells assessed as reduction in ROR mediated... |
Bioorg Med Chem Lett 28: 1446-1455 (2018)
Article DOI: 10.1016/j.bmcl.2018.03.093
BindingDB Entry DOI: 10.7270/Q27W6FPC |
More data for this
Ligand-Target Pair | |
Nociceptin receptor
(Homo sapiens (Human)) | BDBM392076

(US10301272, Example 7/9)Show SMILES CC(C)(C)NS(=O)(=O)c1ccc(-c2sc(nc2CC2CCCCC2)C(=O)N[C@H]2C[C@@H](C2)C(O)=O)c2ccccc12 |r,wU:27.28,wD:29.33,(8.76,1.15,;7.67,.06,;6.19,.46,;7.28,1.55,;8.07,-1.42,;6.98,-2.51,;6.59,-4,;8.07,-3.6,;5.5,-2.11,;5.1,-.63,;3.61,-.23,;2.52,-1.32,;1.03,-.92,;.56,.55,;-.98,.55,;-1.46,-.92,;-.21,-1.82,;-.21,-3.36,;-1.55,-4.13,;-2.88,-3.36,;-4.21,-4.13,;-4.21,-5.67,;-2.88,-6.44,;-1.55,-5.67,;-2.07,1.64,;-1.67,3.12,;-3.56,1.24,;-4.65,2.33,;-6.19,2.33,;-6.19,3.87,;-4.65,3.87,;-7.28,4.96,;-6.88,6.44,;-8.76,4.56,;2.92,-2.8,;1.83,-3.89,;2.23,-5.38,;3.72,-5.78,;4.81,-4.69,;4.41,-3.2,)| Show InChI InChI=1S/C30H37N3O5S2/c1-30(2,3)33-40(37,38)25-14-13-23(21-11-7-8-12-22(21)25)26-24(15-18-9-5-4-6-10-18)32-28(39-26)27(34)31-20-16-19(17-20)29(35)36/h7-8,11-14,18-20,33H,4-6,9-10,15-17H2,1-3H3,(H,31,34)(H,35,36)/t19-,20- | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.980 | n/a | n/a | n/a | n/a | n/a | n/a |
Phenex Pharmaceuticals AG
Curated by ChEMBL
| Assay Description
Displacement of [3H]nociceptin from human recombinant NOP receptor after 60 mins by scintillation counting analysis |
Bioorg Med Chem Lett 28: 1446-1455 (2018)
Article DOI: 10.1016/j.bmcl.2018.03.093
BindingDB Entry DOI: 10.7270/Q27W6FPC |
More data for this
Ligand-Target Pair | |
Nuclear receptor ROR-gamma
(Homo sapiens (Human)) | BDBM50271670
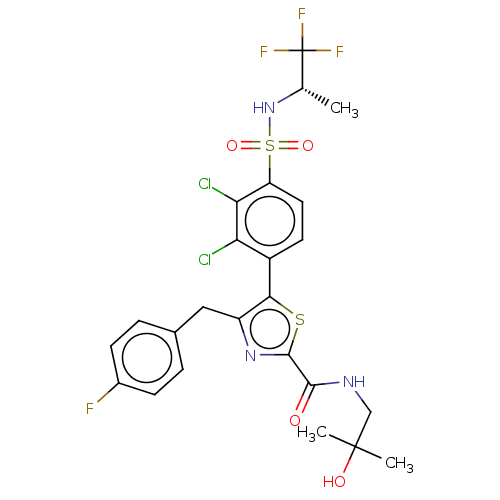
(CHEMBL4126317)Show SMILES C[C@H](NS(=O)(=O)c1ccc(-c2sc(nc2Cc2ccc(F)cc2)C(=O)NCC(C)(C)O)c(Cl)c1Cl)C(F)(F)F |r| Show InChI InChI=1S/C24H23Cl2F4N3O4S2/c1-12(24(28,29)30)33-39(36,37)17-9-8-15(18(25)19(17)26)20-16(10-13-4-6-14(27)7-5-13)32-22(38-20)21(34)31-11-23(2,3)35/h4-9,12,33,35H,10-11H2,1-3H3,(H,31,34)/t12-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Phenex Pharmaceuticals AG
Curated by ChEMBL
| Assay Description
Inverse agonist activity at human GAL4 DBD-fused RORgammat LBD (237 to 497 residues) expressed in HEK293T cells assessed as reduction in ROR mediated... |
