

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM364562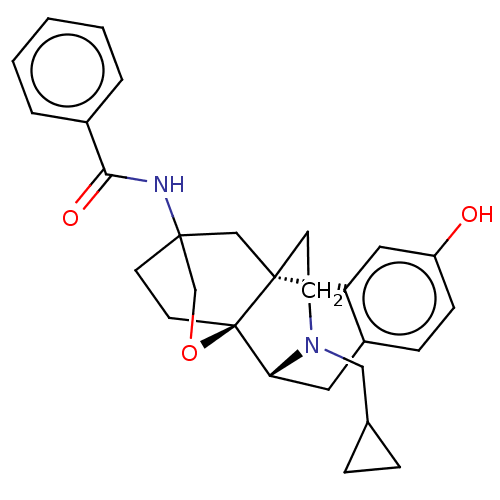 (N-((4bR,6S,8aS,9R)-11- (cyclopropylmethyl)- 3-hydr...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.0190 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GNF | Assay Description Radioligand dose-displacement binding assays for μ-opioid receptors used 0.3 nM [3H]-diprenorphine (Perkin Elmer, Shelton, Conn.), with 5 mg mem... | J Med Chem 50: 4255-9 (2007) BindingDB Entry DOI: 10.7270/Q2222X2R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM364547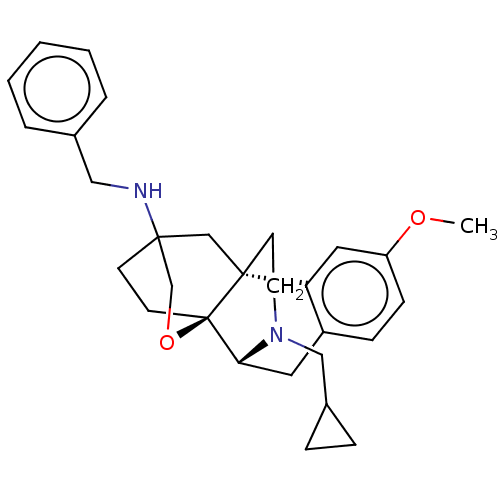 ((4bR,6S,8aS,9R)-N-benzyl-11- (cyclopropylmethyl)-3...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.0260 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GNF | Assay Description Radioligand dose displacement assays used 0.4 nM [3H]-U69,593 (GE Healthcare, Piscataway, N.J.; 40 Ci/mmole) with 15 μg membrane protein (recomb... | J Med Chem 50: 4255-9 (2007) BindingDB Entry DOI: 10.7270/Q2222X2R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM364552 (N-((4bR,6S,8aS,9R)-11- (cyclopropylmethyl)- 3-meth...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.0320 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GNF | Assay Description Radioligand dose displacement assays used 0.4 nM [3H]-U69,593 (GE Healthcare, Piscataway, N.J.; 40 Ci/mmole) with 15 μg membrane protein (recomb... | J Med Chem 50: 4255-9 (2007) BindingDB Entry DOI: 10.7270/Q2222X2R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM364548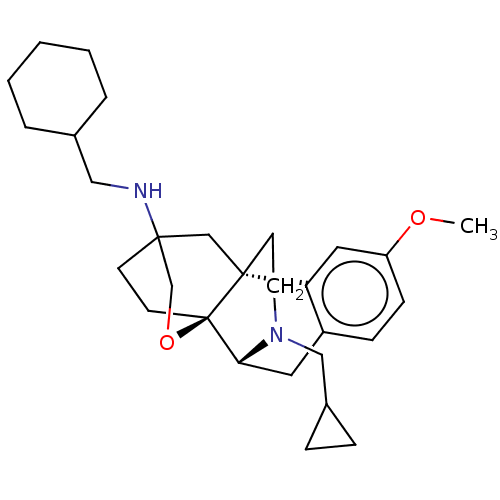 ((4bR,6S,8aS,9R)-N-(cyclohexylmethyl)-11- (cyclopro...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.0520 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GNF | Assay Description Radioligand dose displacement assays used 0.4 nM [3H]-U69,593 (GE Healthcare, Piscataway, N.J.; 40 Ci/mmole) with 15 μg membrane protein (recomb... | J Med Chem 50: 4255-9 (2007) BindingDB Entry DOI: 10.7270/Q2222X2R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM364561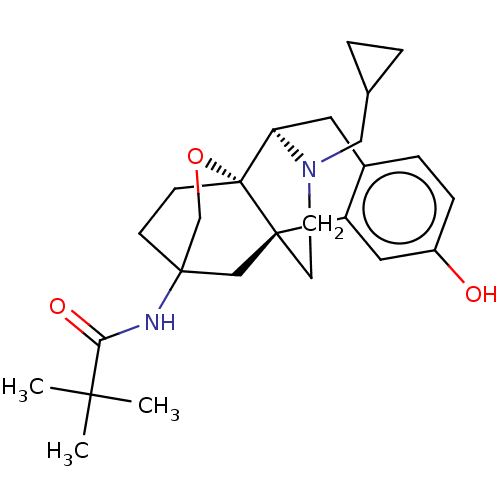 (N-((4bR,6S,8aS,9R)-11- (cyclopropylmethyl)- 3-hydr...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.0560 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GNF | Assay Description Radioligand dose-displacement binding assays for μ-opioid receptors used 0.3 nM [3H]-diprenorphine (Perkin Elmer, Shelton, Conn.), with 5 mg mem... | J Med Chem 50: 4255-9 (2007) BindingDB Entry DOI: 10.7270/Q2222X2R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM364559 (4-Chloro-N-((4bR,6S,8aS,9R)-11- (cyclopropylmethyl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.0570 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GNF | Assay Description Radioligand dose displacement assays used 0.4 nM [3H]-U69,593 (GE Healthcare, Piscataway, N.J.; 40 Ci/mmole) with 15 μg membrane protein (recomb... | J Med Chem 50: 4255-9 (2007) BindingDB Entry DOI: 10.7270/Q2222X2R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM364561 (N-((4bR,6S,8aS,9R)-11- (cyclopropylmethyl)- 3-hydr...