 Found 599 hits with Last Name = 'yu' and Initial = 'xy'
Found 599 hits with Last Name = 'yu' and Initial = 'xy' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Cathepsin K
(Homo sapiens (Human)) | BDBM50517806

(CHEMBL4541720)Show SMILES CC(C)CC(NC(=O)c1ccc(cc1)-c1ccc(cc1)S(C)(=O)=O)C(=O)N(C)N(C)C#N Show InChI InChI=1S/C23H28N4O4S/c1-16(2)14-21(23(29)27(4)26(3)15-24)25-22(28)19-8-6-17(7-9-19)18-10-12-20(13-11-18)32(5,30)31/h6-13,16,21H,14H2,1-5H3,(H,25,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Jilin University
Curated by ChEMBL
| Assay Description
Inhibition of recombinant His-tagged human cathepsin K expressed in Escherichia coli |
Bioorg Med Chem 27: 1034-1042 (2019)
Article DOI: 10.1016/j.bmc.2019.02.003
BindingDB Entry DOI: 10.7270/Q2M048TS |
More data for this
Ligand-Target Pair | |
Bifunctional glutamate/proline--tRNA ligase
(Homo sapiens (Human)) | BDBM50097096

(2-(4-Bromo-phenyl)-6-chloro-8-methyl-quinoline-4-c...)Show InChI InChI=1S/C17H11BrClNO2/c1-9-6-12(19)7-13-14(17(21)22)8-15(20-16(9)13)10-2-4-11(18)5-3-10/h2-8H,1H3,(H,21,22) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 5.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cubist Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Binding affinity towards 5-hydroxytryptamine 3 receptor |
Bioorg Med Chem Lett 11: 541-4 (2001)
BindingDB Entry DOI: 10.7270/Q2Q23ZH8 |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Homo sapiens (Human)) | BDBM50517805

(CHEMBL4454648)Show SMILES CC(C)CC(NC(=O)c1cccc(c1)-c1ccc(cc1)S(C)(=O)=O)C(=O)N(C)N(C)C#N Show InChI InChI=1S/C23H28N4O4S/c1-16(2)13-21(23(29)27(4)26(3)15-24)25-22(28)19-8-6-7-18(14-19)17-9-11-20(12-10-17)32(5,30)31/h6-12,14,16,21H,13H2,1-5H3,(H,25,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 7.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Jilin University
Curated by ChEMBL
| Assay Description
Inhibition of recombinant His-tagged human cathepsin K expressed in Escherichia coli |
Bioorg Med Chem 27: 1034-1042 (2019)
Article DOI: 10.1016/j.bmc.2019.02.003
BindingDB Entry DOI: 10.7270/Q2M048TS |
More data for this
Ligand-Target Pair | |
Bifunctional glutamate/proline--tRNA ligase
(Homo sapiens (Human)) | BDBM50097096

(2-(4-Bromo-phenyl)-6-chloro-8-methyl-quinoline-4-c...)Show InChI InChI=1S/C17H11BrClNO2/c1-9-6-12(19)7-13-14(17(21)22)8-15(20-16(9)13)10-2-4-11(18)5-3-10/h2-8H,1H3,(H,21,22) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 15 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cubist Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Binding affinity towards 5-hydroxytryptamine 3 receptor |
Bioorg Med Chem Lett 11: 541-4 (2001)
BindingDB Entry DOI: 10.7270/Q2Q23ZH8 |
More data for this
Ligand-Target Pair | |
Procathepsin L
(Homo sapiens (Human)) | BDBM50517806

(CHEMBL4541720)Show SMILES CC(C)CC(NC(=O)c1ccc(cc1)-c1ccc(cc1)S(C)(=O)=O)C(=O)N(C)N(C)C#N Show InChI InChI=1S/C23H28N4O4S/c1-16(2)14-21(23(29)27(4)26(3)15-24)25-22(28)19-8-6-17(7-9-19)18-10-12-20(13-11-18)32(5,30)31/h6-13,16,21H,14H2,1-5H3,(H,25,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 195 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Jilin University
Curated by ChEMBL
| Assay Description
Inhibition of human liver cathepsin L |
Bioorg Med Chem 27: 1034-1042 (2019)
Article DOI: 10.1016/j.bmc.2019.02.003
BindingDB Entry DOI: 10.7270/Q2M048TS |
More data for this
Ligand-Target Pair | |
Cathepsin S
(Homo sapiens (Human)) | BDBM50517806

(CHEMBL4541720)Show SMILES CC(C)CC(NC(=O)c1ccc(cc1)-c1ccc(cc1)S(C)(=O)=O)C(=O)N(C)N(C)C#N Show InChI InChI=1S/C23H28N4O4S/c1-16(2)14-21(23(29)27(4)26(3)15-24)25-22(28)19-8-6-17(7-9-19)18-10-12-20(13-11-18)32(5,30)31/h6-13,16,21H,14H2,1-5H3,(H,25,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 239 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Jilin University
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human cathepsin S expressed in Escherichia coli |
Bioorg Med Chem 27: 1034-1042 (2019)
Article DOI: 10.1016/j.bmc.2019.02.003
BindingDB Entry DOI: 10.7270/Q2M048TS |
More data for this
Ligand-Target Pair | |
Procathepsin L
(Homo sapiens (Human)) | BDBM50517805

(CHEMBL4454648)Show SMILES CC(C)CC(NC(=O)c1cccc(c1)-c1ccc(cc1)S(C)(=O)=O)C(=O)N(C)N(C)C#N Show InChI InChI=1S/C23H28N4O4S/c1-16(2)13-21(23(29)27(4)26(3)15-24)25-22(28)19-8-6-7-18(14-19)17-9-11-20(12-10-17)32(5,30)31/h6-12,14,16,21H,13H2,1-5H3,(H,25,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 406 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Jilin University
Curated by ChEMBL
| Assay Description
Inhibition of human liver cathepsin L |
Bioorg Med Chem 27: 1034-1042 (2019)
Article DOI: 10.1016/j.bmc.2019.02.003
BindingDB Entry DOI: 10.7270/Q2M048TS |
More data for this
Ligand-Target Pair | |
Cathepsin S
(Homo sapiens (Human)) | BDBM50517805

(CHEMBL4454648)Show SMILES CC(C)CC(NC(=O)c1cccc(c1)-c1ccc(cc1)S(C)(=O)=O)C(=O)N(C)N(C)C#N Show InChI InChI=1S/C23H28N4O4S/c1-16(2)13-21(23(29)27(4)26(3)15-24)25-22(28)19-8-6-7-18(14-19)17-9-11-20(12-10-17)32(5,30)31/h6-12,14,16,21H,13H2,1-5H3,(H,25,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 735 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Jilin University
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human cathepsin S expressed in Escherichia coli |
Bioorg Med Chem 27: 1034-1042 (2019)
Article DOI: 10.1016/j.bmc.2019.02.003
BindingDB Entry DOI: 10.7270/Q2M048TS |
More data for this
Ligand-Target Pair | |
Cathepsin B
(Homo sapiens (Human)) | BDBM50517806

