

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Thymidylate synthase (Lactobacillus casei) | BDBM50028122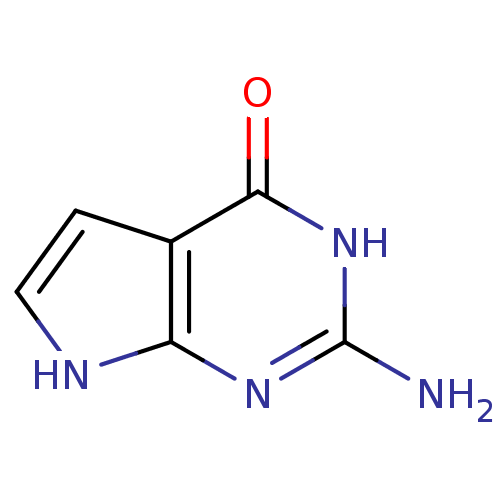 (2-Amino-3,7-dihydro-pyrrolo[2,3-d]pyrimidin-4-one ...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase MMDB PC cid PC sid PDB UniChem Patents | PubMed | 550 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eisai Company, Ltd. Curated by ChEMBL | Assay Description Binding affinity against TS | J Med Chem 37: 1616-24 (1994) BindingDB Entry DOI: 10.7270/Q21G0MWV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Mus musculus (Mouse)) | BDBM50028122 (2-Amino-3,7-dihydro-pyrrolo[2,3-d]pyrimidin-4-one ...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase MMDB PC cid PC sid PDB UniChem Patents | PubMed | 560 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eisai Company, Ltd. Curated by ChEMBL | Assay Description Binding affinity against dihydrofolate reductase | J Med Chem 37: 1616-24 (1994) BindingDB Entry DOI: 10.7270/Q21G0MWV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Lactobacillus casei) | BDBM18050 (2-[(4-{[(2,4-diaminopteridin-6-yl)methyl](methyl)a...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB PubMed | n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Eisai Company, Ltd. Curated by ChEMBL | Assay Description Compound was tested for the inhibition of dihydrofolate reductase in Bovine liver | J Med Chem 37: 1616-24 (1994) BindingDB Entry DOI: 10.7270/Q21G0MWV | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Dihydrofolate reductase (Mus musculus (Mouse)) | BDBM50041670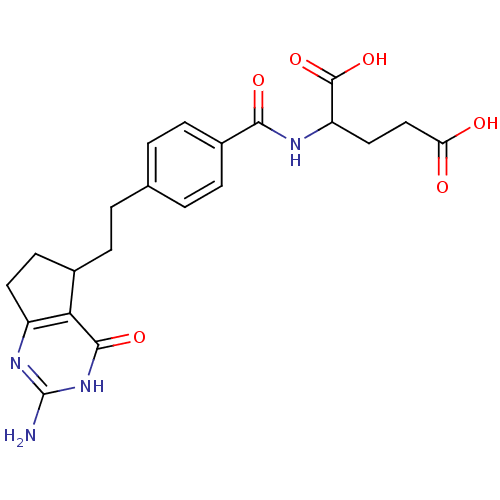 (2-{4-[2-(2-Amino-4-hydroxy-6,7-dihydro-5H-cyclopen...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Eisai Company, Ltd. Curated by ChEMBL | Assay Description Compound was tested for the inhibition of dihydrofolate reductase in P388 cells | J Med Chem 37: 1616-24 (1994) BindingDB Entry DOI: 10.7270/Q21G0MWV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Lactobacillus casei) | BDBM50041672 (2-{4-[3-(2,4-Diamino-6,7-dihydro-5H-cyclopentapyri...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Eisai Company, Ltd. Curated by ChEMBL | Assay Description Compound was tested for the inhibition of dihydrofolate reductase in Bovine liver | J Med Chem 37: 1616-24 (1994) BindingDB Entry DOI: 10.7270/Q21G0MWV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Lactobacillus casei) | BDBM50016460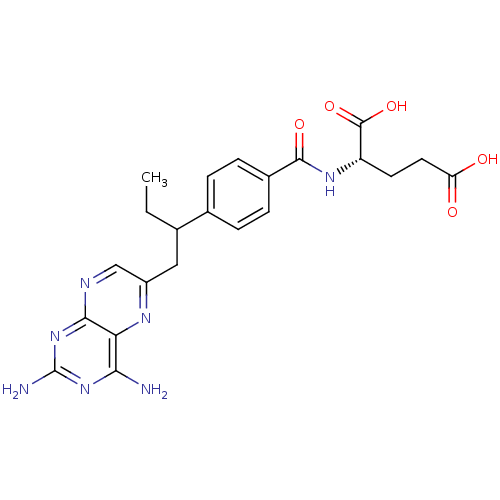 ((S)-2-{4-[1-(2,4-Diamino-pteridin-6-ylmethyl)-prop...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 3.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Eisai Company, Ltd. Curated by ChEMBL | Assay Description Compound was tested for the inhibition of dihydrofolate reductase in Bovine liver | J Med Chem 37: 1616-24 (1994) BindingDB Entry DOI: 10.7270/Q21G0MWV | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Dihydrofolate reductase (Mus musculus (Mouse)) | BDBM18050 (2-[(4-{[(2,4-diaminopteridin-6-yl)methyl](methyl)a...