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.0590 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GNF | Assay Description Radioligand dose displacement assays used 0.4 nM [3H]-U69,593 (GE Healthcare, Piscataway, N.J.; 40 Ci/mmole) with 15 μg membrane protein (recomb... | J Med Chem 50: 4255-9 (2007) BindingDB Entry DOI: 10.7270/Q2222X2R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM364562 (N-((4bR,6S,8aS,9R)-11- (cyclopropylmethyl)- 3-hydr...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.0620 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GNF | Assay Description Radioligand dose displacement assays used 0.4 nM [3H]-U69,593 (GE Healthcare, Piscataway, N.J.; 40 Ci/mmole) with 15 μg membrane protein (recomb... | J Med Chem 50: 4255-9 (2007) BindingDB Entry DOI: 10.7270/Q2222X2R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM364549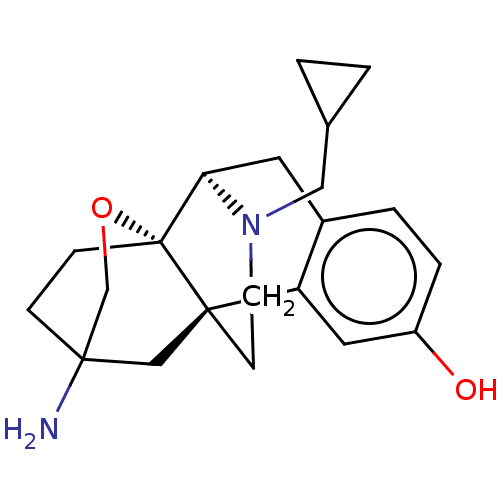 ((4bR,6S,8aS,9R)-6-amino-11- (cyclopropylmethyl)-5,...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.0960 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GNF | Assay Description Radioligand dose displacement assays used 0.4 nM [3H]-U69,593 (GE Healthcare, Piscataway, N.J.; 40 Ci/mmole) with 15 μg membrane protein (recomb... | J Med Chem 50: 4255-9 (2007) BindingDB Entry DOI: 10.7270/Q2222X2R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM151177 (BDBM151178 | US8987287, 7) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.200 | -55.4 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
Purdue Pharma L.P. US Patent | Assay Description Radioligand dose displacement assays used 0.4 nM [3H]-U69,593 (GE Healthcare, Piscataway, N.J.; 40 Ci/mmole) with 15 ug membrane protein (recombinant... | US Patent US8987287 (2015) BindingDB Entry DOI: 10.7270/Q24F1PFT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM151177 (BDBM151178 | US8987287, 7) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.230 | -55.0 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
Purdue Pharma L.P. US Patent | Assay Description Radioligand dose displacement assays used 0.4 nM [3H]-U69,593 (GE Healthcare, Piscataway, N.J.; 40 Ci/mmole) with 15 ug membrane protein (recombinant... | US Patent US8987287 (2015) BindingDB Entry DOI: 10.7270/Q24F1PFT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM364559 (4-Chloro-N-((4bR,6S,8aS,9R)-11- (cyclopropylmethyl...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.290 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GNF | Assay Description Radioligand dose-displacement binding assays for μ-opioid receptors used 0.3 nM [3H]-diprenorphine (Perkin Elmer, Shelton, Conn.), with 5 mg mem... | J Med Chem 50: 4255-9 (2007) BindingDB Entry DOI: 10.7270/Q2222X2R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily V member 1 (Homo sapiens (Human)) | BDBM50147083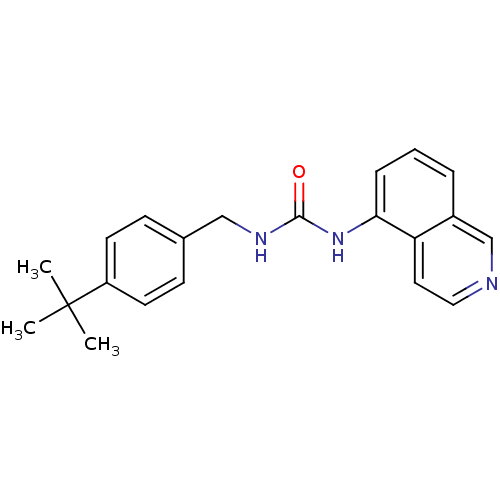 (1-(4-tert-Butyl-benzyl)-3-isoquinolin-5-yl-urea | ...