(CHEMBL4541720)Show SMILES CC(C)CC(NC(=O)c1ccc(cc1)-c1ccc(cc1)S(C)(=O)=O)C(=O)N(C)N(C)C#N Show InChI InChI=1S/C23H28N4O4S/c1-16(2)14-21(23(29)27(4)26(3)15-24)25-22(28)19-8-6-17(7-9-19)18-10-12-20(13-11-18)32(5,30)31/h6-13,16,21H,14H2,1-5H3,(H,25,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 909 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Jilin University
Curated by ChEMBL
| Assay Description
Inhibition of human liver cathepsin B |
Bioorg Med Chem 27: 1034-1042 (2019)
Article DOI: 10.1016/j.bmc.2019.02.003
BindingDB Entry DOI: 10.7270/Q2M048TS |
More data for this
Ligand-Target Pair | |
Cathepsin B
(Homo sapiens (Human)) | BDBM50517805

(CHEMBL4454648)Show SMILES CC(C)CC(NC(=O)c1cccc(c1)-c1ccc(cc1)S(C)(=O)=O)C(=O)N(C)N(C)C#N Show InChI InChI=1S/C23H28N4O4S/c1-16(2)13-21(23(29)27(4)26(3)15-24)25-22(28)19-8-6-7-18(14-19)17-9-11-20(12-10-17)32(5,30)31/h6-12,14,16,21H,13H2,1-5H3,(H,25,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 3.35E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Jilin University
Curated by ChEMBL
| Assay Description
Inhibition of human liver cathepsin B |
Bioorg Med Chem 27: 1034-1042 (2019)
Article DOI: 10.1016/j.bmc.2019.02.003
BindingDB Entry DOI: 10.7270/Q2M048TS |
More data for this
Ligand-Target Pair | |
Leucine--tRNA ligase, cytoplasmic
(Homo sapiens (Human)) | BDBM50075067

(((S)-2-Amino-4-methyl-pentanoyl)-sulfamic acid (2R...)Show SMILES CC(C)C[C@H](N)C(=O)NS(=O)(=O)OC[C@H]1O[C@H]([C@H](O)[C@@H]1O)c1nc(cs1)-c1ccc(Oc2ccccc2)cc1 Show InChI InChI=1S/C26H31N3O8S2/c1-15(2)12-19(27)25(32)29-39(33,34)35-13-21-22(30)23(31)24(37-21)26-28-20(14-38-26)16-8-10-18(11-9-16)36-17-6-4-3-5-7-17/h3-11,14-15,19,21-24,30-31H,12-13,27H2,1-2H3,(H,29,32)/t19-,21+,22+,23+,24+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Cubist Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Compound tested for the inhibition of E. coli Leucyl-tRNA synthetase |
Bioorg Med Chem Lett 9: 375-80 (1999)
BindingDB Entry DOI: 10.7270/Q2M32TX1 |
More data for this
Ligand-Target Pair | |
Leucine--tRNA ligase, cytoplasmic
(Homo sapiens (Human)) | BDBM50075069

(((S)-2-Amino-4-methyl-pentanoyl)-sulfamic acid (2R...)Show SMILES CC(C)C[C@H](N)C(=O)NS(=O)(=O)OC[C@H]1O[C@H]([C@H](O)[C@@H]1O)c1nc(CCc2ccccc2)cs1 Show InChI InChI=1S/C22H31N3O7S2/c1-13(2)10-16(23)21(28)25-34(29,30)31-11-17-18(26)19(27)20(32-17)22-24-15(12-33-22)9-8-14-6-4-3-5-7-14/h3-7,12-13,16-20,26-27H,8-11,23H2,1-2H3,(H,25,28)/t16-,17+,18+,19+,20+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | <2 | n/a | n/a | n/a | n/a | n/a | n/a |
Cubist Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Compound tested for the inhibition of E. coli Leucyl-tRNA synthetase |
Bioorg Med Chem Lett 9: 375-80 (1999)
BindingDB Entry DOI: 10.7270/Q2M32TX1 |
More data for this
Ligand-Target Pair | |
Phenylalanine--tRNA ligase alpha subunit
(Streptococcus pyogenes serotype M18) | BDBM50140706

(5-(3,4-dichlorophenyl)-3-phenyl-(3R,3aR,6aS)-spiro...)Show SMILES Oc1c2C(OC3(C(=O)c4ccccc4C3=O)c2c(O)n1-c1ccc(Cl)c(Cl)c1)c1ccccc1 Show InChI InChI=1S/C26H15Cl2NO5/c27-17-11-10-14(12-18(17)28)29-24(32)19-20(25(29)33)26(34-21(19)13-6-2-1-3-7-13)22(30)15-8-4-5-9-16(15)23(26)31/h1-12,21,32-33H | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Cubist Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Inhibitory activity against human phenylalanyl-tRNA synthetase was determined |
Bioorg Med Chem Lett 14: 1339-42 (2004)
Article DOI: 10.1016/j.bmcl.2003.11.081
BindingDB Entry DOI: 10.7270/Q2PR7VDS |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Homo sapiens (Human)) | BDBM50517806

(CHEMBL4541720)Show SMILES CC(C)CC(NC(=O)c1ccc(cc1)-c1ccc(cc1)S(C)(=O)=O)C(=O)N(C)N(C)C#N Show InChI InChI=1S/C23H28N4O4S/c1-16(2)14-21(23(29)27(4)26(3)15-24)25-22(28)19-8-6-17(7-9-19)18-10-12-20(13-11-18)32(5,30)31/h6-13,16,21H,14H2,1-5H3,(H,25,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Jilin University
Curated by ChEMBL
| Assay Description
Inhibition of recombinant His-tagged human cathepsin K expressed in Escherichia coli |
Bioorg Med Chem 27: 1034-1042 (2019)
Article DOI: 10.1016/j.bmc.2019.02.003
BindingDB Entry DOI: 10.7270/Q2M048TS |
More data for this
Ligand-Target Pair | |
Proline--tRNA ligase, cytoplasmic
(Candida albicans) | BDBM50097096

(2-(4-Bromo-phenyl)-6-chloro-8-methyl-quinoline-4-c...)Show InChI InChI=1S/C17H11BrClNO2/c1-9-6-12(19)7-13-14(17(21)22)8-15(20-16(9)13)10-2-4-11(18)5-3-10/h2-8H,1H3,(H,21,22) | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Cubist Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Aminoacylation activity against Candida albicans prolyl-tRNA synthetase |
Bioorg Med Chem Lett 11: 541-4 (2001)
BindingDB Entry DOI: 10.7270/Q2Q23ZH8 |
More data for this
Ligand-Target Pair | |
Phenylalanine--tRNA ligase alpha subunit
(Streptococcus pyogenes serotype M18) | BDBM50140706

