 Found 1833 hits with Last Name = 'yuan' and Initial = 'y'
Found 1833 hits with Last Name = 'yuan' and Initial = 'y' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
E3 ubiquitin-protein ligase Mdm2
(Homo sapiens (Human)) | BDBM50513202
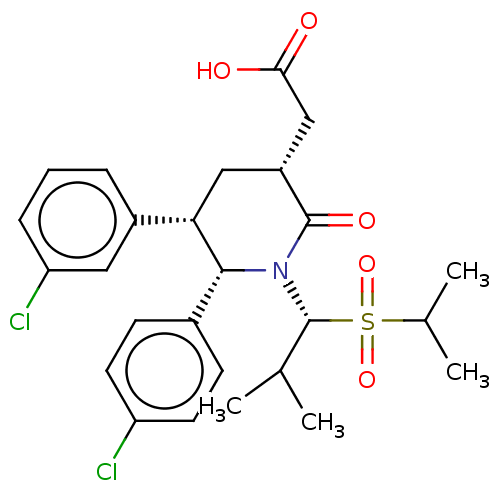
(CHEMBL4463050)Show SMILES CC(C)[C@H](N1[C@@H]([C@@H](C[C@H](CC(O)=O)C1=O)c1cccc(Cl)c1)c1ccc(Cl)cc1)S(=O)(=O)C(C)C |r| Show InChI InChI=1S/C26H31Cl2NO5S/c1-15(2)26(35(33,34)16(3)4)29-24(17-8-10-20(27)11-9-17)22(18-6-5-7-21(28)12-18)13-19(25(29)32)14-23(30)31/h5-12,15-16,19,22,24,26H,13-14H2,1-4H3,(H,30,31)/t19-,22+,24-,26-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0450 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sichuan University
Curated by ChEMBL
| Assay Description
Inhibition of p53 protein binding to MDM2 in human SJSA1 cells |
Eur J Med Chem 176: 92-104 (2019)
Article DOI: 10.1016/j.ejmech.2019.05.018
BindingDB Entry DOI: 10.7270/Q2V12851 |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50352114
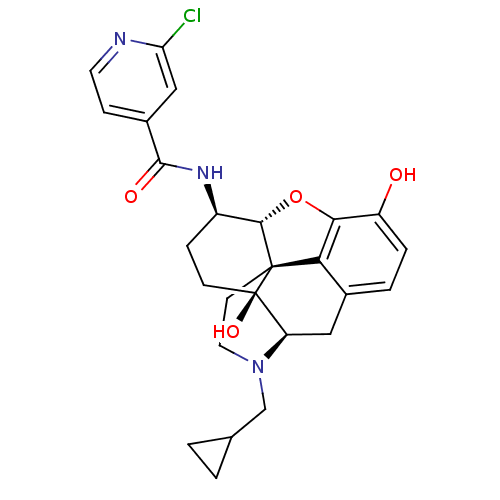
(CHEMBL1824509 | CHEMBL1852788)Show SMILES Oc1ccc2C[C@H]3N(CC4CC4)CC[C@@]45[C@@H](Oc1c24)[C@@H](CC[C@@]35O)NC(=O)c1ccnc(Cl)c1 |r| Show InChI InChI=1S/C26H28ClN3O4/c27-20-12-16(6-9-28-20)24(32)29-17-5-7-26(33)19-11-15-3-4-18(31)22-21(15)25(26,23(17)34-22)8-10-30(19)13-14-1-2-14/h3-4,6,9,12,14,17,19,23,31,33H,1-2,5,7-8,10-11,13H2,(H,29,32)/t17-,19-,23+,25+,26-/m1/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Commonwealth University
Curated by ChEMBL
| Assay Description
Displacement of [3H]Naloxone from mu opioid receptor expressed in CHO cells after 1 hr |
J Med Chem 55: 10118-29 (2012)
Article DOI: 10.1021/jm301247n
BindingDB Entry DOI: 10.7270/Q29P32SK |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50352114