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Binding affinity towards human vanilloid receptor subtype 1 expressed in HEK293 cell membrane using [3H]-RTX as radioligand. | Bioorg Med Chem Lett 14: 3053-6 (2004) Article DOI: 10.1016/j.bmcl.2004.04.038 BindingDB Entry DOI: 10.7270/Q2FB52D6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM364557 (N-((4bR,6S,8aS,9R)-11- (cyclopropylmethyl)- 3-meth...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GNF | Assay Description Radioligand dose displacement assays used 0.4 nM [3H]-U69,593 (GE Healthcare, Piscataway, N.J.; 40 Ci/mmole) with 15 μg membrane protein (recomb... | J Med Chem 50: 4255-9 (2007) BindingDB Entry DOI: 10.7270/Q2222X2R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM151184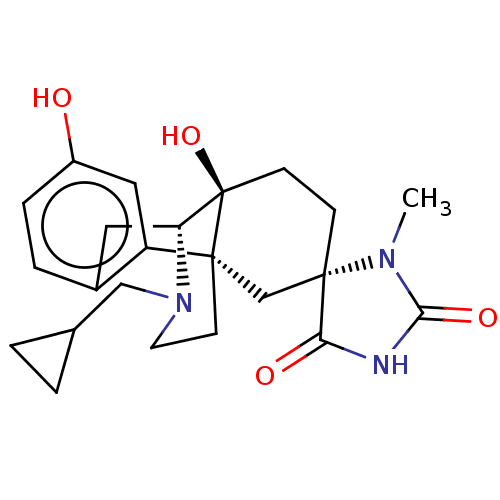 (US8987287, 63) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.300 | -54.4 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
Purdue Pharma L.P. US Patent | Assay Description Radioligand dose displacement assays used 0.4 nM [3H]-U69,593 (GE Healthcare, Piscataway, N.J.; 40 Ci/mmole) with 15 ug membrane protein (recombinant... | US Patent US8987287 (2015) BindingDB Entry DOI: 10.7270/Q24F1PFT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM364556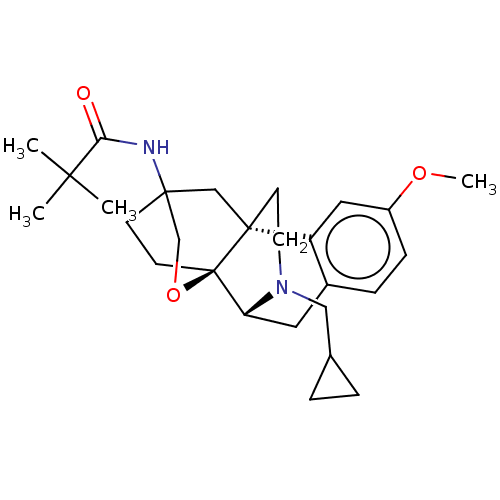 (N-((4bR,6S,8aS,9R)-11- (cyclopropylmethyl)- 3-meth...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.330 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GNF | Assay Description Radioligand dose displacement assays used 0.4 nM [3H]-U69,593 (GE Healthcare, Piscataway, N.J.; 40 Ci/mmole) with 15 μg membrane protein (recomb... | J Med Chem 50: 4255-9 (2007) BindingDB Entry DOI: 10.7270/Q2222X2R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM151183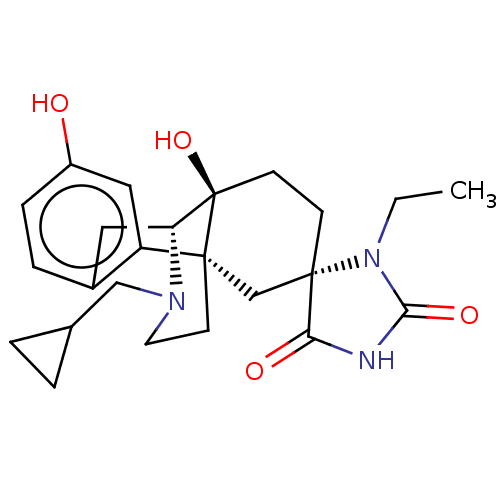 (US8987287, 49) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.470 | -53.2 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
Purdue Pharma L.P. US Patent | Assay Description Radioligand dose displacement assays used 0.4 nM [3H]-U69,593 (GE Healthcare, Piscataway, N.J.; 40 Ci/mmole) with 15 ug membrane protein (recombinant... | US Patent US8987287 (2015) BindingDB Entry DOI: 10.7270/Q24F1PFT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM151173 (US8987287, 3) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.600 | -52.6 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
