 Found 56 hits with Last Name = 'yasuda' and Initial = 'm'
Found 56 hits with Last Name = 'yasuda' and Initial = 'm' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Aldo-keto reductase family 1 member C3
(Homo sapiens (Human)) | BDBM50438582
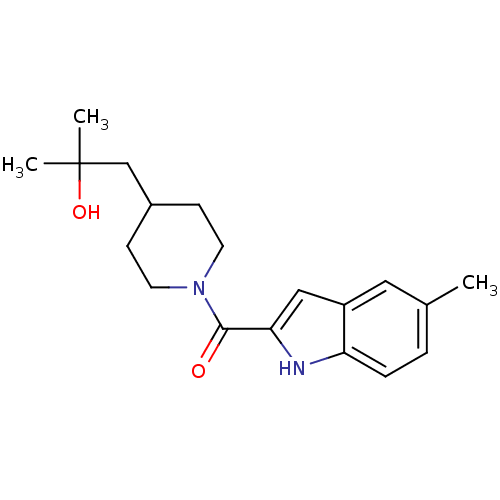
(CHEMBL2413849)Show SMILES Cc1ccc2[nH]c(cc2c1)C(=O)N1CCC(CC(C)(C)O)CC1 Show InChI InChI=1S/C19H26N2O2/c1-13-4-5-16-15(10-13)11-17(20-16)18(22)21-8-6-14(7-9-21)12-19(2,3)23/h4-5,10-11,14,20,23H,6-9,12H2,1-3H3 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.340 | n/a | n/a | n/a | n/a | n/a | n/a |
Astellas Pharma Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human 17beta-HSD5 expressed in HEK293 cells using androstenedione as substrate assessed as testosterone synthesis after 4 hrs |
Bioorg Med Chem 21: 5261-70 (2013)
Article DOI: 10.1016/j.bmc.2013.06.025
BindingDB Entry DOI: 10.7270/Q2GF0VXW |
More data for this
Ligand-Target Pair | |
Aldo-keto reductase family 1 member C3
(Homo sapiens (Human)) | BDBM50438582

(CHEMBL2413849)Show SMILES Cc1ccc2[nH]c(cc2c1)C(=O)N1CCC(CC(C)(C)O)CC1 Show InChI InChI=1S/C19H26N2O2/c1-13-4-5-16-15(10-13)11-17(20-16)18(22)21-8-6-14(7-9-21)12-19(2,3)23/h4-5,10-11,14,20,23H,6-9,12H2,1-3H3 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Astellas Pharma Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human 17beta-HSD5 expressed in human CWR22R cells using androstenedione as substrate assessed as testosterone synthesis after 4 hrs |
Bioorg Med Chem 21: 5261-70 (2013)
Article DOI: 10.1016/j.bmc.2013.06.025
BindingDB Entry DOI: 10.7270/Q2GF0VXW |
More data for this
Ligand-Target Pair | |
Aldo-keto reductase family 1 member C3
(Homo sapiens (Human)) | BDBM50438585

(CHEMBL2413850)Show InChI InChI=1S/C18H24N2O2/c1-18(2,22)12-13-7-9-20(10-8-13)17(21)16-11-14-5-3-4-6-15(14)19-16/h3-6,11,13,19,22H,7-10,12H2,1-2H3 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Astellas Pharma Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human 17beta-HSD5 expressed in HEK293 cells using androstenedione as substrate assessed as testosterone synthesis after 4 hrs |
Bioorg Med Chem 21: 5261-70 (2013)
Article DOI: 10.1016/j.bmc.2013.06.025
BindingDB Entry DOI: 10.7270/Q2GF0VXW |
More data for this
Ligand-Target Pair | |
Aldo-keto reductase family 1 member C3
(Homo sapiens (Human)) | BDBM50438594

(CHEMBL2413860)Show InChI InChI=1S/C16H20N2O2/c19-10-7-12-5-8-18(9-6-12)16(20)15-11-13-3-1-2-4-14(13)17-15/h1-4,11-12,17,19H,5-10H2 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Astellas Pharma Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human 17beta-HSD5 expressed in HEK293 cells using androstenedione as substrate assessed as testosterone synthesis after 4 hrs |
Bioorg Med Chem 21: 5261-70 (2013)
Article DOI: 10.1016/j.bmc.2013.06.025
BindingDB Entry DOI: 10.7270/Q2GF0VXW |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM19130

((2E,4E,6R)-7-[4-(dimethylamino)phenyl]-N-hydroxy-4...)Show SMILES CC(C=CC(=O)NO)C=C(C)C(=O)c1ccc(cc1)N(C)C |w:8.7,2.1| Show InChI InChI=1S/C17H22N2O3/c1-12(5-10-16(20)18-22)11-13(2)17(21)14-6-8-15(9-7-14)19(3)4/h5-12,22H,1-4H3,(H,18,20) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Kansai University
Curated by ChEMBL
| Assay Description
Inhibition of HDAC1 |
Bioorg Med Chem Lett 22: 1926-30 (2012)
Article DOI: 10.1016/j.bmcl.2012.01.053
BindingDB Entry DOI: 10.7270/Q2FQ9X48 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM19130

((2E,4E,6R)-7-[4-(dimethylamino)phenyl]-N-hydroxy-4...)Show SMILES CC(C=CC(=O)NO)C=C(C)C(=O)c1ccc(cc1)N(C)C |w:8.7,2.1| Show InChI InChI=1S/C17H22N2O3/c1-12(5-10-16(20)18-22)11-13(2)17(21)14-6-8-15(9-7-14)19(3)4/h5-12,22H,1-4H3,(H,18,20) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 9.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Kansai University
Curated by ChEMBL
| Assay Description
Inhibition of HDAC1 |
Bioorg Med Chem 18: 3925-33 (2010)
Article DOI: 10.1016/j.bmc.2010.04.033
BindingDB Entry DOI: 10.7270/Q29S1R6H |
More data for this
Ligand-Target Pair | |
Aldo-keto reductase family 1 member C3
(Homo sapiens (Human)) | BDBM50438586

(CHEMBL2413851)Show InChI InChI=1S/C17H22N2O2/c1-12(20)10-13-6-8-19(9-7-13)17(21)16-11-14-4-2-3-5-15(14)18-16/h2-5,11-13,18,20H,6-10H2,1H3 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Astellas Pharma Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human 17beta-HSD5 expressed in HEK293 cells using androstenedione as substrate assessed as testosterone synthesis after 4 hrs |
Bioorg Med Chem 21: 5261-70 (2013)
Article DOI: 10.1016/j.bmc.2013.06.025
BindingDB Entry DOI: 10.7270/Q2GF0VXW |
More data for this
Ligand-Target Pair | |
Aldo-keto reductase family 1 member C3
(Homo sapiens (Human)) | BDBM50438587