Bioorg Med Chem Lett 28: 1446-1455 (2018)
Article DOI: 10.1016/j.bmcl.2018.03.093
BindingDB Entry DOI: 10.7270/Q27W6FPC |
More data for this
Ligand-Target Pair | |
Leukotriene A-4 hydrolase
(Homo sapiens (Human)) | BDBM50402382
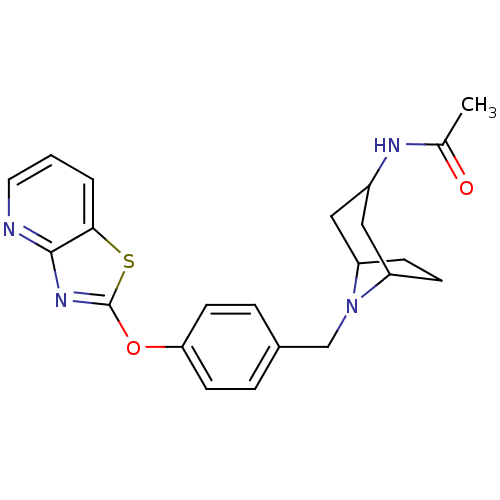
(CHEMBL2207751)Show SMILES CC(=O)NC1CC2CCC(C1)N2Cc1ccc(Oc2nc3ncccc3s2)cc1 |TLB:3:4:11:7.8| Show InChI InChI=1S/C22H24N4O2S/c1-14(27)24-16-11-17-6-7-18(12-16)26(17)13-15-4-8-19(9-5-15)28-22-25-21-20(29-22)3-2-10-23-21/h2-5,8-10,16-18H,6-7,11-13H2,1H3,(H,24,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Research& Development LLC
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant LTA4 hydrolase expressed in SF9 cells using LTA4 as substrate assessed as LTB4 production incubated for 10 mins prior... |
Bioorg Med Chem Lett 22: 7504-11 (2012)
Article DOI: 10.1016/j.bmcl.2012.10.036
BindingDB Entry DOI: 10.7270/Q2ZG6TDQ |
More data for this
Ligand-Target Pair | |
Vasopressin V1a receptor
(Homo sapiens (Human)) | BDBM392076

(US10301272, Example 7/9)Show SMILES CC(C)(C)NS(=O)(=O)c1ccc(-c2sc(nc2CC2CCCCC2)C(=O)N[C@H]2C[C@@H](C2)C(O)=O)c2ccccc12 |r,wU:27.28,wD:29.33,(8.76,1.15,;7.67,.06,;6.19,.46,;7.28,1.55,;8.07,-1.42,;6.98,-2.51,;6.59,-4,;8.07,-3.6,;5.5,-2.11,;5.1,-.63,;3.61,-.23,;2.52,-1.32,;1.03,-.92,;.56,.55,;-.98,.55,;-1.46,-.92,;-.21,-1.82,;-.21,-3.36,;-1.55,-4.13,;-2.88,-3.36,;-4.21,-4.13,;-4.21,-5.67,;-2.88,-6.44,;-1.55,-5.67,;-2.07,1.64,;-1.67,3.12,;-3.56,1.24,;-4.65,2.33,;-6.19,2.33,;-6.19,3.87,;-4.65,3.87,;-7.28,4.96,;-6.88,6.44,;-8.76,4.56,;2.92,-2.8,;1.83,-3.89,;2.23,-5.38,;3.72,-5.78,;4.81,-4.69,;4.41,-3.2,)| Show InChI InChI=1S/C30H37N3O5S2/c1-30(2,3)33-40(37,38)25-14-13-23(21-11-7-8-12-22(21)25)26-24(15-18-9-5-4-6-10-18)32-28(39-26)27(34)31-20-16-19(17-20)29(35)36/h7-8,11-14,18-20,33H,4-6,9-10,15-17H2,1-3H3,(H,31,34)(H,35,36)/t19-,20- | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Phenex Pharmaceuticals AG
Curated by ChEMBL
| Assay Description
Displacement of [3H]AVP from human recombinant V1a receptor after 60 mins by scintillation counting analysis |
Bioorg Med Chem Lett 28: 1446-1455 (2018)