(5-(3,4-dichlorophenyl)-3-phenyl-(3R,3aR,6aS)-spiro...)Show SMILES Oc1c2C(OC3(C(=O)c4ccccc4C3=O)c2c(O)n1-c1ccc(Cl)c(Cl)c1)c1ccccc1 Show InChI InChI=1S/C26H15Cl2NO5/c27-17-11-10-14(12-18(17)28)29-24(32)19-20(25(29)33)26(34-21(19)13-6-2-1-3-7-13)22(30)15-8-4-5-9-16(15)23(26)31/h1-12,21,32-33H | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Cubist Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Inhibitory activity against phenylalanyl-tRNA synthetase from Enterococcus faecalis |
Bioorg Med Chem Lett 14: 1339-42 (2004)
Article DOI: 10.1016/j.bmcl.2003.11.081
BindingDB Entry DOI: 10.7270/Q2PR7VDS |
More data for this
Ligand-Target Pair | |
Leucine--tRNA ligase, cytoplasmic
(Homo sapiens (Human)) | BDBM50075055
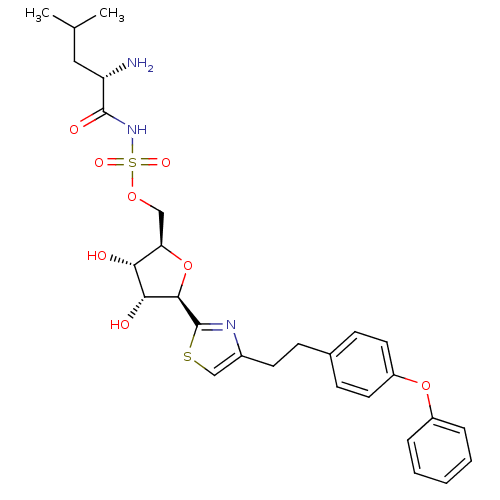
(((S)-2-Amino-4-methyl-pentanoyl)-sulfamic acid (2R...)Show SMILES CC(C)C[C@H](N)C(=O)NS(=O)(=O)OC[C@H]1O[C@H]([C@H](O)[C@@H]1O)c1nc(CCc2ccc(Oc3ccccc3)cc2)cs1 Show InChI InChI=1S/C28H35N3O8S2/c1-17(2)14-22(29)27(34)31-41(35,36)37-15-23-24(32)25(33)26(39-23)28-30-19(16-40-28)11-8-18-9-12-21(13-10-18)38-20-6-4-3-5-7-20/h3-7,9-10,12-13,16-17,22-26,32-33H,8,11,14-15,29H2,1-2H3,(H,31,34)/t22-,23+,24+,25+,26+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Cubist Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Compound tested for the inhibition of human isoleucyl-tRNA synthetase |
Bioorg Med Chem Lett 9: 375-80 (1999)
BindingDB Entry DOI: 10.7270/Q2M32TX1 |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Homo sapiens (Human)) | BDBM50517805

(CHEMBL4454648)Show SMILES CC(C)CC(NC(=O)c1cccc(c1)-c1ccc(cc1)S(C)(=O)=O)C(=O)N(C)N(C)C#N Show InChI InChI=1S/C23H28N4O4S/c1-16(2)13-21(23(29)27(4)26(3)15-24)25-22(28)19-8-6-7-18(14-19)17-9-11-20(12-10-17)32(5,30)31/h6-12,14,16,21H,13H2,1-5H3,(H,25,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Jilin University
Curated by ChEMBL
| Assay Description
Inhibition of recombinant His-tagged human cathepsin K expressed in Escherichia coli |
Bioorg Med Chem 27: 1034-1042 (2019)
Article DOI: 10.1016/j.bmc.2019.02.003
BindingDB Entry DOI: 10.7270/Q2M048TS |
More data for this
Ligand-Target Pair | |
Isoleucine--tRNA ligase, cytoplasmic
(Homo sapiens (Human)) | BDBM50075058

(((S)-2-Amino-3-methyl-pentanoyl)-sulfamic acid (2R...)Show SMILES CCC(C)[C@H](N)C(=O)NS(=O)(=O)OC[C@H]1O[C@H]([C@H](O)[C@@H]1O)c1nc(cs1)-c1cccc(OC)c1 Show InChI InChI=1S/C21H29N3O8S2/c1-4-11(2)16(22)20(27)24-34(28,29)31-9-15-17(25)18(26)19(32-15)21-23-14(10-33-21)12-6-5-7-13(8-12)30-3/h5-8,10-11,15-19,25-26H,4,9,22H2,1-3H3,(H,24,27)/t11?,15-,16+,17-,18-,19-/m1/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Cubist Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Compound tested for the inhibition of human isoleucyl-tRNA synthetase |
Bioorg Med Chem Lett 9: 375-80 (1999)
BindingDB Entry DOI: 10.7270/Q2M32TX1 |
More data for this
Ligand-Target Pair | |
Proline--tRNA ligase, cytoplasmic
(Candida albicans) | BDBM50097082

(2-(4-Bromo-phenyl)-6-iodo-quinoline-4-carboxylic a...)Show InChI InChI=1S/C16H9BrINO2/c17-10-3-1-9(2-4-10)15-8-13(16(20)21)12-7-11(18)5-6-14(12)19-15/h1-8H,(H,20,21) | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
Cubist Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Aminoacylation activity against Candida albicans prolyl-tRNA synthetase |
Bioorg Med Chem Lett 11: 541-4 (2001)
BindingDB Entry DOI: 10.7270/Q2Q23ZH8 |
More data for this
Ligand-Target Pair | |
Proline--tRNA ligase, cytoplasmic
(Candida albicans) | BDBM50097095

(2-(4-Bromo-phenyl)-6-chloro-8-methyl-quinoline-4-c...)Show SMILES Cc1cc(Cl)cc2c(cc(nc12)-c1ccc(Br)cc1)C(=O)OCc1ccc(Cl)c(Cl)c1 Show InChI InChI=1S/C24H15BrCl3NO2/c1-13-8-17(26)10-18-19(24(30)31-12-14-2-7-20(27)21(28)9-14)11-22(29-23(13)18)15-3-5-16(25)6-4-15/h2-11H,12H2,1H3 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Cubist Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Aminoacylation activity against Candida albicans prolyl-tRNA synthetase |
Bioorg Med Chem Lett 11: 541-4 (2001)
BindingDB Entry DOI: 10.7270/Q2Q23ZH8 |
More data for this
Ligand-Target Pair | |
Isoleucine--tRNA ligase, cytoplasmic
(Homo sapiens (Human)) | BDBM50075061
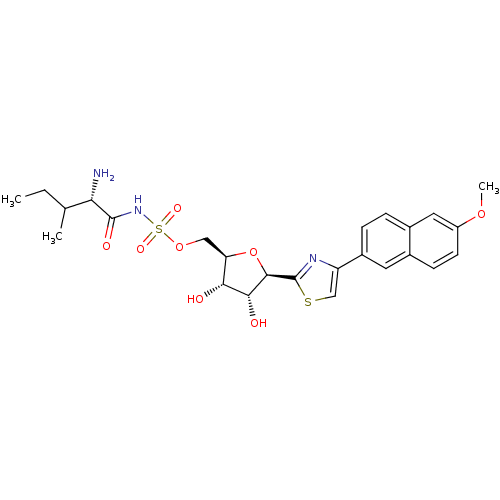
(((S)-2-Amino-3-methyl-pentanoyl)-sulfamic acid (2R...)Show SMILES CCC(C)[C@H](N)C(=O)NS(=O)(=O)OC[C@H]1O[C@H]([C@H](O)[C@@H]1O)c1nc(cs1)-c1ccc2cc(OC)ccc2c1 Show InChI InChI=1S/C25H31N3O8S2/c1-4-13(2)20(26)24(31)28-38(32,33)35-11-19-21(29)22(30)23(36-19)25-27-18(12-37-25)16-6-5-15-10-17(34-3)8-7-14(15)9-16/h5-10,12-13,19-23,29-30H,4,11,26H2,1-3H3,(H,28,31)/t13?,19-,20+,21-,22-,23-/m1/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Cubist Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of E. coli isoleucyl-tRNA synthetase |
Bioorg Med Chem Lett 9: 375-80 (1999)
BindingDB Entry DOI: 10.7270/Q2M32TX1 |
More data for this
Ligand-Target Pair | |
Proline--tRNA ligase, cytoplasmic
(Candida albicans) | BDBM50097105
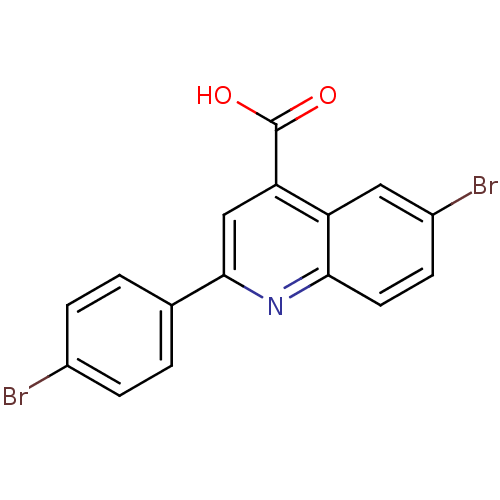
(6-Bromo-2-(4-bromo-phenyl)-quinoline-4-carboxylic ...)Show InChI InChI=1S/C16H9Br2NO2/c17-10-3-1-9(2-4-10)15-8-13(16(20)21)12-7-11(18)5-6-14(12)19-15/h1-8H,(H,20,21) | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a | n/a |
Cubist Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Aminoacylation activity against Candida albicans prolyl-tRNA synthetase |
Bioorg Med Chem Lett 11: 541-4 (2001)
BindingDB Entry DOI: 10.7270/Q2Q23ZH8 |
More data for this
Ligand-Target Pair | |
Proline--tRNA ligase, cytoplasmic
(Candida albicans) | BDBM50097106