Purdue Pharma L.P. US Patent | Assay Description Radioligand dose displacement assays used 0.4 nM [3H]-U69,593 (GE Healthcare, Piscataway, N.J.; 40 Ci/mmole) with 15 ug membrane protein (recombinant... | US Patent US8987287 (2015) BindingDB Entry DOI: 10.7270/Q24F1PFT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM364555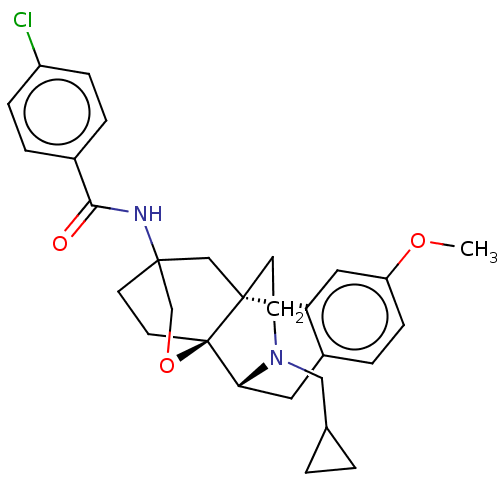 (N-((4bR,6S,8aS,9R)-11- (cyclopropylmethyl)- 3-meth...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GNF | Assay Description Radioligand dose displacement assays used 0.4 nM [3H]-U69,593 (GE Healthcare, Piscataway, N.J.; 40 Ci/mmole) with 15 μg membrane protein (recomb... | J Med Chem 50: 4255-9 (2007) BindingDB Entry DOI: 10.7270/Q2222X2R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM364558 (N-((4bR,6S,8aS,9R)-11- (cyclopropylmethyl)- 3-meth...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.660 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GNF | Assay Description Radioligand dose displacement assays used 0.4 nM [3H]-U69,593 (GE Healthcare, Piscataway, N.J.; 40 Ci/mmole) with 15 μg membrane protein (recomb... | J Med Chem 50: 4255-9 (2007) BindingDB Entry DOI: 10.7270/Q2222X2R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM364549 ((4bR,6S,8aS,9R)-6-amino-11- (cyclopropylmethyl)-5,...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.790 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GNF | Assay Description Radioligand dose-displacement binding assays for μ-opioid receptors used 0.3 nM [3H]-diprenorphine (Perkin Elmer, Shelton, Conn.), with 5 mg mem... | J Med Chem 50: 4255-9 (2007) BindingDB Entry DOI: 10.7270/Q2222X2R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily V member 1 (Homo sapiens (Human)) | BDBM50223336 (1-(1-(cyclopropylmethyl)-6-fluoro-1,2,3,4-tetrahyd...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Displacement of [3H]RTX from human TRPV1 expressed in HEK293 cells | Bioorg Med Chem Lett 17: 6160-3 (2007) Article DOI: 10.1016/j.bmcl.2007.09.036 BindingDB Entry DOI: 10.7270/Q28G8KFT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily V member 1 (Homo sapiens (Human)) | BDBM50147069 (1-(4-Chloro-3-trifluoromethyl-benzyl)-3-isoquinoli...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Binding affinity towards human vanilloid receptor subtype 1 expressed in HEK293 cell membrane using [3H]-RTX as radioligand. | Bioorg Med Chem Lett 14: 3053-6 (2004) Article DOI: 10.1016/j.bmcl.2004.04.038 BindingDB Entry DOI: 10.7270/Q2FB52D6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM151184 (US8987287, 63) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.860 | -56.1 | n/a | n/a | n/a | n/a | n/a | 7.4 | 50 |
Purdue Pharma L.P. US Patent | Assay Description mu-Opioid Receptor Binding Assay Procedures: Radioligand dose-displacement binding assays for mu-opioid receptors used 0.3 nM [3H]-diprenorphine (Per... | US Patent US8987287 (2015) BindingDB Entry DOI: 10.7270/Q24F1PFT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM364548 ((4bR,6S,8aS,9R)-N-(cyclohexylmethyl)-11- (cyclopro...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.930 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GNF | Assay Description Radioligand dose-displacement binding assays for μ-opioid receptors used 0.3 nM [3H]-diprenorphine (Perkin Elmer, Shelton, Conn.), with 5 mg mem... | J Med Chem 50: 4255-9 (2007) BindingDB Entry DOI: 10.7270/Q2222X2R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM151175 (US8987287, 5) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.940 | -51.5 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