(CHEMBL2413852)Show InChI InChI=1S/C17H20N2O2/c1-12(20)10-13-6-8-19(9-7-13)17(21)16-11-14-4-2-3-5-15(14)18-16/h2-5,11,13,18H,6-10H2,1H3 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Astellas Pharma Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human 17beta-HSD5 expressed in HEK293 cells using androstenedione as substrate assessed as testosterone synthesis after 4 hrs |
Bioorg Med Chem 21: 5261-70 (2013)
Article DOI: 10.1016/j.bmc.2013.06.025
BindingDB Entry DOI: 10.7270/Q2GF0VXW |
More data for this
Ligand-Target Pair | |
Histone deacetylase 2
(Homo sapiens (Human)) | BDBM19130

((2E,4E,6R)-7-[4-(dimethylamino)phenyl]-N-hydroxy-4...)Show SMILES CC(C=CC(=O)NO)C=C(C)C(=O)c1ccc(cc1)N(C)C |w:8.7,2.1| Show InChI InChI=1S/C17H22N2O3/c1-12(5-10-16(20)18-22)11-13(2)17(21)14-6-8-15(9-7-14)19(3)4/h5-12,22H,1-4H3,(H,18,20) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
Kansai University
Curated by ChEMBL
| Assay Description
Inhibition of HDAC2 |
Bioorg Med Chem Lett 22: 1926-30 (2012)
Article DOI: 10.1016/j.bmcl.2012.01.053
BindingDB Entry DOI: 10.7270/Q2FQ9X48 |
More data for this
Ligand-Target Pair | |
Aldo-keto reductase family 1 member C3
(Homo sapiens (Human)) | BDBM50438582

(CHEMBL2413849)Show SMILES Cc1ccc2[nH]c(cc2c1)C(=O)N1CCC(CC(C)(C)O)CC1 Show InChI InChI=1S/C19H26N2O2/c1-13-4-5-16-15(10-13)11-17(20-16)18(22)21-8-6-14(7-9-21)12-19(2,3)23/h4-5,10-11,14,20,23H,6-9,12H2,1-3H3 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 24 | n/a | n/a | n/a | n/a | n/a | n/a |
Astellas Pharma Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human AKR1C3-mediated 9,10-phenanthrenequinone reduction after 10 to 20 mins by spectrophotometry in presence of NADPH |
Bioorg Med Chem 21: 5261-70 (2013)
Article DOI: 10.1016/j.bmc.2013.06.025
BindingDB Entry DOI: 10.7270/Q2GF0VXW |
More data for this
Ligand-Target Pair | |
Aldo-keto reductase family 1 member C3
(Homo sapiens (Human)) | BDBM50438588

(CHEMBL2413853)Show InChI InChI=1S/C17H22N2O2/c1-21-11-8-13-6-9-19(10-7-13)17(20)16-12-14-4-2-3-5-15(14)18-16/h2-5,12-13,18H,6-11H2,1H3 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 33 | n/a | n/a | n/a | n/a | n/a | n/a |
Astellas Pharma Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human 17beta-HSD5 expressed in HEK293 cells using androstenedione as substrate assessed as testosterone synthesis after 4 hrs |
Bioorg Med Chem 21: 5261-70 (2013)
Article DOI: 10.1016/j.bmc.2013.06.025
BindingDB Entry DOI: 10.7270/Q2GF0VXW |
More data for this
Ligand-Target Pair | |
Aldo-keto reductase family 1 member C3
(Homo sapiens (Human)) | BDBM50438583
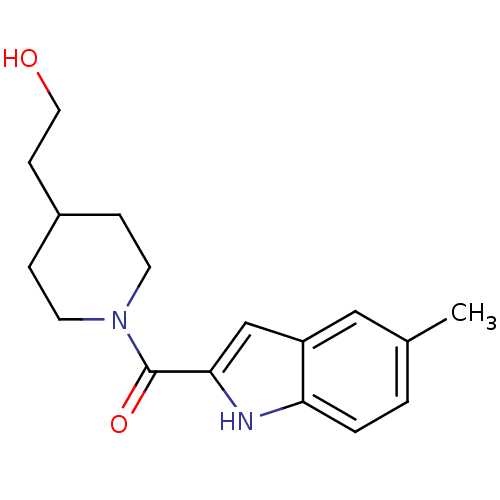
(CHEMBL2413863)Show InChI InChI=1S/C17H22N2O2/c1-12-2-3-15-14(10-12)11-16(18-15)17(21)19-7-4-13(5-8-19)6-9-20/h2-3,10-11,13,18,20H,4-9H2,1H3 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 37 | n/a | n/a | n/a | n/a | n/a | n/a |
Astellas Pharma Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human 17beta-HSD5 expressed in HEK293 cells using androstenedione as substrate assessed as testosterone synthesis after 4 hrs |
Bioorg Med Chem 21: 5261-70 (2013)
Article DOI: 10.1016/j.bmc.2013.06.025
BindingDB Entry DOI: 10.7270/Q2GF0VXW |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50229194
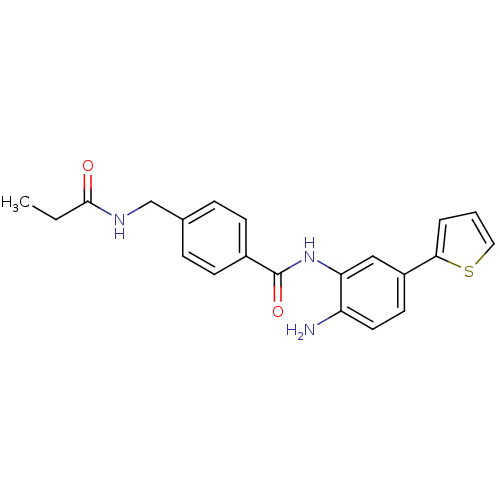
(CHEMBL251819 | N-(2-amino-5-(thiophen-2-yl)phenyl)...)Show SMILES CCC(=O)NCc1ccc(cc1)C(=O)Nc1cc(ccc1N)-c1cccs1 Show InChI InChI=1S/C21H21N3O2S/c1-2-20(25)23-13-14-5-7-15(8-6-14)21(26)24-18-12-16(9-10-17(18)22)19-4-3-11-27-19/h3-12H,2,13,22H2,1H3,(H,23,25)(H,24,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
Kansai University
Curated by ChEMBL
| Assay Description
Inhibition of HDAC1 |
Bioorg Med Chem Lett 22: 1926-30 (2012)
Article DOI: 10.1016/j.bmcl.2012.01.053
BindingDB Entry DOI: 10.7270/Q2FQ9X48 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50365501