(2-(4-Bromo-phenyl)-8-chloro-6-methyl-quinoline-4-c...)Show InChI InChI=1S/C17H11BrClNO2/c1-9-6-12-13(17(21)22)8-15(20-16(12)14(19)7-9)10-2-4-11(18)5-3-10/h2-8H,1H3,(H,21,22) | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a | n/a |
Cubist Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Aminoacylation activity against Candida albicans prolyl-tRNA synthetase |
Bioorg Med Chem Lett 11: 541-4 (2001)
BindingDB Entry DOI: 10.7270/Q2Q23ZH8 |
More data for this
Ligand-Target Pair | |
Proline--tRNA ligase, cytoplasmic
(Candida albicans) | BDBM50097090
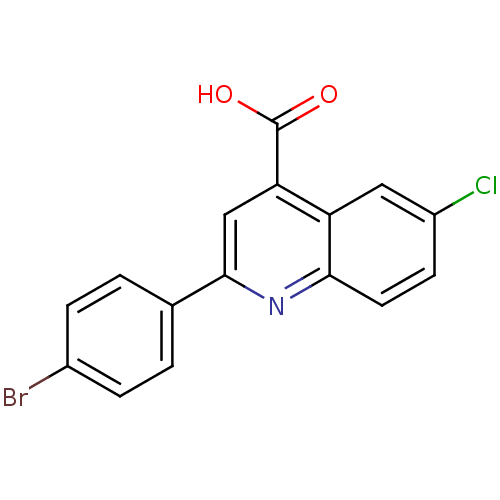
(2-(4-Bromo-phenyl)-6-chloro-quinoline-4-carboxylic...)Show InChI InChI=1S/C16H9BrClNO2/c17-10-3-1-9(2-4-10)15-8-13(16(20)21)12-7-11(18)5-6-14(12)19-15/h1-8H,(H,20,21) | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 26 | n/a | n/a | n/a | n/a | n/a | n/a |
Cubist Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Aminoacylation activity against Candida albicans prolyl-tRNA synthetase |
Bioorg Med Chem Lett 11: 541-4 (2001)
BindingDB Entry DOI: 10.7270/Q2Q23ZH8 |
More data for this
Ligand-Target Pair | |
Proline--tRNA ligase, cytoplasmic
(Candida albicans) | BDBM50097086
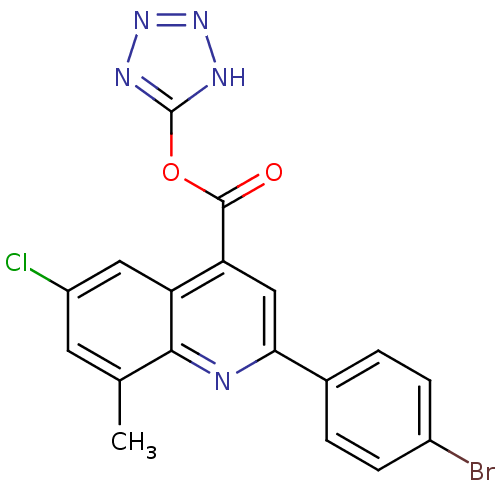
(2-(4-Bromo-phenyl)-6-chloro-8-methyl-quinoline-4-c...)Show SMILES Cc1cc(Cl)cc2c(cc(nc12)-c1ccc(Br)cc1)C(=O)Oc1nnn[nH]1 Show InChI InChI=1S/C18H11BrClN5O2/c1-9-6-12(20)7-13-14(17(26)27-18-22-24-25-23-18)8-15(21-16(9)13)10-2-4-11(19)5-3-10/h2-8H,1H3,(H,22,23,24,25) | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
Cubist Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Aminoacylation activity against Candida albicans prolyl-tRNA synthetase |
Bioorg Med Chem Lett 11: 541-4 (2001)
BindingDB Entry DOI: 10.7270/Q2Q23ZH8 |
More data for this
Ligand-Target Pair | |
Isoleucine--tRNA ligase, cytoplasmic
(Homo sapiens (Human)) | BDBM50075056

(((S)-2-Amino-3-methyl-pentanoyl)-sulfamic acid (2R...)Show SMILES CCC(C)[C@H](N)C(=O)NS(=O)(=O)OC[C@H]1O[C@H]([C@H](O)[C@@H]1O)c1nc(CCc2ccccc2)cs1 Show InChI InChI=1S/C22H31N3O7S2/c1-3-13(2)17(23)21(28)25-34(29,30)31-11-16-18(26)19(27)20(32-16)22-24-15(12-33-22)10-9-14-7-5-4-6-8-14/h4-8,12-13,16-20,26-27H,3,9-11,23H2,1-2H3,(H,25,28)/t13?,16-,17+,18-,19-,20-/m1/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
Cubist Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of E. coli isoleucyl-tRNA synthetase |
Bioorg Med Chem Lett 9: 375-80 (1999)
BindingDB Entry DOI: 10.7270/Q2M32TX1 |
More data for this
Ligand-Target Pair | |
Isoleucine--tRNA ligase, cytoplasmic
(Homo sapiens (Human)) | BDBM50075059

(((S)-2-Amino-3-methyl-pentanoyl)-sulfamic acid (2R...)Show SMILES CCC(C)[C@H](N)C(=O)NS(=O)(=O)OC[C@H]1O[C@H]([C@H](O)[C@@H]1O)c1nc(cs1)-c1ccccc1OC Show InChI InChI=1S/C21H29N3O8S2/c1-4-11(2)16(22)20(27)24-34(28,29)31-9-15-17(25)18(26)19(32-15)21-23-13(10-33-21)12-7-5-6-8-14(12)30-3/h5-8,10-11,15-19,25-26H,4,9,22H2,1-3H3,(H,24,27)/t11?,15-,16+,17-,18-,19-/m1/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
Cubist Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of E. coli isoleucyl-tRNA synthetase |
Bioorg Med Chem Lett 9: 375-80 (1999)
BindingDB Entry DOI: 10.7270/Q2M32TX1 |
More data for this
Ligand-Target Pair | |
Isoleucine--tRNA ligase, cytoplasmic
(Homo sapiens (Human)) | BDBM50075058