Purdue Pharma L.P. US Patent | Assay Description Radioligand dose displacement assays used 0.4 nM [3H]-U69,593 (GE Healthcare, Piscataway, N.J.; 40 Ci/mmole) with 15 ug membrane protein (recombinant... | US Patent US8987287 (2015) BindingDB Entry DOI: 10.7270/Q24F1PFT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM364546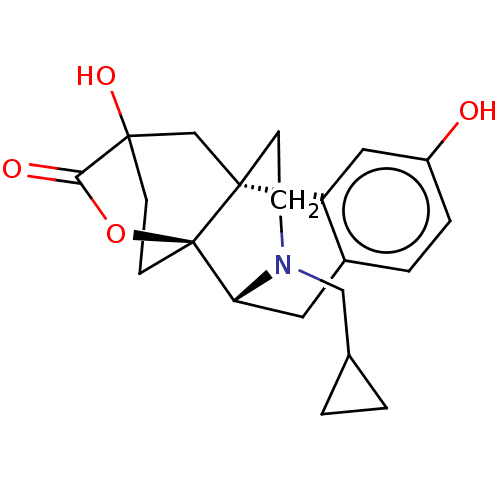 ((4bR,6S,8aS,9R)-11-(cyclopropylmethyl)- 3,6-dihydr...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.950 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GNF | Assay Description Radioligand dose displacement assays used 0.4 nM [3H]-U69,593 (GE Healthcare, Piscataway, N.J.; 40 Ci/mmole) with 15 μg membrane protein (recomb... | J Med Chem 50: 4255-9 (2007) BindingDB Entry DOI: 10.7270/Q2222X2R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily V member 1 (Homo sapiens (Human)) | BDBM50223313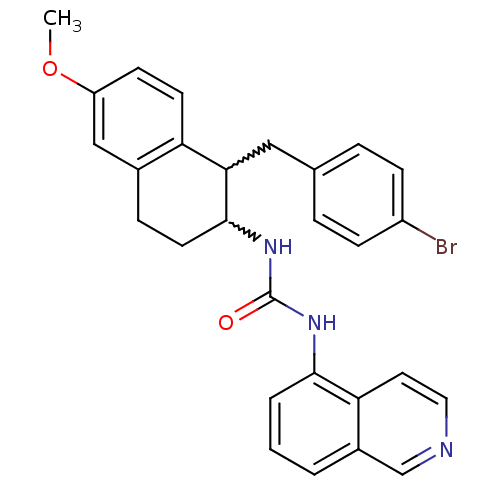 (1-(1-(4-bromobenzyl)-6-methoxy-1,2,3,4-tetrahydron...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Displacement of [3H]RTX from human TRPV1 expressed in HEK293 cells | Bioorg Med Chem Lett 17: 6160-3 (2007) Article DOI: 10.1016/j.bmcl.2007.09.036 BindingDB Entry DOI: 10.7270/Q28G8KFT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily V member 1 (Homo sapiens (Human)) | BDBM50223314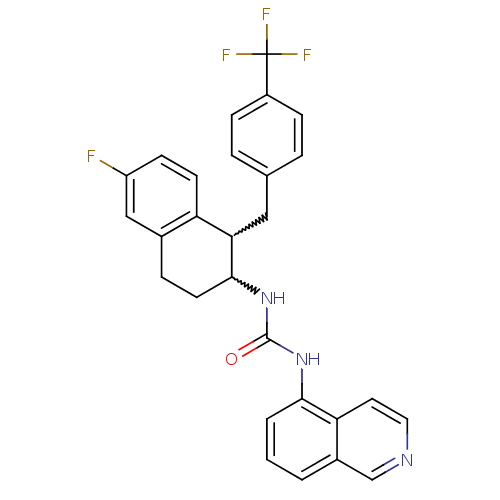 (1-(1-(4-(trifluoromethyl)benzyl)-6-fluoro-1,2,3,4-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Displacement of [3H]RTX from human TRPV1 expressed in HEK293 cells | Bioorg Med Chem Lett 17: 6160-3 (2007) Article DOI: 10.1016/j.bmcl.2007.09.036 BindingDB Entry DOI: 10.7270/Q28G8KFT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily V member 1 (Homo sapiens (Human)) | BDBM50147080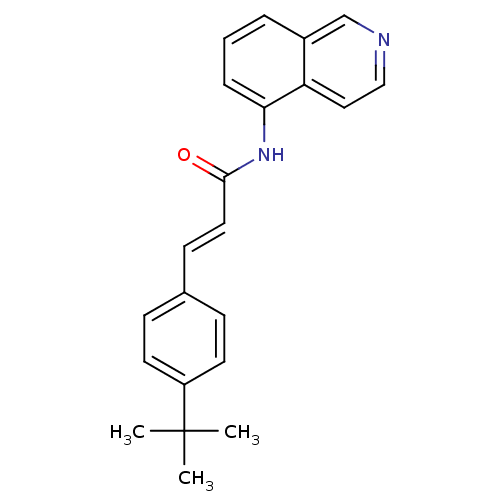 (3-(4-tert-Butyl-phenyl)-N-isoquinolin-5-yl-acrylam...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Antagonistic activity towards human vanilloid receptor subtype 1 expressed in HEK293 cell membrane, as inhibition of agonist-induced intracellular [C... | Bioorg Med Chem Lett 14: 3053-6 (2004) Article DOI: 10.1016/j.bmcl.2004.04.038 BindingDB Entry DOI: 10.7270/Q2FB52D6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily V member 1 (Homo sapiens (Human)) | BDBM50147067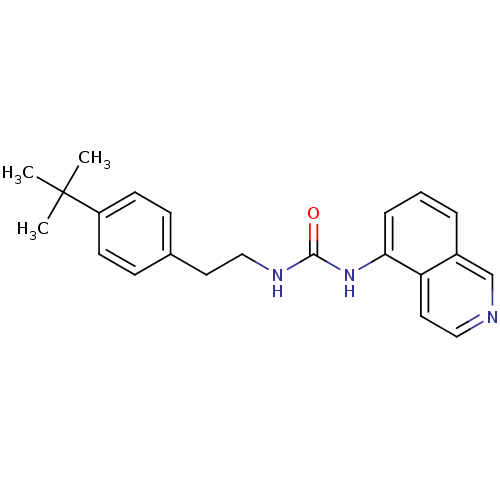 (1-[2-(4-tert-Butyl-phenyl)-ethyl]-3-isoquinolin-5-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Binding affinity towards human vanilloid receptor subtype 1 expressed in HEK293 cell membrane using [3H]-RTX as radioligand. | Bioorg Med Chem Lett 14: 3053-6 (2004) Article DOI: 10.1016/j.bmcl.2004.04.038 BindingDB Entry DOI: 10.7270/Q2FB52D6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily V member 1 (Homo sapiens (Human)) | BDBM50147063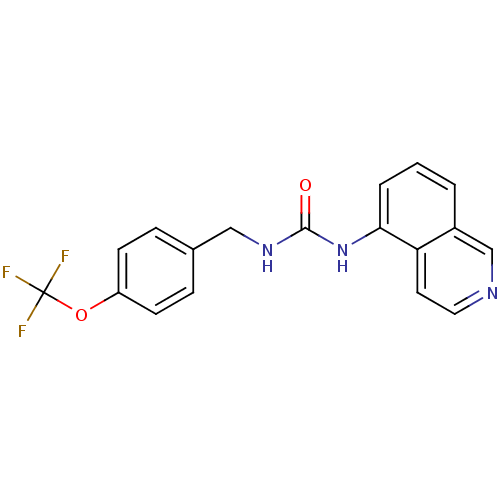 (1-Isoquinolin-5-yl-3-(4-trifluoromethoxy-benzyl)-u...