(CHEMBL1957458)Show SMILES CCN1CCN(C(=O)NCc2ccc(cc2)C(=O)Nc2cc(ccc2N)-c2cccs2)C(=O)C1=O Show InChI InChI=1S/C25H25N5O4S/c1-2-29-11-12-30(24(33)23(29)32)25(34)27-15-16-5-7-17(8-6-16)22(31)28-20-14-18(9-10-19(20)26)21-4-3-13-35-21/h3-10,13-14H,2,11-12,15,26H2,1H3,(H,27,34)(H,28,31) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
Kansai University
Curated by ChEMBL
| Assay Description
Inhibition of HDAC1 |
Bioorg Med Chem Lett 22: 1926-30 (2012)
Article DOI: 10.1016/j.bmcl.2012.01.053
BindingDB Entry DOI: 10.7270/Q2FQ9X48 |
More data for this
Ligand-Target Pair | |
Aldo-keto reductase family 1 member C3
(Homo sapiens (Human)) | BDBM50438583

(CHEMBL2413863)Show InChI InChI=1S/C17H22N2O2/c1-12-2-3-15-14(10-12)11-16(18-15)17(21)19-7-4-13(5-8-19)6-9-20/h2-3,10-11,13,18,20H,4-9H2,1H3 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 58 | n/a | n/a | n/a | n/a | n/a | n/a |
Astellas Pharma Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human 17beta-HSD5 expressed in human CWR22R cells using androstenedione as substrate assessed as testosterone synthesis after 4 hrs |
Bioorg Med Chem 21: 5261-70 (2013)
Article DOI: 10.1016/j.bmc.2013.06.025
BindingDB Entry DOI: 10.7270/Q2GF0VXW |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50365503
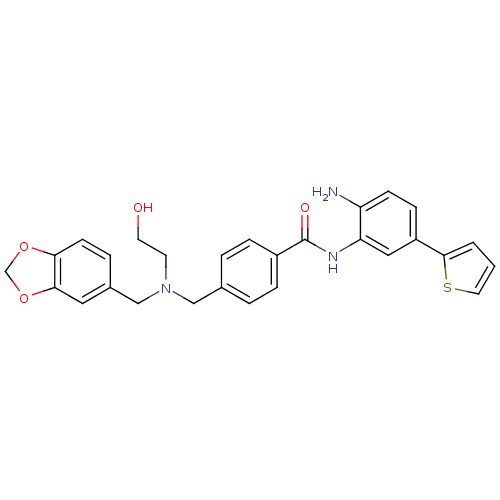
(CHEMBL1957463)Show SMILES Nc1ccc(cc1NC(=O)c1ccc(CN(CCO)Cc2ccc3OCOc3c2)cc1)-c1cccs1 Show InChI InChI=1S/C28H27N3O4S/c29-23-9-8-22(27-2-1-13-36-27)15-24(23)30-28(33)21-6-3-19(4-7-21)16-31(11-12-32)17-20-5-10-25-26(14-20)35-18-34-25/h1-10,13-15,32H,11-12,16-18,29H2,(H,30,33) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 60 | n/a | n/a | n/a | n/a | n/a | n/a |
Kansai University
Curated by ChEMBL
| Assay Description
Inhibition of HDAC1 |
Bioorg Med Chem Lett 22: 1926-30 (2012)
Article DOI: 10.1016/j.bmcl.2012.01.053
BindingDB Entry DOI: 10.7270/Q2FQ9X48 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50365502
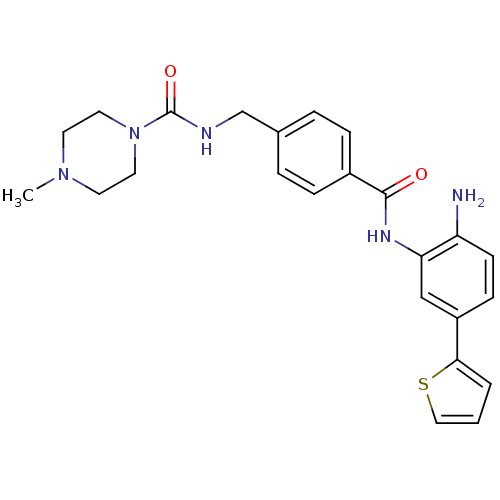
(CHEMBL1957459)Show SMILES CN1CCN(CC1)C(=O)NCc1ccc(cc1)C(=O)Nc1cc(ccc1N)-c1cccs1 Show InChI InChI=1S/C24H27N5O2S/c1-28-10-12-29(13-11-28)24(31)26-16-17-4-6-18(7-5-17)23(30)27-21-15-19(8-9-20(21)25)22-3-2-14-32-22/h2-9,14-15H,10-13,16,25H2,1H3,(H,26,31)(H,27,30) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 80 | n/a | n/a | n/a | n/a | n/a | n/a |
Kansai University
Curated by ChEMBL
| Assay Description
Inhibition of HDAC1 |
Bioorg Med Chem Lett 22: 1926-30 (2012)
Article DOI: 10.1016/j.bmcl.2012.01.053
BindingDB Entry DOI: 10.7270/Q2FQ9X48 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50365505
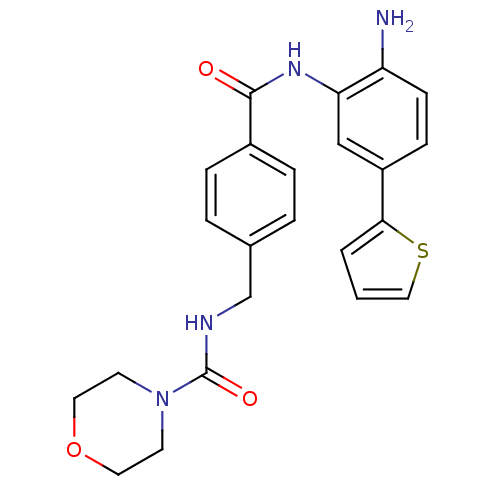
(CHEMBL1957464)Show SMILES Nc1ccc(cc1NC(=O)c1ccc(CNC(=O)N2CCOCC2)cc1)-c1cccs1 Show InChI InChI=1S/C23H24N4O3S/c24-19-8-7-18(21-2-1-13-31-21)14-20(19)26-22(28)17-5-3-16(4-6-17)15-25-23(29)27-9-11-30-12-10-27/h1-8,13-14H,9-12,15,24H2,(H,25,29)(H,26,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
Kansai University
Curated by ChEMBL
| Assay Description
Inhibition of HDAC1 |
Bioorg Med Chem Lett 22: 1926-30 (2012)
Article DOI: 10.1016/j.bmcl.2012.01.053
BindingDB Entry DOI: 10.7270/Q2FQ9X48 |
More data for this
Ligand-Target Pair | |
Aldo-keto reductase family 1 member C3
(Homo sapiens (Human)) | BDBM50438590