(((S)-2-Amino-3-methyl-pentanoyl)-sulfamic acid (2R...)Show SMILES CCC(C)[C@H](N)C(=O)NS(=O)(=O)OC[C@H]1O[C@H]([C@H](O)[C@@H]1O)c1nc(cs1)-c1cccc(OC)c1 Show InChI InChI=1S/C21H29N3O8S2/c1-4-11(2)16(22)20(27)24-34(28,29)31-9-15-17(25)18(26)19(32-15)21-23-14(10-33-21)12-6-5-7-13(8-12)30-3/h5-8,10-11,15-19,25-26H,4,9,22H2,1-3H3,(H,24,27)/t11?,15-,16+,17-,18-,19-/m1/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 34 | n/a | n/a | n/a | n/a | n/a | n/a |
Cubist Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Compound tested for the inhibition of human isoleucyl-tRNA synthetase |
Bioorg Med Chem Lett 9: 375-80 (1999)
BindingDB Entry DOI: 10.7270/Q2M32TX1 |
More data for this
Ligand-Target Pair | |
Phenylalanine--tRNA ligase alpha subunit
(Streptococcus pyogenes serotype M18) | BDBM50140709
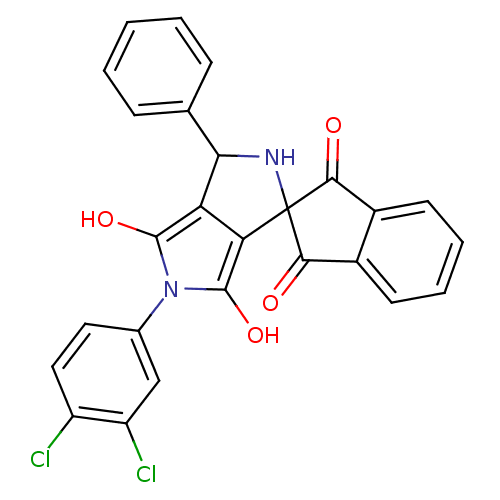
(5'-(3,4-dichlorophenyl)-3'-phenyl-(3'R,3a'S,6a'R)-...)Show SMILES Oc1c2C(NC3(C(=O)c4ccccc4C3=O)c2c(O)n1-c1ccc(Cl)c(Cl)c1)c1ccccc1 Show InChI InChI=1S/C26H16Cl2N2O4/c27-17-11-10-14(12-18(17)28)30-24(33)19-20(25(30)34)26(29-21(19)13-6-2-1-3-7-13)22(31)15-8-4-5-9-16(15)23(26)32/h1-12,21,29,33-34H | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
Cubist Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Inhibitory activity against phenylalanyl-tRNA synthetase from Staphylococcus aureus |
Bioorg Med Chem Lett 14: 1339-42 (2004)
Article DOI: 10.1016/j.bmcl.2003.11.081
BindingDB Entry DOI: 10.7270/Q2PR7VDS |
More data for this
Ligand-Target Pair | |
Isoleucine--tRNA ligase
(Staphylococcus aureus) | BDBM50075056

(((S)-2-Amino-3-methyl-pentanoyl)-sulfamic acid (2R...)Show SMILES CCC(C)[C@H](N)C(=O)NS(=O)(=O)OC[C@H]1O[C@H]([C@H](O)[C@@H]1O)c1nc(CCc2ccccc2)cs1 Show InChI InChI=1S/C22H31N3O7S2/c1-3-13(2)17(23)21(28)25-34(29,30)31-11-16-18(26)19(27)20(32-16)22-24-15(12-33-22)10-9-14-7-5-4-6-8-14/h4-8,12-13,16-20,26-27H,3,9-11,23H2,1-2H3,(H,25,28)/t13?,16-,17+,18-,19-,20-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
Cubist Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Compound tested for the inhibition of S. aureus isoleucyl-tRNA synthetase |
Bioorg Med Chem Lett 9: 375-80 (1999)
BindingDB Entry DOI: 10.7270/Q2M32TX1 |
More data for this
Ligand-Target Pair | |
Isoleucine--tRNA ligase, cytoplasmic
(Homo sapiens (Human)) | BDBM50075056

(((S)-2-Amino-3-methyl-pentanoyl)-sulfamic acid (2R...)Show SMILES CCC(C)[C@H](N)C(=O)NS(=O)(=O)OC[C@H]1O[C@H]([C@H](O)[C@@H]1O)c1nc(CCc2ccccc2)cs1 Show InChI InChI=1S/C22H31N3O7S2/c1-3-13(2)17(23)21(28)25-34(29,30)31-11-16-18(26)19(27)20(32-16)22-24-15(12-33-22)10-9-14-7-5-4-6-8-14/h4-8,12-13,16-20,26-27H,3,9-11,23H2,1-2H3,(H,25,28)/t13?,16-,17+,18-,19-,20-/m1/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
Cubist Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Compound tested for the inhibition of S. aureus isoleucyl-tRNA synthetase |
Bioorg Med Chem Lett 9: 375-80 (1999)
BindingDB Entry DOI: 10.7270/Q2M32TX1 |
More data for this
Ligand-Target Pair | |
Phenylalanine--tRNA ligase alpha subunit
(Streptococcus pyogenes serotype M18) | BDBM50140709

(5'-(3,4-dichlorophenyl)-3'-phenyl-(3'R,3a'S,6a'R)-...)Show SMILES Oc1c2C(NC3(C(=O)c4ccccc4C3=O)c2c(O)n1-c1ccc(Cl)c(Cl)c1)c1ccccc1 Show InChI InChI=1S/C26H16Cl2N2O4/c27-17-11-10-14(12-18(17)28)30-24(33)19-20(25(30)34)26(29-21(19)13-6-2-1-3-7-13)22(31)15-8-4-5-9-16(15)23(26)32/h1-12,21,29,33-34H | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
Cubist Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Inhibitory activity against phenylalanyl-tRNA synthetase from Enterococcus faecalis |
Bioorg Med Chem Lett 14: 1339-42 (2004)
Article DOI: 10.1016/j.bmcl.2003.11.081
BindingDB Entry DOI: 10.7270/Q2PR7VDS |
More data for this
Ligand-Target Pair | |
Isoleucine--tRNA ligase, cytoplasmic
(Homo sapiens (Human)) | BDBM50075070
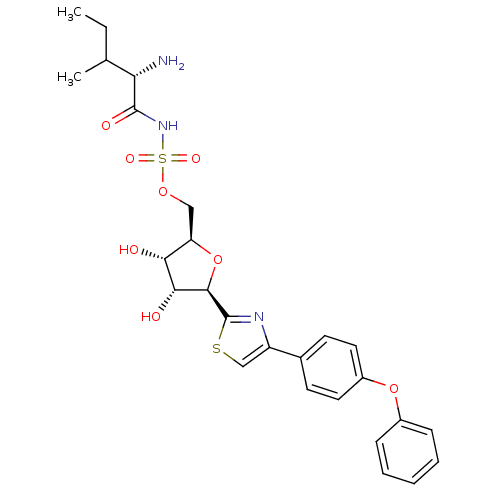
(((S)-2-Amino-3-methyl-pentanoyl)-sulfamic acid (2R...)Show SMILES CCC(C)[C@H](N)C(=O)NS(=O)(=O)OC[C@H]1O[C@H]([C@H](O)[C@@H]1O)c1nc(cs1)-c1ccc(Oc2ccccc2)cc1 Show InChI InChI=1S/C26H31N3O8S2/c1-3-15(2)21(27)25(32)29-39(33,34)35-13-20-22(30)23(31)24(37-20)26-28-19(14-38-26)16-9-11-18(12-10-16)36-17-7-5-4-6-8-17/h4-12,14-15,20-24,30-31H,3,13,27H2,1-2H3,(H,29,32)/t15?,20-,21+,22-,23-,24-/m1/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 45 | n/a | n/a | n/a | n/a | n/a | n/a |
Cubist Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Compound tested for the inhibition of human Leucyl-tRNA synthetase |
Bioorg Med Chem Lett 9: 375-80 (1999)
BindingDB Entry DOI: 10.7270/Q2M32TX1 |
More data for this
Ligand-Target Pair | |
Isoleucine--tRNA ligase
(Staphylococcus aureus) | BDBM50075058