Article DOI: 10.1016/j.bmcl.2018.03.093
BindingDB Entry DOI: 10.7270/Q27W6FPC |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM50594233
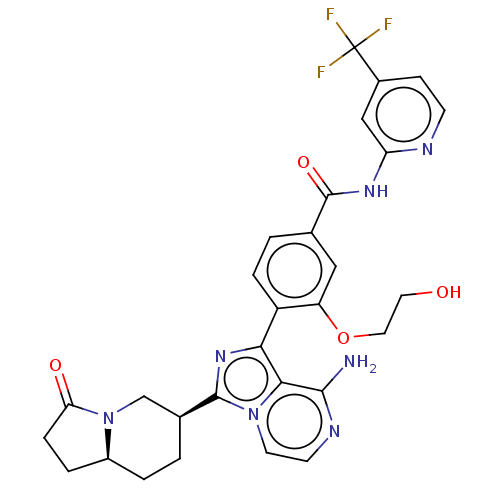
(CHEMBL5175002)Show SMILES [H][C@]12CCC(=O)N1C[C@H](CC2)c1nc(-c2ccc(cc2OCCO)C(=O)Nc2cc(ccn2)C(F)(F)F)c2c(N)nccn12 |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00030
BindingDB Entry DOI: 10.7270/Q2FX7FHQ |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM392076

(US10301272, Example 7/9)Show SMILES CC(C)(C)NS(=O)(=O)c1ccc(-c2sc(nc2CC2CCCCC2)C(=O)N[C@H]2C[C@@H](C2)C(O)=O)c2ccccc12 |r,wU:27.28,wD:29.33,(8.76,1.15,;7.67,.06,;6.19,.46,;7.28,1.55,;8.07,-1.42,;6.98,-2.51,;6.59,-4,;8.07,-3.6,;5.5,-2.11,;5.1,-.63,;3.61,-.23,;2.52,-1.32,;1.03,-.92,;.56,.55,;-.98,.55,;-1.46,-.92,;-.21,-1.82,;-.21,-3.36,;-1.55,-4.13,;-2.88,-3.36,;-4.21,-4.13,;-4.21,-5.67,;-2.88,-6.44,;-1.55,-5.67,;-2.07,1.64,;-1.67,3.12,;-3.56,1.24,;-4.65,2.33,;-6.19,2.33,;-6.19,3.87,;-4.65,3.87,;-7.28,4.96,;-6.88,6.44,;-8.76,4.56,;2.92,-2.8,;1.83,-3.89,;2.23,-5.38,;3.72,-5.78,;4.81,-4.69,;4.41,-3.2,)| Show InChI InChI=1S/C30H37N3O5S2/c1-30(2,3)33-40(37,38)25-14-13-23(21-11-7-8-12-22(21)25)26-24(15-18-9-5-4-6-10-18)32-28(39-26)27(34)31-20-16-19(17-20)29(35)36/h7-8,11-14,18-20,33H,4-6,9-10,15-17H2,1-3H3,(H,31,34)(H,35,36)/t19-,20- | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Phenex Pharmaceuticals AG
Curated by ChEMBL
| Assay Description
Displacement of [3H]U69593 from human kappa receptor after 60 mins by scintillation counting analysis |
Bioorg Med Chem Lett 28: 1446-1455 (2018)
Article DOI: 10.1016/j.bmcl.2018.03.093
BindingDB Entry DOI: 10.7270/Q27W6FPC |
More data for this
Ligand-Target Pair | |
Nuclear receptor ROR-gamma
(Homo sapiens (Human)) | BDBM50044179
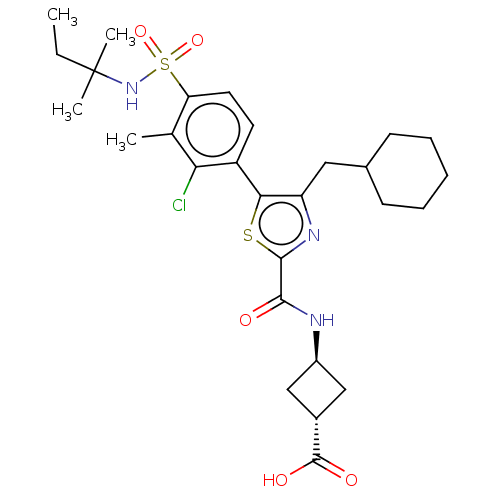
(CHEMBL3314002 | US10301272, Example 7/4)Show SMILES CCC(C)(C)NS(=O)(=O)c1ccc(-c2sc(nc2CC2CCCCC2)C(=O)N[C@H]2C[C@@H](C2)C(O)=O)c(Cl)c1C |r,wU:30.34,wD:28.29,(19.83,-3.9,;18.5,-4.68,;18.5,-6.22,;19.84,-6.98,;19.83,-5.44,;17.17,-6.99,;15.83,-6.22,;15.06,-4.88,;16.6,-4.88,;14.51,-7,;14.51,-8.55,;13.17,-9.32,;11.84,-8.55,;10.51,-9.32,;10.34,-10.85,;8.84,-11.17,;8.07,-9.83,;9.1,-8.69,;8.78,-7.18,;7.32,-6.7,;7,-5.19,;5.54,-4.71,;4.39,-5.73,;4.71,-7.24,;6.17,-7.73,;8.21,-12.57,;9.11,-13.82,;6.68,-12.73,;6.05,-14.14,;4.62,-14.69,;5.17,-16.13,;6.61,-15.58,;4.54,-17.54,;3.01,-17.7,;5.45,-18.79,;11.84,-7.01,;10.51,-6.24,;13.17,-6.24,;13.17,-4.7,)| Show InChI InChI=1S/C28H38ClN3O5S2/c1-5-28(3,4)32-39(36,37)22-12-11-20(23(29)16(22)2)24-21(13-17-9-7-6-8-10-17)31-26(38-24)25(33)30-19-14-18(15-19)27(34)35/h11-12,17-19,32H,5-10,13-15H2,1-4H3,(H,30,33)(H,34,35)/t18-,19- | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Phenex Pharmaceuticals AG
Curated by ChEMBL
| Assay Description
Inverse agonist activity at human GAL4 DBD-fused RORgammat LBD (237 to 497 residues) expressed in HEK293T cells assessed as reduction in ROR mediated... |
Bioorg Med Chem Lett 28: 1446-1455 (2018)
Article DOI: 10.1016/j.bmcl.2018.03.093
BindingDB Entry DOI: 10.7270/Q27W6FPC |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM392076

(US10301272, Example 7/9)Show SMILES CC(C)(C)NS(=O)(=O)c1ccc(-c2sc(nc2CC2CCCCC2)C(=O)N[C@H]2C[C@@H](C2)C(O)=O)c2ccccc12 |r,wU:27.28,wD:29.33,(8.76,1.15,;7.67,.06,;6.19,.46,;7.28,1.55,;8.07,-1.42,;6.98,-2.51,;6.59,-4,;8.07,-3.6,;5.5,-2.11,;5.1,-.63,;3.61,-.23,;2.52,-1.32,;1.03,-.92,;.56,.55,;-.98,.55,;-1.46,-.92,;-.21,-1.82,;-.21,-3.36,;-1.55,-4.13,;-2.88,-3.36,;-4.21,-4.13,;-4.21,-5.67,;-2.88,-6.44,;-1.55,-5.67,;-2.07,1.64,;-1.67,3.12,;-3.56,1.24,;-4.65,2.33,;-6.19,2.33,;-6.19,3.87,;-4.65,3.87,;-7.28,4.96,;-6.88,6.44,;-8.76,4.56,;2.92,-2.8,;1.83,-3.89,;2.23,-5.38,;3.72,-5.78,;4.81,-4.69,;4.41,-3.2,)| Show InChI InChI=1S/C30H37N3O5S2/c1-30(2,3)33-40(37,38)25-14-13-23(21-11-7-8-12-22(21)25)26-24(15-18-9-5-4-6-10-18)32-28(39-26)27(34)31-20-16-19(17-20)29(35)36/h7-8,11-14,18-20,33H,4-6,9-10,15-17H2,1-3H3,(H,31,34)(H,35,36)/t19-,20- | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Phenex Pharmaceuticals AG
Curated by ChEMBL
| Assay Description
Displacement of [3H]DAMGO from human recombinant mu receptor after 120 mins by scintillation counting analysis |
Bioorg Med Chem Lett 28: 1446-1455 (2018)
Article DOI: 10.1016/j.bmcl.2018.03.093
BindingDB Entry DOI: 10.7270/Q27W6FPC |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data