(CHEMBL2413855)Show InChI InChI=1S/C14H16N2O2/c17-6-5-10-8-16(9-10)14(18)13-7-11-3-1-2-4-12(11)15-13/h1-4,7,10,15,17H,5-6,8-9H2 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 140 | n/a | n/a | n/a | n/a | n/a | n/a |
Astellas Pharma Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human 17beta-HSD5 expressed in HEK293 cells using androstenedione as substrate assessed as testosterone synthesis after 4 hrs |
Bioorg Med Chem 21: 5261-70 (2013)
Article DOI: 10.1016/j.bmc.2013.06.025
BindingDB Entry DOI: 10.7270/Q2GF0VXW |
More data for this
Ligand-Target Pair | |
Aldo-keto reductase family 1 member C3
(Homo sapiens (Human)) | BDBM50438596

(CHEMBL2413861)Show InChI InChI=1S/C18H24N2O2/c1-13-4-5-16-15(11-13)12-17(19-16)18(22)20-8-6-14(7-9-20)3-2-10-21/h4-5,11-12,14,19,21H,2-3,6-10H2,1H3 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 160 | n/a | n/a | n/a | n/a | n/a | n/a |
Astellas Pharma Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human 17beta-HSD5 expressed in HEK293 cells using androstenedione as substrate assessed as testosterone synthesis after 4 hrs |
Bioorg Med Chem 21: 5261-70 (2013)
Article DOI: 10.1016/j.bmc.2013.06.025
BindingDB Entry DOI: 10.7270/Q2GF0VXW |
More data for this
Ligand-Target Pair | |
Aldo-keto reductase family 1 member C3
(Homo sapiens (Human)) | BDBM50438592

(CHEMBL2413857)Show InChI InChI=1S/C16H20N2O2/c19-10-8-13-6-3-4-9-18(13)16(20)15-11-12-5-1-2-7-14(12)17-15/h1-2,5,7,11,13,17,19H,3-4,6,8-10H2 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 230 | n/a | n/a | n/a | n/a | n/a | n/a |
Astellas Pharma Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human 17beta-HSD5 expressed in HEK293 cells using androstenedione as substrate assessed as testosterone synthesis after 4 hrs |
Bioorg Med Chem 21: 5261-70 (2013)
Article DOI: 10.1016/j.bmc.2013.06.025
BindingDB Entry DOI: 10.7270/Q2GF0VXW |
More data for this
Ligand-Target Pair | |
Histone deacetylase 2
(Homo sapiens (Human)) | BDBM50229194

(CHEMBL251819 | N-(2-amino-5-(thiophen-2-yl)phenyl)...)Show SMILES CCC(=O)NCc1ccc(cc1)C(=O)Nc1cc(ccc1N)-c1cccs1 Show InChI InChI=1S/C21H21N3O2S/c1-2-20(25)23-13-14-5-7-15(8-6-14)21(26)24-18-12-16(9-10-17(18)22)19-4-3-11-27-19/h3-12H,2,13,22H2,1H3,(H,23,25)(H,24,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 470 | n/a | n/a | n/a | n/a | n/a | n/a |
Kansai University
Curated by ChEMBL
| Assay Description
Inhibition of HDAC2 |
Bioorg Med Chem Lett 22: 1926-30 (2012)
Article DOI: 10.1016/j.bmcl.2012.01.053
BindingDB Entry DOI: 10.7270/Q2FQ9X48 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM19410

(CHEMBL27759 | MS-275 | US11377423, MS-275 | US1167...)Show InChI InChI=1S/C21H20N4O3/c22-18-5-1-2-6-19(18)25-20(26)17-9-7-15(8-10-17)13-24-21(27)28-14-16-4-3-11-23-12-16/h1-12H,13-14,22H2,(H,24,27)(H,25,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 480 | n/a | n/a | n/a | n/a | n/a | n/a |
Kansai University
Curated by ChEMBL
| Assay Description
Inhibition of HDAC1 |
Bioorg Med Chem Lett 22: 1926-30 (2012)
Article DOI: 10.1016/j.bmcl.2012.01.053
BindingDB Entry DOI: 10.7270/Q2FQ9X48 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM19410

(CHEMBL27759 | MS-275 | US11377423, MS-275 | US1167...)Show InChI InChI=1S/C21H20N4O3/c22-18-5-1-2-6-19(18)25-20(26)17-9-7-15(8-10-17)13-24-21(27)28-14-16-4-3-11-23-12-16/h1-12H,13-14,22H2,(H,24,27)(H,25,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 500 | n/a | n/a | n/a | n/a | n/a | n/a |
Kansai University
Curated by ChEMBL
| Assay Description
Inhibition of HDAC1 |
Bioorg Med Chem 18: 3925-33 (2010)
Article DOI: 10.1016/j.bmc.2010.04.033
BindingDB Entry DOI: 10.7270/Q29S1R6H |
More data for this
Ligand-Target Pair | |
Histone deacetylase 2
(Homo sapiens (Human)) | BDBM50365501

(CHEMBL1957458)Show SMILES CCN1CCN(C(=O)NCc2ccc(cc2)C(=O)Nc2cc(ccc2N)-c2cccs2)C(=O)C1=O Show InChI InChI=1S/C25H25N5O4S/c1-2-29-11-12-30(24(33)23(29)32)25(34)27-15-16-5-7-17(8-6-16)22(31)28-20-14-18(9-10-19(20)26)21-4-3-13-35-21/h3-10,13-14H,2,11-12,15,26H2,1H3,(H,27,34)(H,28,31) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 670 | n/a | n/a | n/a | n/a | n/a | n/a |
Kansai University
Curated by ChEMBL
| Assay Description
Inhibition of HDAC2 |
Bioorg Med Chem Lett 22: 1926-30 (2012)
Article DOI: 10.1016/j.bmcl.2012.01.053
BindingDB Entry DOI: 10.7270/Q2FQ9X48 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50320762