(((S)-2-Amino-3-methyl-pentanoyl)-sulfamic acid (2R...)Show SMILES CCC(C)[C@H](N)C(=O)NS(=O)(=O)OC[C@H]1O[C@H]([C@H](O)[C@@H]1O)c1nc(cs1)-c1cccc(OC)c1 Show InChI InChI=1S/C21H29N3O8S2/c1-4-11(2)16(22)20(27)24-34(28,29)31-9-15-17(25)18(26)19(32-15)21-23-14(10-33-21)12-6-5-7-13(8-12)30-3/h5-8,10-11,15-19,25-26H,4,9,22H2,1-3H3,(H,24,27)/t11?,15-,16+,17-,18-,19-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
Cubist Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Compound tested for the inhibition of S. aureus isoleucyl-tRNA synthetase |
Bioorg Med Chem Lett 9: 375-80 (1999)
BindingDB Entry DOI: 10.7270/Q2M32TX1 |
More data for this
Ligand-Target Pair | |
Proline--tRNA ligase, cytoplasmic
(Candida albicans) | BDBM50097113
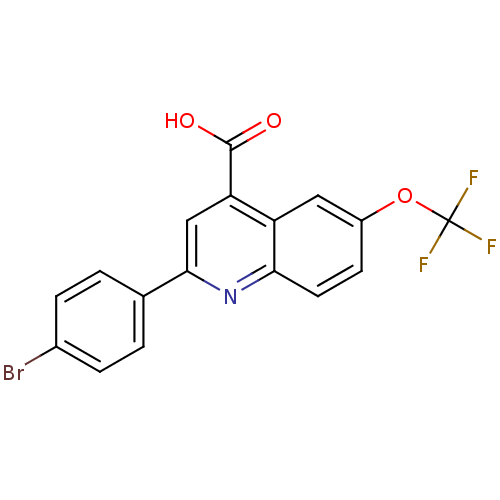
(2-(4-Bromo-phenyl)-6-trifluoromethoxy-quinoline-4-...)Show SMILES OC(=O)c1cc(nc2ccc(OC(F)(F)F)cc12)-c1ccc(Br)cc1 Show InChI InChI=1S/C17H9BrF3NO3/c18-10-3-1-9(2-4-10)15-8-13(16(23)24)12-7-11(25-17(19,20)21)5-6-14(12)22-15/h1-8H,(H,23,24) | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 53 | n/a | n/a | n/a | n/a | n/a | n/a |
Cubist Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Aminoacylation activity against Candida albicans prolyl-tRNA synthetase |
Bioorg Med Chem Lett 11: 541-4 (2001)
BindingDB Entry DOI: 10.7270/Q2Q23ZH8 |
More data for this
Ligand-Target Pair | |
Isoleucine--tRNA ligase
(Staphylococcus aureus) | BDBM50075067

(((S)-2-Amino-4-methyl-pentanoyl)-sulfamic acid (2R...)Show SMILES CC(C)C[C@H](N)C(=O)NS(=O)(=O)OC[C@H]1O[C@H]([C@H](O)[C@@H]1O)c1nc(cs1)-c1ccc(Oc2ccccc2)cc1 Show InChI InChI=1S/C26H31N3O8S2/c1-15(2)12-19(27)25(32)29-39(33,34)35-13-21-22(30)23(31)24(37-21)26-28-20(14-38-26)16-8-10-18(11-9-16)36-17-6-4-3-5-7-17/h3-11,14-15,19,21-24,30-31H,12-13,27H2,1-2H3,(H,29,32)/t19-,21+,22+,23+,24+/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | >54 | n/a | n/a | n/a | n/a | n/a | n/a |
Cubist Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Compound tested for the inhibition of S. aureus Leucyl-tRNA synthetase |
Bioorg Med Chem Lett 9: 375-80 (1999)
BindingDB Entry DOI: 10.7270/Q2M32TX1 |
More data for this
Ligand-Target Pair | |
Phenylalanine--tRNA ligase alpha subunit
(Streptococcus pyogenes serotype M18) | BDBM50140707
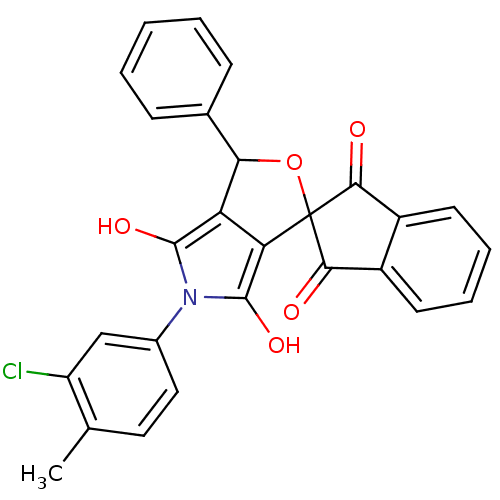
(5-(3-chloro-4-methylphenyl)-3-phenyl-(3R,3aS,6aR)-...)Show SMILES Cc1ccc(cc1Cl)-n1c(O)c2C(OC3(C(=O)c4ccccc4C3=O)c2c1O)c1ccccc1 Show InChI InChI=1S/C27H18ClNO5/c1-14-11-12-16(13-19(14)28)29-25(32)20-21(26(29)33)27(34-22(20)15-7-3-2-4-8-15)23(30)17-9-5-6-10-18(17)24(27)31/h2-13,22,32-33H,1H3 | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 70 | n/a | n/a | n/a | n/a | n/a | n/a |
Cubist Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Inhibitory activity against phenylalanyl-tRNA synthetase from Staphylococcus aureus |
Bioorg Med Chem Lett 14: 1339-42 (2004)
Article DOI: 10.1016/j.bmcl.2003.11.081
BindingDB Entry DOI: 10.7270/Q2PR7VDS |
More data for this
Ligand-Target Pair | |
Proline--tRNA ligase, cytoplasmic
(Candida albicans) | BDBM50097102