(CHEMBL1824509 | CHEMBL1852788)Show SMILES Oc1ccc2C[C@H]3N(CC4CC4)CC[C@@]45[C@@H](Oc1c24)[C@@H](CC[C@@]35O)NC(=O)c1ccnc(Cl)c1 |r| Show InChI InChI=1S/C26H28ClN3O4/c27-20-12-16(6-9-28-20)24(32)29-17-5-7-26(33)19-11-15-3-4-18(31)22-21(15)25(26,23(17)34-22)8-10-30(19)13-14-1-2-14/h3-4,6,9,12,14,17,19,23,31,33H,1-2,5,7-8,10-11,13H2,(H,29,32)/t17-,19-,23+,25+,26-/m1/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Commonwealth University
Curated by ChEMBL
| Assay Description
Displacement of [3H]naloxone from mu opioid receptor expressed in CHO cells after 1 hr |
Bioorg Med Chem Lett 21: 5625-9 (2011)
Article DOI: 10.1016/j.bmcl.2011.06.135
BindingDB Entry DOI: 10.7270/Q21J9B57 |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50492288
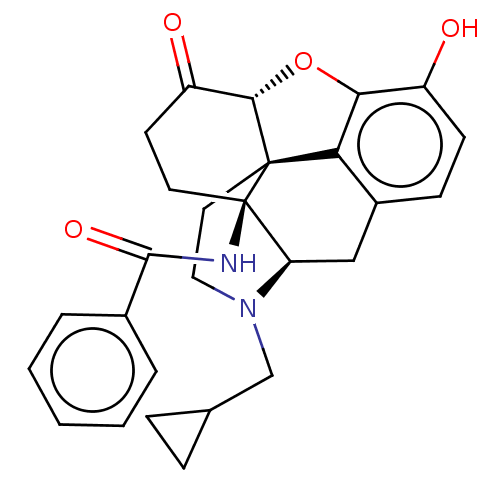
(CHEMBL2397015)Show SMILES [H][C@@]12Oc3c4c(C[C@@]5([H])N(CC6CC6)CC[C@@]14[C@]5(CCC2=O)NC(=O)c1ccccc1)ccc3O |r,THB:10:9:17:4.5.6| Show InChI InChI=1S/C27H28N2O4/c30-19-9-8-18-14-21-27(28-25(32)17-4-2-1-3-5-17)11-10-20(31)24-26(27,22(18)23(19)33-24)12-13-29(21)15-16-6-7-16/h1-5,8-9,16,21,24,30H,6-7,10-15H2,(H,28,32)/t21-,24+,26+,27-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Commonwealth University
Curated by ChEMBL
| Assay Description
Displacement of [3H]DPN from kappa opioid receptor (unknown origin) expressed in CHO cell membranes after 1.5 hrs |
J Med Chem 56: 9156-69 (2013)
Article DOI: 10.1021/jm4012214
BindingDB Entry DOI: 10.7270/Q2JH3Q4C |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50492288

(CHEMBL2397015)Show SMILES [H][C@@]12Oc3c4c(C[C@@]5([H])N(CC6CC6)CC[C@@]14[C@]5(CCC2=O)NC(=O)c1ccccc1)ccc3O |r,THB:10:9:17:4.5.6| Show InChI InChI=1S/C27H28N2O4/c30-19-9-8-18-14-21-27(28-25(32)17-4-2-1-3-5-17)11-10-20(31)24-26(27,22(18)23(19)33-24)12-13-29(21)15-16-6-7-16/h1-5,8-9,16,21,24,30H,6-7,10-15H2,(H,28,32)/t21-,24+,26+,27-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Commonwealth University
Curated by ChEMBL
| Assay Description
Displacement of [3H]DPN from kappa opioid receptor (unknown origin) after 1.5 hrs |
Bioorg Med Chem Lett 23: 3719-22 (2013)
Article DOI: 10.1016/j.bmcl.2013.05.027
BindingDB Entry DOI: 10.7270/Q2HM5CD9 |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50492293

(CHEMBL2397018)Show SMILES [H][C@@]12Oc3c4c(C[C@@]5([H])N(CC6CC6)CC[C@@]14[C@]5(CCC2=O)NC(=O)c1cnc2ccccc2c1)ccc3O |r,THB:10:9:17:4.5.6| Show InChI InChI=1S/C30H29N3O4/c34-22-8-7-19-14-24-30(32-28(36)20-13-18-3-1-2-4-21(18)31-15-20)10-9-23(35)27-29(30,25(19)26(22)37-27)11-12-33(24)16-17-5-6-17/h1-4,7-8,13,15,17,24,27,34H,5-6,9-12,14,16H2,(H,32,36)/t24-,27+,29+,30-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Commonwealth University
Curated by ChEMBL
| Assay Description
Displacement of [3H]DPN from kappa opioid receptor (unknown origin) after 1.5 hrs |
Bioorg Med Chem Lett 23: 3719-22 (2013)
Article DOI: 10.1016/j.bmcl.2013.05.027
BindingDB Entry DOI: 10.7270/Q2HM5CD9 |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50492293