(CHEMBL1164063 | N-(2-Aminophenyl)-4-(((biphenyl-4-...)Show SMILES Nc1ccccc1NC(=O)c1ccc(CN(CCO)Cc2ccc(cc2)-c2ccccc2)cc1 Show InChI InChI=1S/C29H29N3O2/c30-27-8-4-5-9-28(27)31-29(34)26-16-12-23(13-17-26)21-32(18-19-33)20-22-10-14-25(15-11-22)24-6-2-1-3-7-24/h1-17,33H,18-21,30H2,(H,31,34) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 700 | n/a | n/a | n/a | n/a | n/a | n/a |
Kansai University
Curated by ChEMBL
| Assay Description
Inhibition of HDAC1 |
Bioorg Med Chem 18: 3925-33 (2010)
Article DOI: 10.1016/j.bmc.2010.04.033
BindingDB Entry DOI: 10.7270/Q29S1R6H |
More data for this
Ligand-Target Pair | |
Histone deacetylase 2
(Homo sapiens (Human)) | BDBM50365503

(CHEMBL1957463)Show SMILES Nc1ccc(cc1NC(=O)c1ccc(CN(CCO)Cc2ccc3OCOc3c2)cc1)-c1cccs1 Show InChI InChI=1S/C28H27N3O4S/c29-23-9-8-22(27-2-1-13-36-27)15-24(23)30-28(33)21-6-3-19(4-7-21)16-31(11-12-32)17-20-5-10-25-26(14-20)35-18-34-25/h1-10,13-15,32H,11-12,16-18,29H2,(H,30,33) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 740 | n/a | n/a | n/a | n/a | n/a | n/a |
Kansai University
Curated by ChEMBL
| Assay Description
Inhibition of HDAC2 |
Bioorg Med Chem Lett 22: 1926-30 (2012)
Article DOI: 10.1016/j.bmcl.2012.01.053
BindingDB Entry DOI: 10.7270/Q2FQ9X48 |
More data for this
Ligand-Target Pair | |
Aldo-keto reductase family 1 member C3
(Homo sapiens (Human)) | BDBM50438595
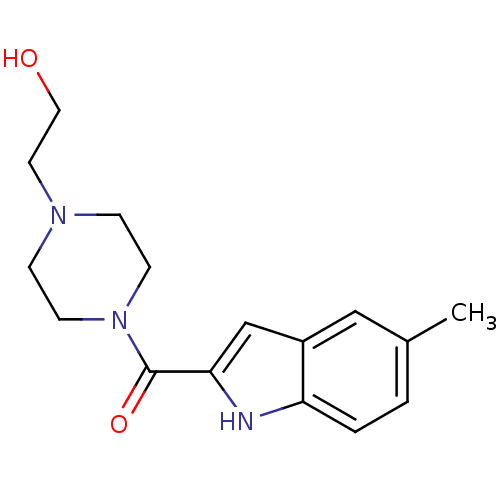
(CHEMBL2413859)Show InChI InChI=1S/C16H21N3O2/c1-12-2-3-14-13(10-12)11-15(17-14)16(21)19-6-4-18(5-7-19)8-9-20/h2-3,10-11,17,20H,4-9H2,1H3 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 770 | n/a | n/a | n/a | n/a | n/a | n/a |
Astellas Pharma Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human 17beta-HSD5 expressed in HEK293 cells using androstenedione as substrate assessed as testosterone synthesis after 4 hrs |
Bioorg Med Chem 21: 5261-70 (2013)
Article DOI: 10.1016/j.bmc.2013.06.025
BindingDB Entry DOI: 10.7270/Q2GF0VXW |
More data for this
Ligand-Target Pair | |
Aldo-keto reductase family 1 member C3
(Homo sapiens (Human)) | BDBM50438597
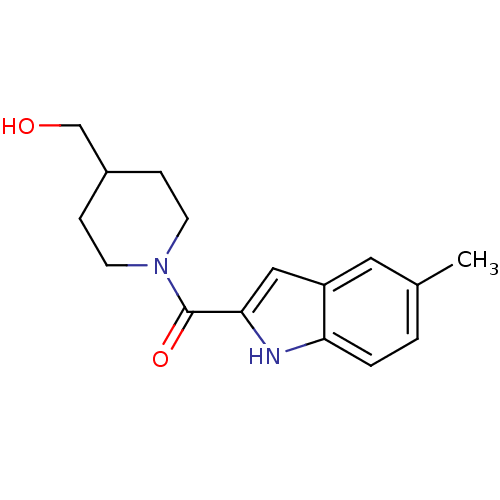
(CHEMBL2413864)Show InChI InChI=1S/C16H20N2O2/c1-11-2-3-14-13(8-11)9-15(17-14)16(20)18-6-4-12(10-19)5-7-18/h2-3,8-9,12,17,19H,4-7,10H2,1H3 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 790 | n/a | n/a | n/a | n/a | n/a | n/a |
Astellas Pharma Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human 17beta-HSD5 expressed in HEK293 cells using androstenedione as substrate assessed as testosterone synthesis after 4 hrs |
Bioorg Med Chem 21: 5261-70 (2013)
Article DOI: 10.1016/j.bmc.2013.06.025
BindingDB Entry DOI: 10.7270/Q2GF0VXW |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50320760

(CHEMBL1165774 | N-(2-Aminophenyl)-4-((3,3-dimethyl...)Show SMILES CN(C)C(=O)N(Cc1ccc(cc1)C(=O)Nc1ccccc1N)Cc1ccc(cc1)-c1cccs1 Show InChI InChI=1S/C28H28N4O2S/c1-31(2)28(34)32(18-20-9-13-22(14-10-20)26-8-5-17-35-26)19-21-11-15-23(16-12-21)27(33)30-25-7-4-3-6-24(25)29/h3-17H,18-19,29H2,1-2H3,(H,30,33) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 800 | n/a | n/a | n/a | n/a | n/a | n/a |
Kansai University
Curated by ChEMBL
| Assay Description
Inhibition of HDAC1 |
Bioorg Med Chem 18: 3925-33 (2010)
Article DOI: 10.1016/j.bmc.2010.04.033
BindingDB Entry DOI: 10.7270/Q29S1R6H |
More data for this
Ligand-Target Pair | |
Histone deacetylase 2
(Homo sapiens (Human)) | BDBM50365502