(2-(4-Bromo-phenyl)-6-chloro-8-methyl-quinoline-4-c...)Show SMILES Cc1cc(Cl)cc2c(cc(nc12)-c1ccc(Br)cc1)C(=O)OCc1ccco1 Show InChI InChI=1S/C22H15BrClNO3/c1-13-9-16(24)10-18-19(22(26)28-12-17-3-2-8-27-17)11-20(25-21(13)18)14-4-6-15(23)7-5-14/h2-11H,12H2,1H3 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 70 | n/a | n/a | n/a | n/a | n/a | n/a |
Cubist Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Aminoacylation activity against Candida albicans prolyl-tRNA synthetase |
Bioorg Med Chem Lett 11: 541-4 (2001)
BindingDB Entry DOI: 10.7270/Q2Q23ZH8 |
More data for this
Ligand-Target Pair | |
Isoleucine--tRNA ligase
(Staphylococcus aureus) | BDBM50075069

(((S)-2-Amino-4-methyl-pentanoyl)-sulfamic acid (2R...)Show SMILES CC(C)C[C@H](N)C(=O)NS(=O)(=O)OC[C@H]1O[C@H]([C@H](O)[C@@H]1O)c1nc(CCc2ccccc2)cs1 Show InChI InChI=1S/C22H31N3O7S2/c1-13(2)10-16(23)21(28)25-34(29,30)31-11-17-18(26)19(27)20(32-17)22-24-15(12-33-22)9-8-14-6-4-3-5-7-14/h3-7,12-13,16-20,26-27H,8-11,23H2,1-2H3,(H,25,28)/t16-,17+,18+,19+,20+/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 90 | n/a | n/a | n/a | n/a | n/a | n/a |
Cubist Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Compound tested for the inhibition of S. aureus Leucyl-tRNA synthetase |
Bioorg Med Chem Lett 9: 375-80 (1999)
BindingDB Entry DOI: 10.7270/Q2M32TX1 |
More data for this
Ligand-Target Pair | |
Isoleucine--tRNA ligase, cytoplasmic
(Homo sapiens (Human)) | BDBM50075068

(((2S,3S)-2-Amino-3-methyl-pentanoyl)-sulfamic acid...)Show SMILES CC[C@H](C)[C@H](N)C(=O)NS(=O)(=O)OC[C@H]1O[C@H]([C@H](O)[C@@H]1O)c1nc(cs1)-c1ccccc1 Show InChI InChI=1S/C20H27N3O7S2/c1-3-11(2)15(21)19(26)23-32(27,28)29-9-14-16(24)17(25)18(30-14)20-22-13(10-31-20)12-7-5-4-6-8-12/h4-8,10-11,14-18,24-25H,3,9,21H2,1-2H3,(H,23,26)/t11-,14+,15-,16+,17+,18+/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
Cubist Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of E. coli isoleucyl-tRNA synthetase |
Bioorg Med Chem Lett 9: 375-80 (1999)
BindingDB Entry DOI: 10.7270/Q2M32TX1 |
More data for this
Ligand-Target Pair | |
Isoleucine--tRNA ligase
(Staphylococcus aureus) | BDBM50075055

(((S)-2-Amino-4-methyl-pentanoyl)-sulfamic acid (2R...)Show SMILES CC(C)C[C@H](N)C(=O)NS(=O)(=O)OC[C@H]1O[C@H]([C@H](O)[C@@H]1O)c1nc(CCc2ccc(Oc3ccccc3)cc2)cs1 Show InChI InChI=1S/C28H35N3O8S2/c1-17(2)14-22(29)27(34)31-41(35,36)37-15-23-24(32)25(33)26(39-23)28-30-19(16-40-28)11-8-18-9-12-21(13-10-18)38-20-6-4-3-5-7-20/h3-7,9-10,12-13,16-17,22-26,32-33H,8,11,14-15,29H2,1-2H3,(H,31,34)/t22-,23+,24+,25+,26+/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
Cubist Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Compound tested for the inhibition of S. aureus Leucyl-tRNA synthetase |
Bioorg Med Chem Lett 9: 375-80 (1999)
BindingDB Entry DOI: 10.7270/Q2M32TX1 |
More data for this
Ligand-Target Pair | |
Isoleucine--tRNA ligase, cytoplasmic
(Homo sapiens (Human)) | BDBM50075063

(((S)-2-Amino-3-methyl-pentanoyl)-sulfamic acid (2R...)Show SMILES CCC(C)[C@H](N)C(=O)NS(=O)(=O)OC[C@H]1O[C@H]([C@H](O)[C@@H]1O)c1nc(cs1)-c1ccc(OC)cc1 Show InChI InChI=1S/C21H29N3O8S2/c1-4-11(2)16(22)20(27)24-34(28,29)31-9-15-17(25)18(26)19(32-15)21-23-14(10-33-21)12-5-7-13(30-3)8-6-12/h5-8,10-11,15-19,25-26H,4,9,22H2,1-3H3,(H,24,27)/t11?,15-,16+,17-,18-,19-/m1/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
Cubist Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of E. coli isoleucyl-tRNA synthetase |
Bioorg Med Chem Lett 9: 375-80 (1999)
BindingDB Entry DOI: 10.7270/Q2M32TX1 |
More data for this
Ligand-Target Pair | |
Isoleucine--tRNA ligase
(Staphylococcus aureus) | BDBM50075063