(CHEMBL2397018)Show SMILES [H][C@@]12Oc3c4c(C[C@@]5([H])N(CC6CC6)CC[C@@]14[C@]5(CCC2=O)NC(=O)c1cnc2ccccc2c1)ccc3O |r,THB:10:9:17:4.5.6| Show InChI InChI=1S/C30H29N3O4/c34-22-8-7-19-14-24-30(32-28(36)20-13-18-3-1-2-4-21(18)31-15-20)10-9-23(35)27-29(30,25(19)26(22)37-27)11-12-33(24)16-17-5-6-17/h1-4,7-8,13,15,17,24,27,34H,5-6,9-12,14,16H2,(H,32,36)/t24-,27+,29+,30-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Commonwealth University
Curated by ChEMBL
| Assay Description
Displacement of [3H]DPN from kappa opioid receptor (unknown origin) expressed in CHO cell membranes after 1.5 hrs |
J Med Chem 56: 9156-69 (2013)
Article DOI: 10.1021/jm4012214
BindingDB Entry DOI: 10.7270/Q2JH3Q4C |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50399663
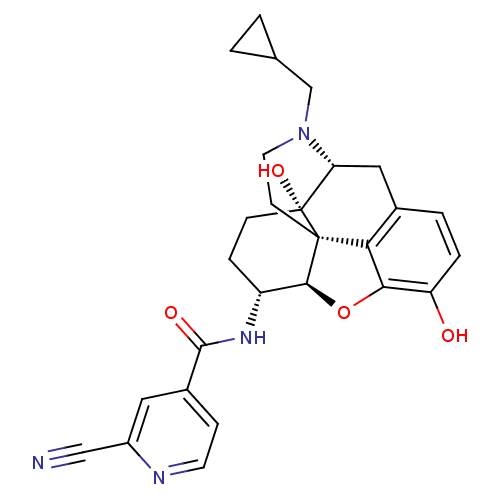
(CHEMBL2177697)Show SMILES Oc1ccc2C[C@H]3N(CC4CC4)CC[C@@]45[C@@H](Oc1c24)[C@@H](CC[C@@]35O)NC(=O)c1ccnc(c1)C#N |r| Show InChI InChI=1S/C27H28N4O4/c28-13-18-11-17(6-9-29-18)25(33)30-19-5-7-27(34)21-12-16-3-4-20(32)23-22(16)26(27,24(19)35-23)8-10-31(21)14-15-1-2-15/h3-4,6,9,11,15,19,21,24,32,34H,1-2,5,7-8,10,12,14H2,(H,30,33)/t19-,21-,24+,26+,27-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Commonwealth University
Curated by ChEMBL
| Assay Description
Displacement of [3H]DPN from kappa opioid receptor expressed in CHO cells after 1 hr |
J Med Chem 55: 10118-29 (2012)
Article DOI: 10.1021/jm301247n
BindingDB Entry DOI: 10.7270/Q29P32SK |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50266857
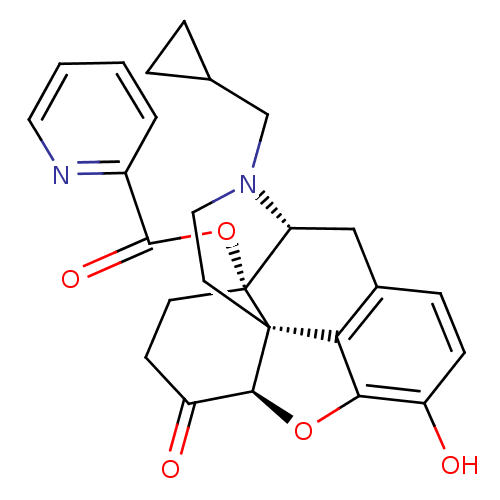
((1S,5R,13R,17S)-4-(cyclopropylmethyl)-10-hydroxy-1...)Show SMILES Oc1ccc2C[C@H]3N(CC4CC4)CC[C@@]45[C@@H](Oc1c24)C(=O)CC[C@@]35OC(=O)c1ccccn1 |r| Show InChI InChI=1S/C26H26N2O5/c29-18-7-6-16-13-20-26(33-24(31)17-3-1-2-11-27-17)9-8-19(30)23-25(26,21(16)22(18)32-23)10-12-28(20)14-15-4-5-15/h1-3,6-7,11,15,20,23,29H,4-5,8-10,12-14H2/t20-,23+,25+,26-/m1/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Commonwealth University
Curated by ChEMBL
| Assay Description
Displacement of [3H]NLX from mu opioid receptor (unknown origin) expressed in CHO cell membranes after 1.5 hrs |
J Med Chem 56: 9156-69 (2013)
Article DOI: 10.1021/jm4012214
BindingDB Entry DOI: 10.7270/Q2JH3Q4C |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50494375
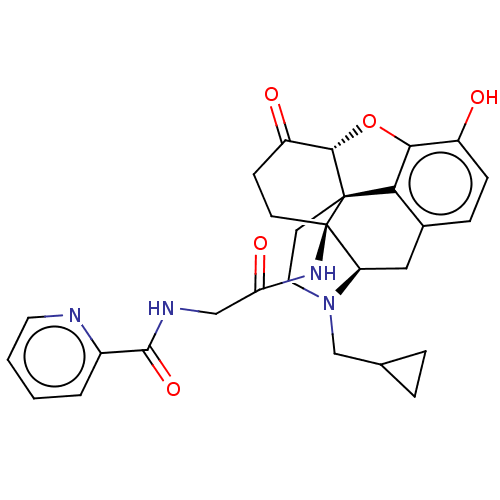
(CHEMBL3086756)Show SMILES Cl.[H][C@@]12Oc3c4c(C[C@@]5([H])N(CC6CC6)CC[C@@]14[C@]5(CCC2=O)NC(=O)CNC(=O)c1ccccn1)ccc3O |r,THB:11:10:18:5.6.7| Show InChI InChI=1S/C28H30N4O5.ClH/c33-19-7-6-17-13-21-28(31-22(35)14-30-26(36)18-3-1-2-11-29-18)9-8-20(34)25-27(28,23(17)24(19)37-25)10-12-32(21)15-16-4-5-16;/h1-3,6-7,11,16,21,25,33H,4-5,8-10,12-15H2,(H,30,36)(H,31,35);1H/t21-,25+,27+,28-;/m1./s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Commonwealth University
Curated by ChEMBL
| Assay Description
Displacement of [3H]DPN from kappa opioid receptor (unknown origin) expressed in CHO cell membranes after 1.5 hrs |
J Med Chem 56: 9156-69 (2013)
Article DOI: 10.1021/jm4012214
BindingDB Entry DOI: 10.7270/Q2JH3Q4C |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50352114