(CHEMBL1957459)Show SMILES CN1CCN(CC1)C(=O)NCc1ccc(cc1)C(=O)Nc1cc(ccc1N)-c1cccs1 Show InChI InChI=1S/C24H27N5O2S/c1-28-10-12-29(13-11-28)24(31)26-16-17-4-6-18(7-5-17)23(30)27-21-15-19(8-9-20(21)25)22-3-2-14-32-22/h2-9,14-15H,10-13,16,25H2,1H3,(H,26,31)(H,27,30) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 800 | n/a | n/a | n/a | n/a | n/a | n/a |
Kansai University
Curated by ChEMBL
| Assay Description
Inhibition of HDAC2 |
Bioorg Med Chem Lett 22: 1926-30 (2012)
Article DOI: 10.1016/j.bmcl.2012.01.053
BindingDB Entry DOI: 10.7270/Q2FQ9X48 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50320761
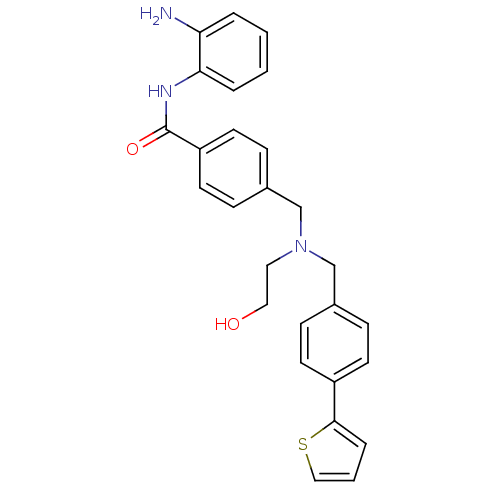
(CHEMBL1163973 | N-(2-Aminophenyl)-4-(((2-hydroxyet...)Show SMILES Nc1ccccc1NC(=O)c1ccc(CN(CCO)Cc2ccc(cc2)-c2cccs2)cc1 Show InChI InChI=1S/C27H27N3O2S/c28-24-4-1-2-5-25(24)29-27(32)23-13-9-21(10-14-23)19-30(15-16-31)18-20-7-11-22(12-8-20)26-6-3-17-33-26/h1-14,17,31H,15-16,18-19,28H2,(H,29,32) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 800 | n/a | n/a | n/a | n/a | n/a | n/a |
Kansai University
Curated by ChEMBL
| Assay Description
Inhibition of HDAC1 |
Bioorg Med Chem 18: 3925-33 (2010)
Article DOI: 10.1016/j.bmc.2010.04.033
BindingDB Entry DOI: 10.7270/Q29S1R6H |
More data for this
Ligand-Target Pair | |
Histone deacetylase 2
(Homo sapiens (Human)) | BDBM50365505

(CHEMBL1957464)Show SMILES Nc1ccc(cc1NC(=O)c1ccc(CNC(=O)N2CCOCC2)cc1)-c1cccs1 Show InChI InChI=1S/C23H24N4O3S/c24-19-8-7-18(21-2-1-13-31-21)14-20(19)26-22(28)17-5-3-16(4-6-17)15-25-23(29)27-9-11-30-12-10-27/h1-8,13-14H,9-12,15,24H2,(H,25,29)(H,26,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 880 | n/a | n/a | n/a | n/a | n/a | n/a |
Kansai University
Curated by ChEMBL
| Assay Description
Inhibition of HDAC2 |
Bioorg Med Chem Lett 22: 1926-30 (2012)
Article DOI: 10.1016/j.bmcl.2012.01.053
BindingDB Entry DOI: 10.7270/Q2FQ9X48 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50320757

(CHEMBL1165443 | N-(2-aminophenyl)-4-((biphenyl-4-y...)Show SMILES Nc1ccccc1NC(=O)c1ccc(CNCc2ccc(cc2)-c2ccccc2)cc1 Show InChI InChI=1S/C27H25N3O/c28-25-8-4-5-9-26(25)30-27(31)24-16-12-21(13-17-24)19-29-18-20-10-14-23(15-11-20)22-6-2-1-3-7-22/h1-17,29H,18-19,28H2,(H,30,31) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 900 | n/a | n/a | n/a | n/a | n/a | n/a |
Kansai University
Curated by ChEMBL
| Assay Description
Inhibition of HDAC1 |
Bioorg Med Chem 18: 3925-33 (2010)
Article DOI: 10.1016/j.bmc.2010.04.033
BindingDB Entry DOI: 10.7270/Q29S1R6H |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50320754

(CHEMBL1163887 | N-(2-aminophenyl)-4-((4-(thiophen-...)Show SMILES Nc1ccccc1NC(=O)c1ccc(CNCc2ccc(cc2)-c2cccs2)cc1 Show InChI InChI=1S/C25H23N3OS/c26-22-4-1-2-5-23(22)28-25(29)21-13-9-19(10-14-21)17-27-16-18-7-11-20(12-8-18)24-6-3-15-30-24/h1-15,27H,16-17,26H2,(H,28,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Kansai University
Curated by ChEMBL
| Assay Description
Inhibition of HDAC1 |
Bioorg Med Chem 18: 3925-33 (2010)
Article DOI: 10.1016/j.bmc.2010.04.033
BindingDB Entry DOI: 10.7270/Q29S1R6H |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50320755

(CHEMBL1165102 | N-(2-aminophenyl)-4-((benzo[b]thio...)Show InChI InChI=1S/C23H21N3OS/c24-20-6-2-3-7-21(20)26-23(27)17-11-9-16(10-12-17)14-25-15-19-13-18-5-1-4-8-22(18)28-19/h1-13,25H,14-15,24H2,(H,26,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Kansai University
Curated by ChEMBL
| Assay Description
Inhibition of HDAC1 |
Bioorg Med Chem 18: 3925-33 (2010)
Article DOI: 10.1016/j.bmc.2010.04.033
BindingDB Entry DOI: 10.7270/Q29S1R6H |
More data for this
Ligand-Target Pair | |
Aldo-keto reductase family 1 member C3
(Homo sapiens (Human)) | BDBM50438591