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Binding affinity towards human vanilloid receptor subtype 1 expressed in HEK293 cell membrane using [3H]-RTX as radioligand. | Bioorg Med Chem Lett 14: 3053-6 (2004) Article DOI: 10.1016/j.bmcl.2004.04.038 BindingDB Entry DOI: 10.7270/Q2FB52D6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily V member 1 (Homo sapiens (Human)) | BDBM50147077 (1-(3,5-Bis-trifluoromethyl-phenyl)-3-isoquinolin-5...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Antagonistic activity towards human vanilloid receptor subtype 1 expressed in HEK293 cell membrane, as inhibition of agonist-induced intracellular [C... | Bioorg Med Chem Lett 14: 3053-6 (2004) Article DOI: 10.1016/j.bmcl.2004.04.038 BindingDB Entry DOI: 10.7270/Q2FB52D6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM364553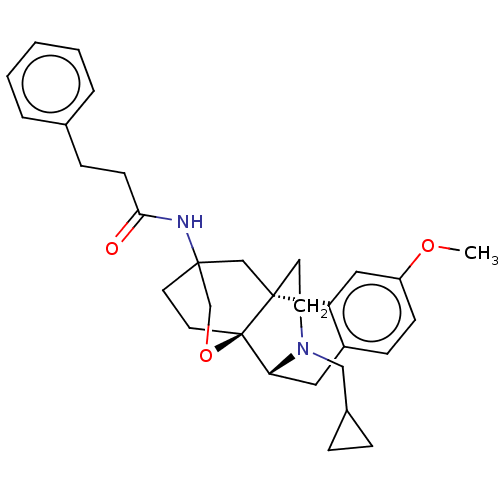 (N-((4bR,6S,8aS,9R)-11- (cyclopropylmethyl)- 3-meth...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 1.58 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GNF | Assay Description Radioligand dose displacement assays used 0.4 nM [3H]-U69,593 (GE Healthcare, Piscataway, N.J.; 40 Ci/mmole) with 15 μg membrane protein (recomb... | J Med Chem 50: 4255-9 (2007) BindingDB Entry DOI: 10.7270/Q2222X2R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily V member 1 (Homo sapiens (Human)) | BDBM50223328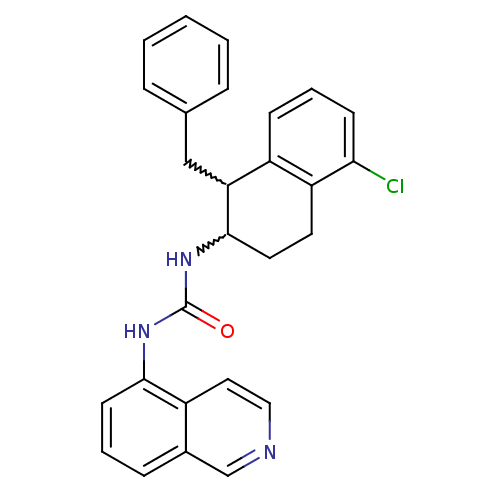 (1-(1-benzyl-5-chloro-1,2,3,4-tetrahydronaphthalen-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Displacement of [3H]RTX from human TRPV1 expressed in HEK293 cells | Bioorg Med Chem Lett 17: 6160-3 (2007) Article DOI: 10.1016/j.bmcl.2007.09.036 BindingDB Entry DOI: 10.7270/Q28G8KFT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily V member 1 (Homo sapiens (Human)) | BDBM50223330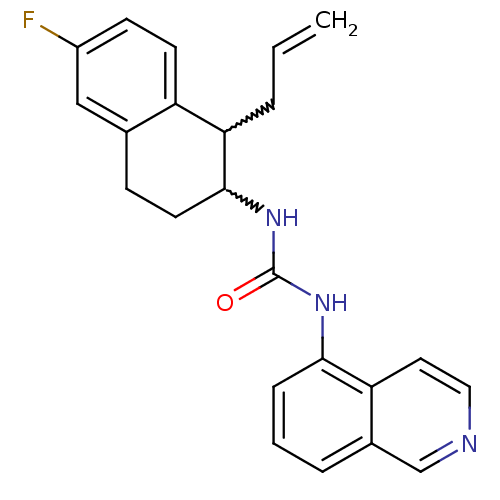 (1-(1-allyl-6-fluoro-1,2,3,4-tetrahydronaphthalen-2...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Displacement of [3H]RTX from human TRPV1 expressed in HEK293 cells | Bioorg Med Chem Lett 17: 6160-3 (2007) Article DOI: 10.1016/j.bmcl.2007.09.036 BindingDB Entry DOI: 10.7270/Q28G8KFT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily V member 1 (Homo sapiens (Human)) | BDBM50223331 (1-(1-benzyl-6-bromo-1,2,3,4-tetrahydronaphthalen-2...