(CHEMBL1824509 | CHEMBL1852788)Show SMILES Oc1ccc2C[C@H]3N(CC4CC4)CC[C@@]45[C@@H](Oc1c24)[C@@H](CC[C@@]35O)NC(=O)c1ccnc(Cl)c1 |r| Show InChI InChI=1S/C26H28ClN3O4/c27-20-12-16(6-9-28-20)24(32)29-17-5-7-26(33)19-11-15-3-4-18(31)22-21(15)25(26,23(17)34-22)8-10-30(19)13-14-1-2-14/h3-4,6,9,12,14,17,19,23,31,33H,1-2,5,7-8,10-11,13H2,(H,29,32)/t17-,19-,23+,25+,26-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Commonwealth University
Curated by ChEMBL
| Assay Description
Displacement of [3H]DPN from kappa opioid receptor expressed in CHO cells after 1 hr |
J Med Chem 55: 10118-29 (2012)
Article DOI: 10.1021/jm301247n
BindingDB Entry DOI: 10.7270/Q29P32SK |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50352114

(CHEMBL1824509 | CHEMBL1852788)Show SMILES Oc1ccc2C[C@H]3N(CC4CC4)CC[C@@]45[C@@H](Oc1c24)[C@@H](CC[C@@]35O)NC(=O)c1ccnc(Cl)c1 |r| Show InChI InChI=1S/C26H28ClN3O4/c27-20-12-16(6-9-28-20)24(32)29-17-5-7-26(33)19-11-15-3-4-18(31)22-21(15)25(26,23(17)34-22)8-10-30(19)13-14-1-2-14/h3-4,6,9,12,14,17,19,23,31,33H,1-2,5,7-8,10-11,13H2,(H,29,32)/t17-,19-,23+,25+,26-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Commonwealth University
Curated by ChEMBL
| Assay Description
Displacement of [3H]-norBNI from kappa opioid receptor expressed in CHO cells after 1 hr |
Bioorg Med Chem Lett 21: 5625-9 (2011)
Article DOI: 10.1016/j.bmcl.2011.06.135
BindingDB Entry DOI: 10.7270/Q21J9B57 |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50492292

(CHEMBL2397021)Show SMILES [H][C@@]12Oc3c4c(C[C@@]5([H])N(CC6CC6)CC[C@@]14[C@]5(CCC2=O)NC(=O)c1cccnc1)ccc3O |r,THB:10:9:17:4.5.6| Show InChI InChI=1S/C26H27N3O4/c30-18-6-5-16-12-20-26(28-24(32)17-2-1-10-27-13-17)8-7-19(31)23-25(26,21(16)22(18)33-23)9-11-29(20)14-15-3-4-15/h1-2,5-6,10,13,15,20,23,30H,3-4,7-9,11-12,14H2,(H,28,32)/t20-,23+,25+,26-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Commonwealth University
Curated by ChEMBL
| Assay Description
Displacement of [3H]DPN from kappa opioid receptor (unknown origin) expressed in CHO cell membranes after 1.5 hrs |
J Med Chem 56: 9156-69 (2013)
Article DOI: 10.1021/jm4012214
BindingDB Entry DOI: 10.7270/Q2JH3Q4C |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50492292