(CHEMBL2413856)Show InChI InChI=1S/C15H18N2O2/c18-8-6-11-5-7-17(10-11)15(19)14-9-12-3-1-2-4-13(12)16-14/h1-4,9,11,16,18H,5-8,10H2 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Astellas Pharma Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human 17beta-HSD5 expressed in HEK293 cells using androstenedione as substrate assessed as testosterone synthesis after 4 hrs |
Bioorg Med Chem 21: 5261-70 (2013)
Article DOI: 10.1016/j.bmc.2013.06.025
BindingDB Entry DOI: 10.7270/Q2GF0VXW |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50365504
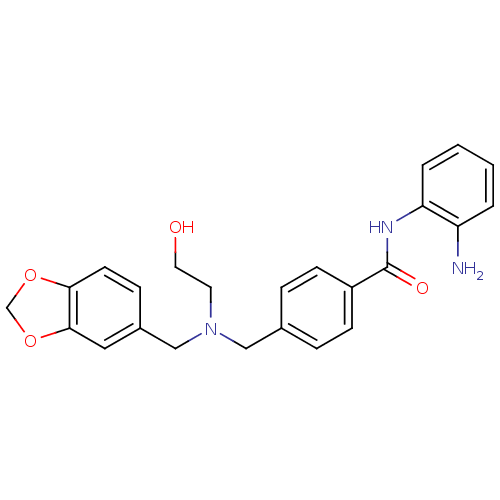
(CHEMBL407420)Show SMILES Nc1ccccc1NC(=O)c1ccc(CN(CCO)Cc2ccc3OCOc3c2)cc1 Show InChI InChI=1S/C24H25N3O4/c25-20-3-1-2-4-21(20)26-24(29)19-8-5-17(6-9-19)14-27(11-12-28)15-18-7-10-22-23(13-18)31-16-30-22/h1-10,13,28H,11-12,14-16,25H2,(H,26,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.17E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Kansai University
Curated by ChEMBL
| Assay Description
Inhibition of HDAC1 |
Bioorg Med Chem Lett 22: 1926-30 (2012)
Article DOI: 10.1016/j.bmcl.2012.01.053
BindingDB Entry DOI: 10.7270/Q2FQ9X48 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50320759

(CHEMBL1164440 | N-(2-Aminophenyl)-4-((N-(4-(thioph...)Show SMILES CS(=O)(=O)N(Cc1ccc(cc1)C(=O)Nc1ccccc1N)Cc1ccc(cc1)-c1cccs1 Show InChI InChI=1S/C26H25N3O3S2/c1-34(31,32)29(17-19-8-12-21(13-9-19)25-7-4-16-33-25)18-20-10-14-22(15-11-20)26(30)28-24-6-3-2-5-23(24)27/h2-16H,17-18,27H2,1H3,(H,28,30) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Kansai University
Curated by ChEMBL
| Assay Description
Inhibition of HDAC1 |
Bioorg Med Chem 18: 3925-33 (2010)
Article DOI: 10.1016/j.bmc.2010.04.033
BindingDB Entry DOI: 10.7270/Q29S1R6H |
More data for this
Ligand-Target Pair | |
Aldo-keto reductase family 1 member C3
(Homo sapiens (Human)) | BDBM50438589
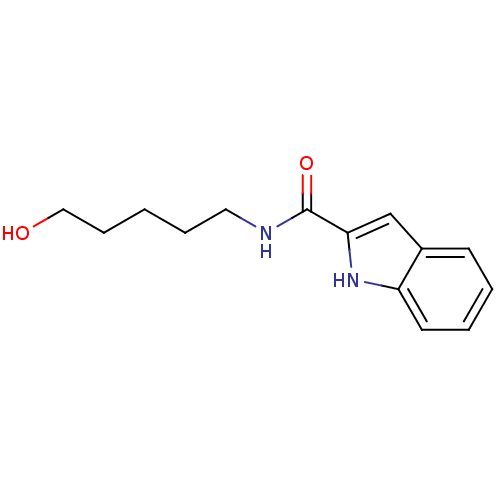
(CHEMBL2413854)Show InChI InChI=1S/C14H18N2O2/c17-9-5-1-4-8-15-14(18)13-10-11-6-2-3-7-12(11)16-13/h2-3,6-7,10,16-17H,1,4-5,8-9H2,(H,15,18) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Astellas Pharma Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human 17beta-HSD5 expressed in HEK293 cells using androstenedione as substrate assessed as testosterone synthesis after 4 hrs |
Bioorg Med Chem 21: 5261-70 (2013)
Article DOI: 10.1016/j.bmc.2013.06.025
BindingDB Entry DOI: 10.7270/Q2GF0VXW |
More data for this
Ligand-Target Pair | |
Histone deacetylase 2
(Homo sapiens (Human)) | BDBM19410

(CHEMBL27759 | MS-275 | US11377423, MS-275 | US1167...)Show InChI InChI=1S/C21H20N4O3/c22-18-5-1-2-6-19(18)25-20(26)17-9-7-15(8-10-17)13-24-21(27)28-14-16-4-3-11-23-12-16/h1-12H,13-14,22H2,(H,24,27)(H,25,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.51E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Kansai University
Curated by ChEMBL
| Assay Description
Inhibition of HDAC2 |
Bioorg Med Chem Lett 22: 1926-30 (2012)
Article DOI: 10.1016/j.bmcl.2012.01.053
BindingDB Entry DOI: 10.7270/Q2FQ9X48 |
More data for this
Ligand-Target Pair | |
Aldo-keto reductase family 1 member C3
(Homo sapiens (Human)) | BDBM50438593

(CHEMBL2413858)Show InChI InChI=1S/C16H20N2O2/c19-9-7-12-4-3-8-18(11-12)16(20)15-10-13-5-1-2-6-14(13)17-15/h1-2,5-6,10,12,17,19H,3-4,7-9,11H2 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Astellas Pharma Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human 17beta-HSD5 expressed in HEK293 cells using androstenedione as substrate assessed as testosterone synthesis after 4 hrs |
Bioorg Med Chem 21: 5261-70 (2013)
Article DOI: 10.1016/j.bmc.2013.06.025
BindingDB Entry DOI: 10.7270/Q2GF0VXW |
More data for this
Ligand-Target Pair | |
Aldo-keto reductase family 1 member C3
(Homo sapiens (Human)) | BDBM50438598
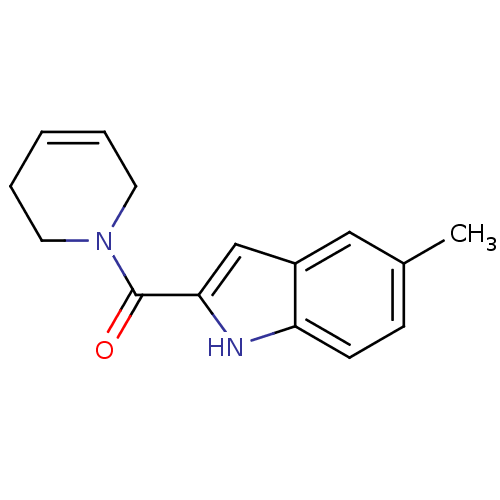
(CHEMBL2413862)Show InChI InChI=1S/C15H16N2O/c1-11-5-6-13-12(9-11)10-14(16-13)15(18)17-7-3-2-4-8-17/h2-3,5-6,9-10,16H,4,7-8H2,1H3 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | 2.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Astellas Pharma Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human 17beta-HSD5 expressed in HEK293 cells using androstenedione as substrate assessed as testosterone synthesis after 4 hrs |
Bioorg Med Chem 21: 5261-70 (2013)
Article DOI: 10.1016/j.bmc.2013.06.025
BindingDB Entry DOI: 10.7270/Q2GF0VXW |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50320756