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB PubMed | n/a | n/a | 6.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Eisai Company, Ltd. Curated by ChEMBL | Assay Description Compound was tested for the inhibition of dihydrofolate reductase in P388 cells | J Med Chem 37: 1616-24 (1994) BindingDB Entry DOI: 10.7270/Q21G0MWV | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Dihydrofolate reductase (Mus musculus (Mouse)) | BDBM50041672 (2-{4-[3-(2,4-Diamino-6,7-dihydro-5H-cyclopentapyri...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 7.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Eisai Company, Ltd. Curated by ChEMBL | Assay Description Compound was tested for the inhibition of dihydrofolate reductase in P388 cells | J Med Chem 37: 1616-24 (1994) BindingDB Entry DOI: 10.7270/Q21G0MWV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Mus musculus (Mouse)) | BDBM50016460 ((S)-2-{4-[1-(2,4-Diamino-pteridin-6-ylmethyl)-prop...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Eisai Company, Ltd. Curated by ChEMBL | Assay Description Compound was tested for the inhibition of dihydrofolate reductase in P388 cells | J Med Chem 37: 1616-24 (1994) BindingDB Entry DOI: 10.7270/Q21G0MWV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Lactobacillus casei) | BDBM50041673 (2-{4-[2-(2,4-Diamino-6,7-dihydro-5H-cyclopentapyri...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
Eisai Company, Ltd. Curated by ChEMBL | Assay Description Compound was tested for the inhibition of dihydrofolate reductase in Bovine liver | J Med Chem 37: 1616-24 (1994) BindingDB Entry DOI: 10.7270/Q21G0MWV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Mus musculus (Mouse)) | BDBM50041671 (2-{4-[3-(2-Amino-4-hydroxy-6,7-dihydro-5H-cyclopen...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 60 | n/a | n/a | n/a | n/a | n/a | n/a |
Eisai Company, Ltd. Curated by ChEMBL | Assay Description Compound was tested for the inhibition of dihydrofolate reductase in P388 cells | J Med Chem 37: 1616-24 (1994) BindingDB Entry DOI: 10.7270/Q21G0MWV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Mus musculus (Mouse)) | BDBM50041673 (2-{4-[2-(2,4-Diamino-6,7-dihydro-5H-cyclopentapyri...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 72 | n/a | n/a | n/a | n/a | n/a | n/a |
Eisai Company, Ltd. Curated by ChEMBL | Assay Description Compound was tested for the inhibition of dihydrofolate reductase in P388 cells | J Med Chem 37: 1616-24 (1994) BindingDB Entry DOI: 10.7270/Q21G0MWV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Similar to alpha-tubulin isoform 1 (Bos taurus) | BDBM50120465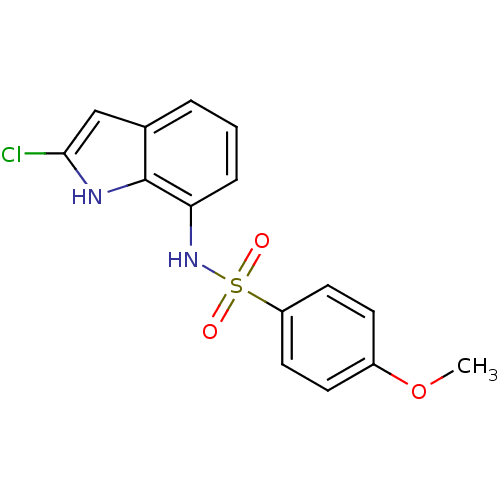 (CHEMBL281995 | N-(2-Chloro-1H-indol-7-yl)-4-methox...) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Eisai Co., Ltd. Curated by ChEMBL | Assay Description Inhibitory activity against tubulin polymerization. | J Med Chem 45: 4913-22 (2002) Article DOI: 10.1021/jm0201060 BindingDB Entry DOI: 10.7270/Q2668GZX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Similar to alpha-tubulin isoform 1 (Bos taurus) | BDBM50101086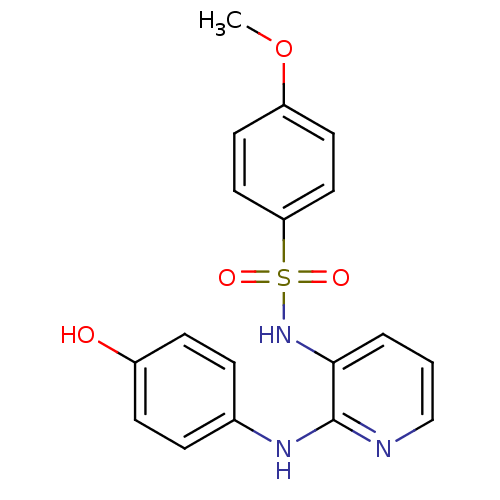 (CHEMBL20684 | E-7010 | N-(2-(4-hydroxyphenylamino)...) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents | Article PubMed | n/a | n/a | 2.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Eisai Co., Ltd. Curated by ChEMBL | Assay Description Inhibitory activity against tubulin polymerization. | J Med Chem 45: 4913-22 (2002) Article DOI: 10.1021/jm0201060 BindingDB Entry DOI: 10.7270/Q2668GZX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Similar to alpha-tubulin isoform 1 (Bos taurus) | BDBM50120462 (CHEMBL20289 | N-(3-Chloro-1H-indol-7-yl)-4-methoxy...) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 9.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Eisai Co., Ltd. Curated by ChEMBL | Assay Description Inhibitory activity against tubulin polymerization. | J Med Chem 45: 4913-22 (2002) Article DOI: 10.1021/jm0201060 BindingDB Entry DOI: 10.7270/Q2668GZX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Similar to alpha-tubulin isoform 1 (Bos taurus) | BDBM50473974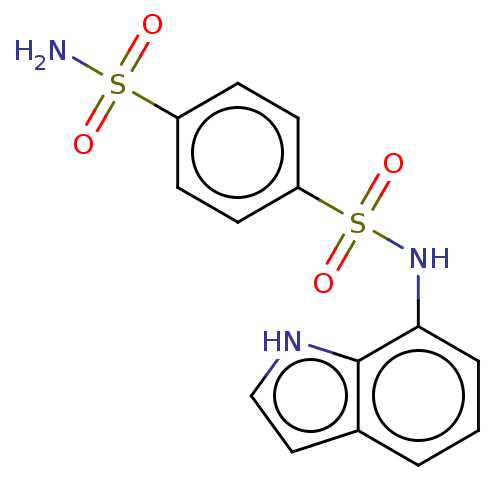 (CHEMBL146194) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Eisai Co., Ltd. Curated by ChEMBL | Assay Description Inhibitory activity against tubulin polymerization. | J Med Chem 45: 4913-22 (2002) Article DOI: 10.1021/jm0201060 BindingDB Entry DOI: 10.7270/Q2668GZX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Similar to alpha-tubulin isoform 1 (Bos taurus) | BDBM50473975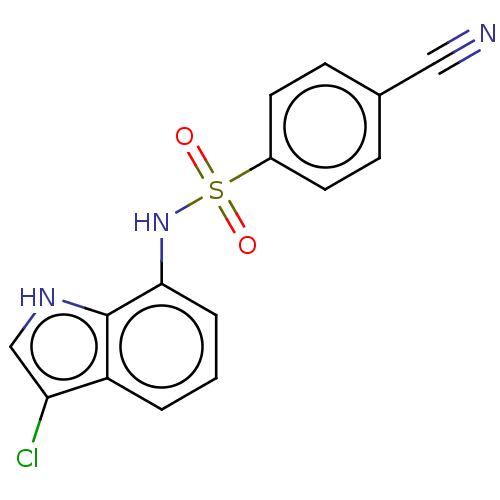 (CHEMBL20814) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Eisai Co., Ltd. Curated by ChEMBL | Assay Description Inhibitory activity against tubulin polymerization. | J Med Chem 45: 4913-22 (2002) Article DOI: 10.1021/jm0201060 BindingDB Entry DOI: 10.7270/Q2668GZX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Similar to alpha-tubulin isoform 1 (Bos taurus) | BDBM10890 (1-N-(3-chloro-1H-indol-7-yl)benzene-1,4-disulfonam...) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Eisai Co., Ltd. Curated by ChEMBL | Assay Description Inhibitory activity against tubulin polymerization. | J Med Chem 45: 4913-22 (2002) Article DOI: 10.1021/jm0201060 BindingDB Entry DOI: 10.7270/Q2668GZX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Similar to alpha-tubulin isoform 1 (Bos taurus) | BDBM50473976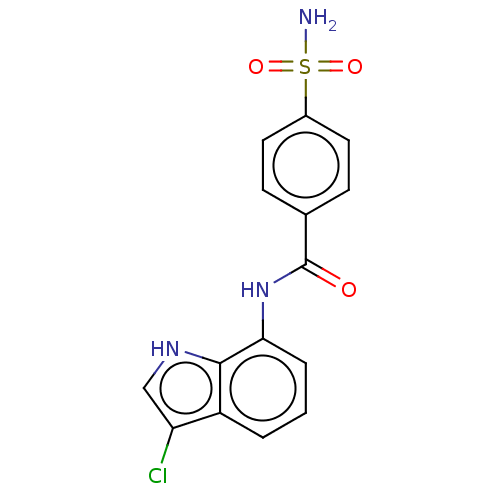 (CHEMBL358114) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Eisai Co., Ltd. Curated by ChEMBL | Assay Description Inhibitory activity against tubulin polymerization. | J Med Chem 45: 4913-22 (2002) Article DOI: 10.1021/jm0201060 BindingDB Entry DOI: 10.7270/Q2668GZX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||