(CHEMBL2397021)Show SMILES [H][C@@]12Oc3c4c(C[C@@]5([H])N(CC6CC6)CC[C@@]14[C@]5(CCC2=O)NC(=O)c1cccnc1)ccc3O |r,THB:10:9:17:4.5.6| Show InChI InChI=1S/C26H27N3O4/c30-18-6-5-16-12-20-26(28-24(32)17-2-1-10-27-13-17)8-7-19(31)23-25(26,21(16)22(18)33-23)9-11-29(20)14-15-3-4-15/h1-2,5-6,10,13,15,20,23,30H,3-4,7-9,11-12,14H2,(H,28,32)/t20-,23+,25+,26-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Commonwealth University
Curated by ChEMBL
| Assay Description
Displacement of [3H]DPN from kappa opioid receptor (unknown origin) after 1.5 hrs |
Bioorg Med Chem Lett 23: 3719-22 (2013)
Article DOI: 10.1016/j.bmcl.2013.05.027
BindingDB Entry DOI: 10.7270/Q2HM5CD9 |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50494377
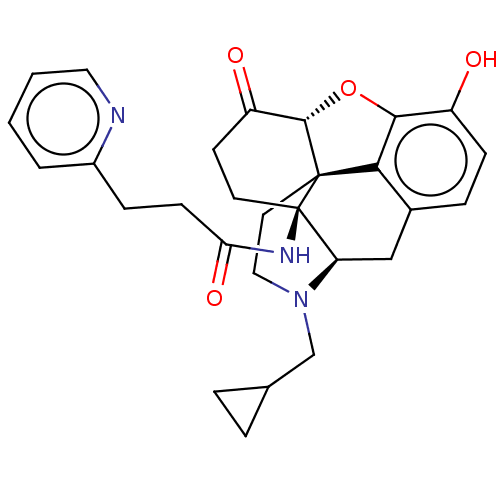
(CHEMBL3086755)Show SMILES Cl.[H][C@@]12Oc3c4c(C[C@@]5([H])N(CC6CC6)CC[C@@]14[C@]5(CCC2=O)NC(=O)CCc1ccccn1)ccc3O |r,THB:11:10:18:5.6.7| Show InChI InChI=1S/C28H31N3O4.ClH/c32-20-8-6-18-15-22-28(30-23(34)9-7-19-3-1-2-13-29-19)11-10-21(33)26-27(28,24(18)25(20)35-26)12-14-31(22)16-17-4-5-17;/h1-3,6,8,13,17,22,26,32H,4-5,7,9-12,14-16H2,(H,30,34);1H/t22-,26+,27+,28-;/m1./s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.170 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Commonwealth University
Curated by ChEMBL
| Assay Description
Displacement of [3H]DPN from kappa opioid receptor (unknown origin) expressed in CHO cell membranes after 1.5 hrs |
J Med Chem 56: 9156-69 (2013)
Article DOI: 10.1021/jm4012214
BindingDB Entry DOI: 10.7270/Q2JH3Q4C |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50352115
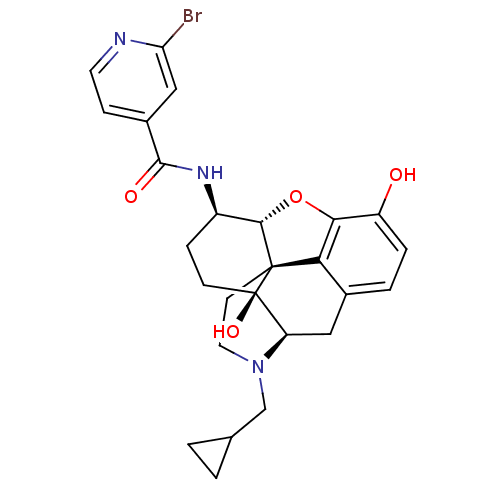
(CHEMBL1824510 | CHEMBL1852385)Show SMILES Oc1ccc2C[C@H]3N(CC4CC4)CC[C@@]45[C@@H](Oc1c24)[C@@H](CC[C@@]35O)NC(=O)c1ccnc(Br)c1 |r| Show InChI InChI=1S/C26H28BrN3O4/c27-20-12-16(6-9-28-20)24(32)29-17-5-7-26(33)19-11-15-3-4-18(31)22-21(15)25(26,23(17)34-22)8-10-30(19)13-14-1-2-14/h3-4,6,9,12,14,17,19,23,31,33H,1-2,5,7-8,10-11,13H2,(H,29,32)/t17-,19-,23+,25+,26-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Commonwealth University
Curated by ChEMBL
| Assay Description
Displacement of [3H]DPN from kappa opioid receptor expressed in CHO cells after 1 hr |
J Med Chem 55: 10118-29 (2012)
Article DOI: 10.1021/jm301247n
BindingDB Entry DOI: 10.7270/Q29P32SK |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50352115