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Displacement of [3H]RTX from human TRPV1 expressed in HEK293 cells | Bioorg Med Chem Lett 17: 6160-3 (2007) Article DOI: 10.1016/j.bmcl.2007.09.036 BindingDB Entry DOI: 10.7270/Q28G8KFT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily V member 1 (Homo sapiens (Human)) | BDBM50223338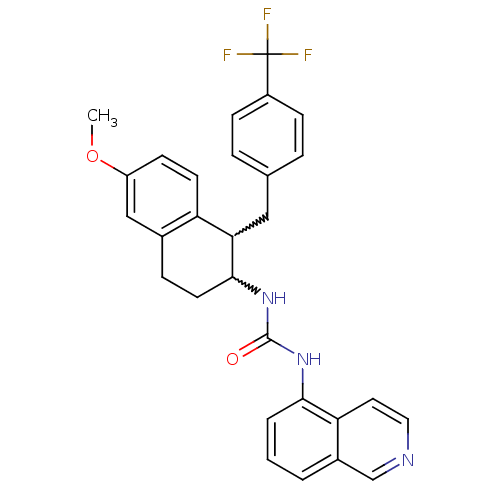 (1-(1-(4-(trifluoromethyl)benzyl)-6-methoxy-1,2,3,4...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Displacement of [3H]RTX from human TRPV1 expressed in HEK293 cells | Bioorg Med Chem Lett 17: 6160-3 (2007) Article DOI: 10.1016/j.bmcl.2007.09.036 BindingDB Entry DOI: 10.7270/Q28G8KFT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily V member 1 (Homo sapiens (Human)) | BDBM50223321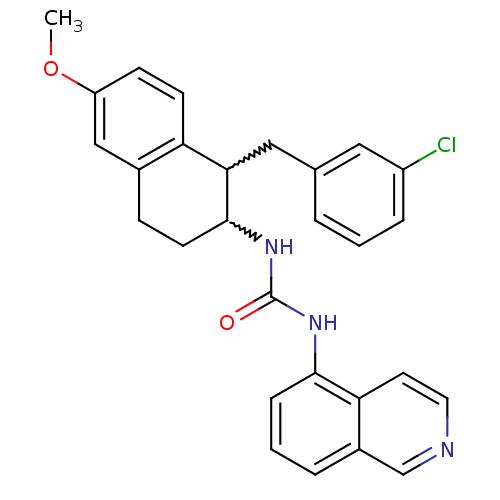 (1-(1-(3-chlorobenzyl)-6-methoxy-1,2,3,4-tetrahydro...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Displacement of [3H]RTX from human TRPV1 expressed in HEK293 cells | Bioorg Med Chem Lett 17: 6160-3 (2007) Article DOI: 10.1016/j.bmcl.2007.09.036 BindingDB Entry DOI: 10.7270/Q28G8KFT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM151183 (US8987287, 49) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 2.28 | -53.5 | n/a | n/a | n/a | n/a | n/a | 7.4 | 50 |
Purdue Pharma L.P. US Patent | Assay Description mu-Opioid Receptor Binding Assay Procedures: Radioligand dose-displacement binding assays for mu-opioid receptors used 0.3 nM [3H]-diprenorphine (Per... | US Patent US8987287 (2015) BindingDB Entry DOI: 10.7270/Q24F1PFT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM364560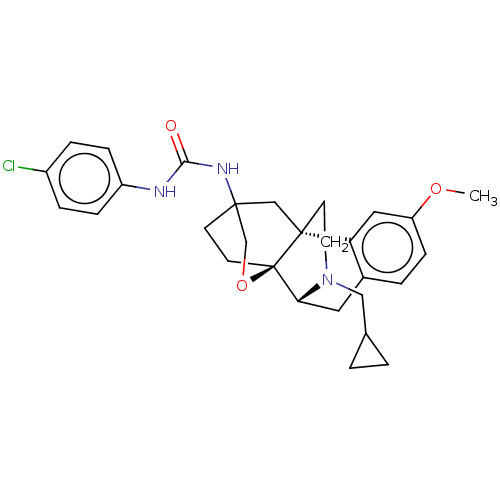 (1-Chlorophenyl-3-((4bR,6S,8aS,9R)-11- (cyclopropyl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 2.56 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GNF | Assay Description Radioligand dose displacement assays used 0.4 nM [3H]-U69,593 (GE Healthcare, Piscataway, N.J.; 40 Ci/mmole) with 15 μg membrane protein (recomb... | J Med Chem 50: 4255-9 (2007) BindingDB Entry DOI: 10.7270/Q2222X2R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily V member 1 (Homo sapiens (Human)) | BDBM50223333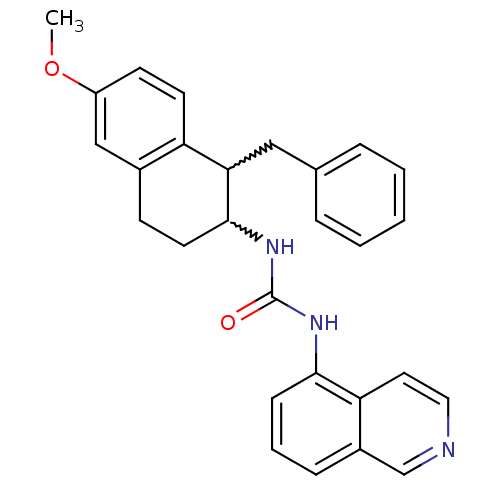 (1-(1-benzyl-6-methoxy-1,2,3,4-tetrahydronaphthalen...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Displacement of [3H]RTX from human TRPV1 expressed in HEK293 cells | Bioorg Med Chem Lett 17: 6160-3 (2007) Article DOI: 10.1016/j.bmcl.2007.09.036 BindingDB Entry DOI: 10.7270/Q28G8KFT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM364560 (1-Chlorophenyl-3-((4bR,6S,8aS,9R)-11- (cyclopropyl...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 3.51 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GNF | Assay Description Radioligand dose-displacement binding assays for μ-opioid receptors used 0.3 nM [3H]-diprenorphine (Perkin Elmer, Shelton, Conn.), with 5 mg mem... | J Med Chem 50: 4255-9 (2007) BindingDB Entry DOI: 10.7270/Q2222X2R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM151175 (US8987287, 5) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 3.55 | -52.3 | n/a | n/a | n/a | n/a | n/a | 7.4 | 50 |