(((S)-2-Amino-3-methyl-pentanoyl)-sulfamic acid (2R...)Show SMILES CCC(C)[C@H](N)C(=O)NS(=O)(=O)OC[C@H]1O[C@H]([C@H](O)[C@@H]1O)c1nc(cs1)-c1ccc(OC)cc1 Show InChI InChI=1S/C21H29N3O8S2/c1-4-11(2)16(22)20(27)24-34(28,29)31-9-15-17(25)18(26)19(32-15)21-23-14(10-33-21)12-5-7-13(30-3)8-6-12/h5-8,10-11,15-19,25-26H,4,9,22H2,1-3H3,(H,24,27)/t11?,15-,16+,17-,18-,19-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
Cubist Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Compound tested for the inhibition of S. aureus isoleucyl-tRNA synthetase |
Bioorg Med Chem Lett 9: 375-80 (1999)
BindingDB Entry DOI: 10.7270/Q2M32TX1 |
More data for this
Ligand-Target Pair | |
Isoleucine--tRNA ligase, cytoplasmic
(Homo sapiens (Human)) | BDBM50075065
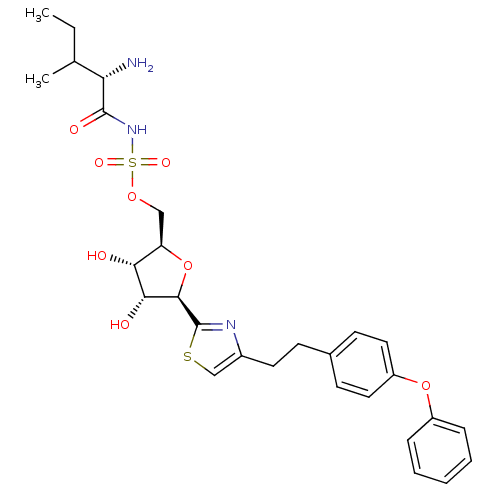
(((S)-2-Amino-3-methyl-pentanoyl)-sulfamic acid (2R...)Show SMILES CCC(C)[C@H](N)C(=O)NS(=O)(=O)OC[C@H]1O[C@H]([C@H](O)[C@@H]1O)c1nc(CCc2ccc(Oc3ccccc3)cc2)cs1 Show InChI InChI=1S/C28H35N3O8S2/c1-3-17(2)23(29)27(34)31-41(35,36)37-15-22-24(32)25(33)26(39-22)28-30-19(16-40-28)12-9-18-10-13-21(14-11-18)38-20-7-5-4-6-8-20/h4-8,10-11,13-14,16-17,22-26,32-33H,3,9,12,15,29H2,1-2H3,(H,31,34)/t17?,22-,23+,24-,25-,26-/m1/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 110 | n/a | n/a | n/a | n/a | n/a | n/a |
Cubist Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of E. coli isoleucyl-tRNA synthetase |
Bioorg Med Chem Lett 9: 375-80 (1999)
BindingDB Entry DOI: 10.7270/Q2M32TX1 |
More data for this
Ligand-Target Pair | |
Isoleucine--tRNA ligase, cytoplasmic
(Homo sapiens (Human)) | BDBM50075062
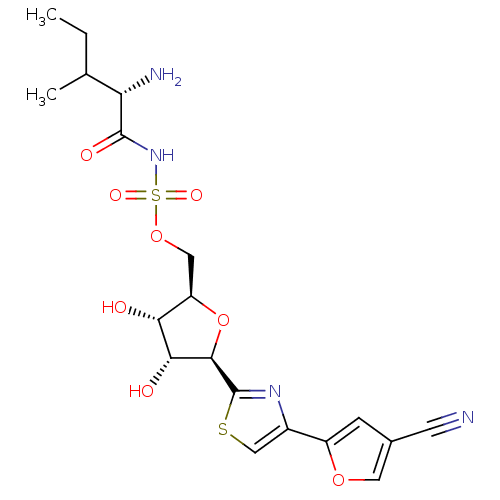
(((S)-2-Amino-3-methyl-pentanoyl)-sulfamic acid (2R...)Show SMILES CCC(C)[C@H](N)C(=O)NS(=O)(=O)OC[C@H]1O[C@H]([C@H](O)[C@@H]1O)c1nc(cs1)-c1cc(co1)C#N Show InChI InChI=1S/C19H24N4O8S2/c1-3-9(2)14(21)18(26)23-33(27,28)30-7-13-15(24)16(25)17(31-13)19-22-11(8-32-19)12-4-10(5-20)6-29-12/h4,6,8-9,13-17,24-25H,3,7,21H2,1-2H3,(H,23,26)/t9?,13-,14+,15-,16-,17-/m1/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 120 | n/a | n/a | n/a | n/a | n/a | n/a |
Cubist Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of E. coli isoleucyl-tRNA synthetase |
Bioorg Med Chem Lett 9: 375-80 (1999)
BindingDB Entry DOI: 10.7270/Q2M32TX1 |
More data for this
Ligand-Target Pair | |
Proline--tRNA ligase, cytoplasmic
(Candida albicans) | BDBM50097107

(2-(4-Bromo-phenyl)-7-fluoro-8-methyl-quinoline-4-c...)Show InChI InChI=1S/C17H11BrFNO2/c1-9-14(19)7-6-12-13(17(21)22)8-15(20-16(9)12)10-2-4-11(18)5-3-10/h2-8H,1H3,(H,21,22) | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 120 | n/a | n/a | n/a | n/a | n/a | n/a |
Cubist Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Aminoacylation activity against Candida albicans prolyl-tRNA synthetase |
Bioorg Med Chem Lett 11: 541-4 (2001)
BindingDB Entry DOI: 10.7270/Q2Q23ZH8 |
More data for this
Ligand-Target Pair | |
Isoleucine--tRNA ligase
(Staphylococcus aureus) | BDBM50075065

(((S)-2-Amino-3-methyl-pentanoyl)-sulfamic acid (2R...)Show SMILES CCC(C)[C@H](N)C(=O)NS(=O)(=O)OC[C@H]1O[C@H]([C@H](O)[C@@H]1O)c1nc(CCc2ccc(Oc3ccccc3)cc2)cs1 Show InChI InChI=1S/C28H35N3O8S2/c1-3-17(2)23(29)27(34)31-41(35,36)37-15-22-24(32)25(33)26(39-22)28-30-19(16-40-28)12-9-18-10-13-21(14-11-18)38-20-7-5-4-6-8-20/h4-8,10-11,13-14,16-17,22-26,32-33H,3,9,12,15,29H2,1-2H3,(H,31,34)/t17?,22-,23+,24-,25-,26-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 120 | n/a | n/a | n/a | n/a | n/a | n/a |
Cubist Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Compound tested for the inhibition of S. aureus isoleucyl-tRNA synthetase |
Bioorg Med Chem Lett 9: 375-80 (1999)
BindingDB Entry DOI: 10.7270/Q2M32TX1 |
More data for this
Ligand-Target Pair | |
Isoleucine--tRNA ligase
(Staphylococcus aureus) | BDBM50075061

(((S)-2-Amino-3-methyl-pentanoyl)-sulfamic acid (2R...)Show SMILES CCC(C)[C@H](N)C(=O)NS(=O)(=O)OC[C@H]1O[C@H]([C@H](O)[C@@H]1O)c1nc(cs1)-c1ccc2cc(OC)ccc2c1 Show InChI InChI=1S/C25H31N3O8S2/c1-4-13(2)20(26)24(31)28-38(32,33)35-11-19-21(29)22(30)23(36-19)25-27-18(12-37-25)16-6-5-15-10-17(34-3)8-7-14(15)9-16/h5-10,12-13,19-23,29-30H,4,11,26H2,1-3H3,(H,28,31)/t13?,19-,20+,21-,22-,23-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 130 | n/a | n/a | n/a | n/a | n/a | n/a |
Cubist Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Compound tested for the inhibition of S. aureus isoleucyl-tRNA synthetase |
Bioorg Med Chem Lett 9: 375-80 (1999)
BindingDB Entry DOI: 10.7270/Q2M32TX1 |
More data for this
Ligand-Target Pair | |
Proline--tRNA ligase, cytoplasmic
(Candida albicans) | BDBM50097091
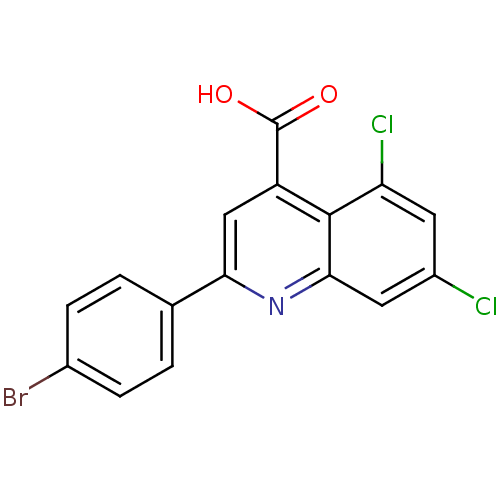
(2-(4-Bromo-phenyl)-5,7-dichloro-quinoline-4-carbox...)Show SMILES OC(=O)c1cc(nc2cc(Cl)cc(Cl)c12)-c1ccc(Br)cc1 Show InChI InChI=1S/C16H8BrCl2NO2/c17-9-3-1-8(2-4-9)13-7-11(16(21)22)15-12(19)5-10(18)6-14(15)20-13/h1-7H,(H,21,22) | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 140 | n/a | n/a | n/a | n/a | n/a | n/a |
Cubist Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Aminoacylation activity against Candida albicans prolyl-tRNA synthetase |
Bioorg Med Chem Lett 11: 541-4 (2001)
BindingDB Entry DOI: 10.7270/Q2Q23ZH8 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data