(CHEMBL1824510 | CHEMBL1852385)Show SMILES Oc1ccc2C[C@H]3N(CC4CC4)CC[C@@]45[C@@H](Oc1c24)[C@@H](CC[C@@]35O)NC(=O)c1ccnc(Br)c1 |r| Show InChI InChI=1S/C26H28BrN3O4/c27-20-12-16(6-9-28-20)24(32)29-17-5-7-26(33)19-11-15-3-4-18(31)22-21(15)25(26,23(17)34-22)8-10-30(19)13-14-1-2-14/h3-4,6,9,12,14,17,19,23,31,33H,1-2,5,7-8,10-11,13H2,(H,29,32)/t17-,19-,23+,25+,26-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Commonwealth University
Curated by ChEMBL
| Assay Description
Displacement of [3H]-norBNI from kappa opioid receptor expressed in CHO cells after 1 hr |
Bioorg Med Chem Lett 21: 5625-9 (2011)
Article DOI: 10.1016/j.bmcl.2011.06.135
BindingDB Entry DOI: 10.7270/Q21J9B57 |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50494376

(CHEMBL3086754)Show SMILES Cl.[H][C@@]12Oc3c4c(C[C@@]5([H])N(CC6CC6)CC[C@@]14[C@]5(CCC2=O)NC(=O)Cc1ccccn1)ccc3O |r,THB:11:10:18:5.6.7| Show InChI InChI=1S/C27H29N3O4.ClH/c31-19-7-6-17-13-21-27(29-22(33)14-18-3-1-2-11-28-18)9-8-20(32)25-26(27,23(17)24(19)34-25)10-12-30(21)15-16-4-5-16;/h1-3,6-7,11,16,21,25,31H,4-5,8-10,12-15H2,(H,29,33);1H/t21-,25+,26+,27-;/m1./s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.190 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Commonwealth University
Curated by ChEMBL
| Assay Description
Displacement of [3H]DPN from kappa opioid receptor (unknown origin) expressed in CHO cell membranes after 1.5 hrs |
J Med Chem 56: 9156-69 (2013)
Article DOI: 10.1021/jm4012214
BindingDB Entry DOI: 10.7270/Q2JH3Q4C |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 5
(Macaca fascicularis) | BDBM50464147

(CHEMBL256907)Show SMILES [H][C@]12CC[C@]([H])(C[C@@H](C1)n1c(C)nnc1C(C)C)N2CC[C@H](NC(=O)C1CCC(F)(F)CC1)c1ccccc1 |r,TLB:9:7:2.3:18| Show InChI InChI=1S/C29H41F2N5O/c1-19(2)27-34-33-20(3)36(27)25-17-23-9-10-24(18-25)35(23)16-13-26(21-7-5-4-6-8-21)32-28(37)22-11-14-29(30,31)15-12-22/h4-8,19,22-26H,9-18H2,1-3H3,(H,32,37)/t23-,24+,25+,26-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
| Article
PubMed
| 0.240 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [125I]MIP-1alpha from CCR5 receptor in rhesus monkey membrane incubated for 120 mins by liquid scintillation counting analysis |
Citation and Details
Article DOI: 10.1016/j.bmc.2016.09.059
BindingDB Entry DOI: 10.7270/Q2HD80CC |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 5
(Macaca fascicularis) | BDBM50334986

(4,4-Difluoro-cyclohexanecarboxylic acid {(S)-3-[(1...)Show SMILES CC(C)c1nnc(C)n1[C@H]1C[C@@H]2CC[C@H](C1)N2CC[C@H](NC(=O)C1CCC(F)(F)CC1)c1ccccc1 |r,TLB:17:16:9.15.10:13.12| Show InChI InChI=1S/C29H41F2N5O/c1-19(2)27-34-33-20(3)36(27)25-17-23-9-10-24(18-25)35(23)16-13-26(21-7-5-4-6-8-21)32-28(37)22-11-14-29(30,31)15-12-22/h4-8,19,22-26H,9-18H2,1-3H3,(H,32,37)/t23-,24+,25-,26-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.240 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Commonwealth University
Curated by ChEMBL
| Assay Description
Displacement of [125I]-MIP-1alpha from CCR5 in rhesus monkey Chem-1 cell membranes after 120 mins by liquid scintillation counting analysis |
Medchemcomm 4: 847-851 (2013)
Article DOI: 10.1039/c3md00080j
BindingDB Entry DOI: 10.7270/Q2474DTH |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50492287