Purdue Pharma L.P. US Patent | Assay Description mu-Opioid Receptor Binding Assay Procedures: Radioligand dose-displacement binding assays for mu-opioid receptors used 0.3 nM [3H]-diprenorphine (Per... | US Patent US8987287 (2015) BindingDB Entry DOI: 10.7270/Q24F1PFT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| B2 bradykinin receptor (Homo sapiens (Human)) | BDBM50126226 (3-[5-(4-Cyano-benzoyl)-1-methyl-1H-pyrrol-2-yl]-N-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Binding affinity towards human bradykinin receptor B2 | Bioorg Med Chem Lett 13: 1341-4 (2003) BindingDB Entry DOI: 10.7270/Q2HM57T1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily V member 1 (Homo sapiens (Human)) | BDBM50223335 (1-(1-benzyl-1,2,3,4-tetrahydronaphthalen-2-yl)-3-(...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Displacement of [3H]RTX from human TRPV1 expressed in HEK293 cells | Bioorg Med Chem Lett 17: 6160-3 (2007) Article DOI: 10.1016/j.bmcl.2007.09.036 BindingDB Entry DOI: 10.7270/Q28G8KFT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily V member 1 (Homo sapiens (Human)) | BDBM50223340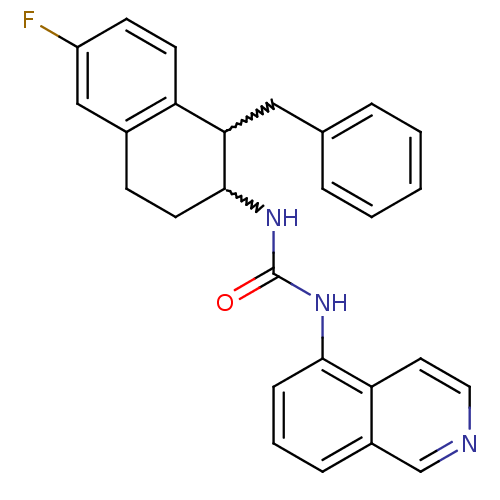 (1-(1-benzyl-6-fluoro-1,2,3,4-tetrahydronaphthalen-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Displacement of [3H]RTX from human TRPV1 expressed in HEK293 cells | Bioorg Med Chem Lett 17: 6160-3 (2007) Article DOI: 10.1016/j.bmcl.2007.09.036 BindingDB Entry DOI: 10.7270/Q28G8KFT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily V member 1 (Homo sapiens (Human)) | BDBM50223323 (1-(isoquinolin-5-yl)-3-(3-phenyl-2-(4-(trifluorome...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Displacement of [3H]RTX from human TRPV1 expressed in HEK293 cells | Bioorg Med Chem Lett 17: 6160-3 (2007) Article DOI: 10.1016/j.bmcl.2007.09.036 BindingDB Entry DOI: 10.7270/Q28G8KFT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily V member 1 (Homo sapiens (Human)) | BDBM50223315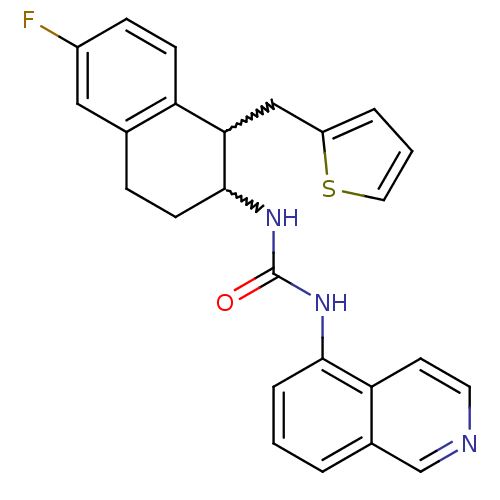 (1-(6-fluoro-1-(thiophen-2-ylmethyl)-1,2,3,4-tetrah...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Displacement of [3H]RTX from human TRPV1 expressed in HEK293 cells | Bioorg Med Chem Lett 17: 6160-3 (2007) Article DOI: 10.1016/j.bmcl.2007.09.036 BindingDB Entry DOI: 10.7270/Q28G8KFT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily V member 1 (Homo sapiens (Human)) | BDBM50147060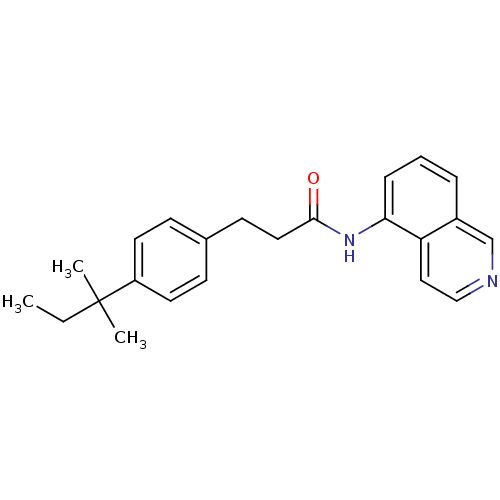 (3-[4-(1,1-Dimethyl-propyl)-phenyl]-N-isoquinolin-5...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Binding affinity towards human vanilloid receptor subtype 1 expressed in HEK293 cell membrane using [3H]-RTX as radioligand. | Bioorg Med Chem Lett 14: 3053-6 (2004) Article DOI: 10.1016/j.bmcl.2004.04.038 BindingDB Entry DOI: 10.7270/Q2FB52D6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 1917 total ) | Next | Last >> |