(CHEMBL1164335 | N-(2-aminophenyl)-4-((3-(thiophen-...)Show SMILES Nc1ccccc1NC(=O)c1ccc(CNCc2cccc(c2)-c2cccs2)cc1 Show InChI InChI=1S/C25H23N3OS/c26-22-7-1-2-8-23(22)28-25(29)20-12-10-18(11-13-20)16-27-17-19-5-3-6-21(15-19)24-9-4-14-30-24/h1-15,27H,16-17,26H2,(H,28,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Kansai University
Curated by ChEMBL
| Assay Description
Inhibition of HDAC1 |
Bioorg Med Chem 18: 3925-33 (2010)
Article DOI: 10.1016/j.bmc.2010.04.033
BindingDB Entry DOI: 10.7270/Q29S1R6H |
More data for this
Ligand-Target Pair | |
Histone deacetylase 2
(Homo sapiens (Human)) | BDBM50365504

(CHEMBL407420)Show SMILES Nc1ccccc1NC(=O)c1ccc(CN(CCO)Cc2ccc3OCOc3c2)cc1 Show InChI InChI=1S/C24H25N3O4/c25-20-3-1-2-4-21(20)26-24(29)19-8-5-17(6-9-19)14-27(11-12-28)15-18-7-10-22-23(13-18)31-16-30-22/h1-10,13,28H,11-12,14-16,25H2,(H,26,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.36E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Kansai University
Curated by ChEMBL
| Assay Description
Inhibition of HDAC2 |
Bioorg Med Chem Lett 22: 1926-30 (2012)
Article DOI: 10.1016/j.bmcl.2012.01.053
BindingDB Entry DOI: 10.7270/Q2FQ9X48 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50320758

(CHEMBL1165580 | N-(2-Aminophenyl)-4-((methyl(4-(th...)Show SMILES CN(Cc1ccc(cc1)C(=O)Nc1ccccc1N)Cc1ccc(cc1)-c1cccs1 Show InChI InChI=1S/C26H25N3OS/c1-29(17-19-8-12-21(13-9-19)25-7-4-16-31-25)18-20-10-14-22(15-11-20)26(30)28-24-6-3-2-5-23(24)27/h2-16H,17-18,27H2,1H3,(H,28,30) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Kansai University
Curated by ChEMBL
| Assay Description
Inhibition of HDAC1 |
Bioorg Med Chem 18: 3925-33 (2010)
Article DOI: 10.1016/j.bmc.2010.04.033
BindingDB Entry DOI: 10.7270/Q29S1R6H |
More data for this
Ligand-Target Pair | |
Aldo-keto reductase family 1 member C3
(Homo sapiens (Human)) | BDBM50438584

(CHEMBL2413848)Show InChI InChI=1S/C17H20N2O3/c1-11-2-3-14-13(8-11)10-15(18-14)17(22)19-6-4-12(5-7-19)9-16(20)21/h2-3,8,10,12,18H,4-7,9H2,1H3,(H,20,21) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Astellas Pharma Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human 17beta-HSD5 expressed in HEK293 cells using androstenedione as substrate assessed as testosterone synthesis after 4 hrs |
Bioorg Med Chem 21: 5261-70 (2013)
Article DOI: 10.1016/j.bmc.2013.06.025
BindingDB Entry DOI: 10.7270/Q2GF0VXW |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50320752

(CHEMBL1163807 | N-(2-Aminophenyl)-4-(((2,3-dihydro...)Show InChI InChI=1S/C23H22N2O3S/c24-19-5-1-2-6-20(19)25-23(26)17-11-9-16(10-12-17)14-29-15-18-13-27-21-7-3-4-8-22(21)28-18/h1-12,18H,13-15,24H2,(H,25,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Kansai University
Curated by ChEMBL
| Assay Description
Inhibition of HDAC1 |
Bioorg Med Chem 18: 3925-33 (2010)
Article DOI: 10.1016/j.bmc.2010.04.033
BindingDB Entry DOI: 10.7270/Q29S1R6H |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50320753

(CHEMBL1163886 | N-(2-Aminophenyl)-4-((4-chlorobenz...)Show InChI InChI=1S/C21H19ClN2OS/c22-18-11-7-16(8-12-18)14-26-13-15-5-9-17(10-6-15)21(25)24-20-4-2-1-3-19(20)23/h1-12H,13-14,23H2,(H,24,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.90E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Kansai University
Curated by ChEMBL
| Assay Description
Inhibition of HDAC1 |
Bioorg Med Chem 18: 3925-33 (2010)
Article DOI: 10.1016/j.bmc.2010.04.033
BindingDB Entry DOI: 10.7270/Q29S1R6H |
More data for this
Ligand-Target Pair | |
Aldo-keto reductase family 1 member C2
(Homo sapiens (Human)) | BDBM50438582

(CHEMBL2413849)Show SMILES Cc1ccc2[nH]c(cc2c1)C(=O)N1CCC(CC(C)(C)O)CC1 Show InChI InChI=1S/C19H26N2O2/c1-13-4-5-16-15(10-13)11-17(20-16)18(22)21-8-6-14(7-9-21)12-19(2,3)23/h4-5,10-11,14,20,23H,6-9,12H2,1-3H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Astellas Pharma Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human AKR1C2-mediated 9,10-phenanthrenequinone reduction after 10 to 20 mins by spectrophotometry in presence of NADPH |
Bioorg Med Chem 21: 5261-70 (2013)
Article DOI: 10.1016/j.bmc.2013.06.025
BindingDB Entry DOI: 10.7270/Q2GF0VXW |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data