(CHEMBL2397017)Show SMILES [H][C@@]12Oc3c4c(C[C@@]5([H])N(CC6CC6)CC[C@@]14[C@]5(CCC2=O)NC(=O)c1ccc2ccccc2n1)ccc3O |r,THB:10:9:17:4.5.6| Show InChI InChI=1S/C30H29N3O4/c34-22-10-8-19-15-24-30(32-28(36)21-9-7-18-3-1-2-4-20(18)31-21)12-11-23(35)27-29(30,25(19)26(22)37-27)13-14-33(24)16-17-5-6-17/h1-4,7-10,17,24,27,34H,5-6,11-16H2,(H,32,36)/t24-,27+,29+,30-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.260 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Commonwealth University
Curated by ChEMBL
| Assay Description
Displacement of [3H]DPN from kappa opioid receptor (unknown origin) after 1.5 hrs |
Bioorg Med Chem Lett 23: 3719-22 (2013)
Article DOI: 10.1016/j.bmcl.2013.05.027
BindingDB Entry DOI: 10.7270/Q2HM5CD9 |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM60212

((4R,4aS,7aR,12bS)-3-(cyclopropylmethyl)-4a,9-bis(o...)Show SMILES Oc1ccc2C[C@H]3N(CC4CC4)CC[C@@]45[C@@H](Oc1c24)C(=O)CC[C@@]35O Show InChI InChI=1S/C20H23NO4/c22-13-4-3-12-9-15-20(24)6-5-14(23)18-19(20,16(12)17(13)25-18)7-8-21(15)10-11-1-2-11/h3-4,11,15,18,22,24H,1-2,5-10H2/t15-,18+,19+,20-/m1/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
KEGG
PC cid
PC sid
UniChem
Similars
| DrugBank
Article
PubMed
| 0.260 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Commonwealth University
Curated by ChEMBL
| Assay Description
Displacement of [3H]NLX from mu opioid receptor (unknown origin) expressed in CHO cell membranes after 1.5 hrs |
J Med Chem 56: 9156-69 (2013)
Article DOI: 10.1021/jm4012214
BindingDB Entry DOI: 10.7270/Q2JH3Q4C |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50492287

(CHEMBL2397017)Show SMILES [H][C@@]12Oc3c4c(C[C@@]5([H])N(CC6CC6)CC[C@@]14[C@]5(CCC2=O)NC(=O)c1ccc2ccccc2n1)ccc3O |r,THB:10:9:17:4.5.6| Show InChI InChI=1S/C30H29N3O4/c34-22-10-8-19-15-24-30(32-28(36)21-9-7-18-3-1-2-4-20(18)31-21)12-11-23(35)27-29(30,25(19)26(22)37-27)13-14-33(24)16-17-5-6-17/h1-4,7-10,17,24,27,34H,5-6,11-16H2,(H,32,36)/t24-,27+,29+,30-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.260 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Commonwealth University
Curated by ChEMBL
| Assay Description
Displacement of [3H]DPN from kappa opioid receptor (unknown origin) expressed in CHO cell membranes after 1.5 hrs |
J Med Chem 56: 9156-69 (2013)
Article DOI: 10.1021/jm4012214
BindingDB Entry DOI: 10.7270/Q2JH3Q4C |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50492291
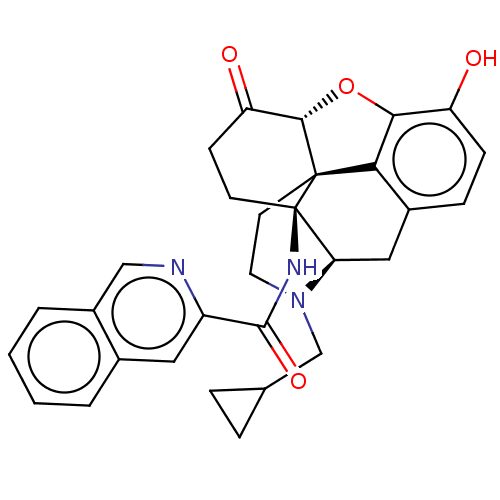
(CHEMBL2397016)Show SMILES [H][C@@]12Oc3c4c(C[C@@]5([H])N(CC6CC6)CC[C@@]14[C@]5(CCC2=O)NC(=O)c1cc2ccccc2cn1)ccc3O |r,THB:10:9:17:4.5.6| Show InChI InChI=1S/C30H29N3O4/c34-22-8-7-19-14-24-30(32-28(36)21-13-18-3-1-2-4-20(18)15-31-21)10-9-23(35)27-29(30,25(19)26(22)37-27)11-12-33(24)16-17-5-6-17/h1-4,7-8,13,15,17,24,27,34H,5-6,9-12,14,16H2,(H,32,36)/t24-,27+,29+,30-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.270 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Commonwealth University
Curated by ChEMBL
| Assay Description
Displacement of [3H]DPN from kappa opioid receptor (unknown origin) expressed in CHO cell membranes after 1.5 hrs |
J Med Chem 56: 9156-69 (2013)
Article DOI: 10.1021/jm4012214
BindingDB Entry DOI: 10.7270/Q2JH3Q4C |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50492291

(CHEMBL2397016)Show SMILES [H][C@@]12Oc3c4c(C[C@@]5([H])N(CC6CC6)CC[C@@]14[C@]5(CCC2=O)NC(=O)c1cc2ccccc2cn1)ccc3O |r,THB:10:9:17:4.5.6| Show InChI InChI=1S/C30H29N3O4/c34-22-8-7-19-14-24-30(32-28(36)21-13-18-3-1-2-4-20(18)15-31-21)10-9-23(35)27-29(30,25(19)26(22)37-27)11-12-33(24)16-17-5-6-17/h1-4,7-8,13,15,17,24,27,34H,5-6,9-12,14,16H2,(H,32,36)/t24-,27+,29+,30-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.270 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Commonwealth University
Curated by ChEMBL
| Assay Description
Displacement of [3H]DPN from kappa opioid receptor (unknown origin) after 1.5 hrs |
Bioorg Med Chem Lett 23: 3719-22 (2013)
Article DOI: 10.1016/j.bmcl.2013.05.027
BindingDB Entry DOI: 10.7270/Q2HM5CD9 |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50494376

(CHEMBL3086754)Show SMILES Cl.[H][C@@]12Oc3c4c(C[C@@]5([H])N(CC6CC6)CC[C@@]14[C@]5(CCC2=O)NC(=O)Cc1ccccn1)ccc3O |r,THB:11:10:18:5.6.7| Show InChI InChI=1S/C27H29N3O4.ClH/c31-19-7-6-17-13-21-27(29-22(33)14-18-3-1-2-11-28-18)9-8-20(32)25-26(27,23(17)24(19)34-25)10-12-30(21)15-16-4-5-16;/h1-3,6-7,11,16,21,25,31H,4-5,8-10,12-15H2,(H,29,33);1H/t21-,25+,26+,27-;/m1./s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.290 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Commonwealth University
Curated by ChEMBL
| Assay Description
Displacement of [3H]NLX from mu opioid receptor (unknown origin) expressed in CHO cell membranes after 1.5 hrs |
J Med Chem 56: 9156-69 (2013)
Article DOI: 10.1021/jm4012214
BindingDB Entry DOI: 10.7270/Q2JH3Q4C |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50494375

(CHEMBL3086756)Show SMILES Cl.[H][C@@]12Oc3c4c(C[C@@]5([H])N(CC6CC6)CC[C@@]14[C@]5(CCC2=O)NC(=O)CNC(=O)c1ccccn1)ccc3O |r,THB:11:10:18:5.6.7| Show InChI InChI=1S/C28H30N4O5.ClH/c33-19-7-6-17-13-21-28(31-22(35)14-30-26(36)18-3-1-2-11-29-18)9-8-20(34)25-27(28,23(17)24(19)37-25)10-12-32(21)15-16-4-5-16;/h1-3,6-7,11,16,21,25,33H,4-5,8-10,12-15H2,(H,30,36)(H,31,35);1H/t21-,25+,27+,28-;/m1./s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Commonwealth University
Curated by ChEMBL
| Assay Description
Displacement of [3H]NLX from mu opioid receptor (unknown origin) expressed in CHO cell membranes after 1.5 hrs |
J Med Chem 56: 9156-69 (2013)
Article DOI: 10.1021/jm4012214
BindingDB Entry DOI: 10.7270/Q2JH3Q4C |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50494377

(CHEMBL3086755)Show SMILES Cl.[H][C@@]12Oc3c4c(C[C@@]5([H])N(CC6CC6)CC[C@@]14[C@]5(CCC2=O)NC(=O)CCc1ccccn1)ccc3O |r,THB:11:10:18:5.6.7| Show InChI InChI=1S/C28H31N3O4.ClH/c32-20-8-6-18-15-22-28(30-23(34)9-7-19-3-1-2-13-29-19)11-10-21(33)26-27(28,24(18)25(20)35-26)12-14-31(22)16-17-4-5-17;/h1-3,6,8,13,17,22,26,32H,4-5,7,9-12,14-16H2,(H,30,34);1H/t22-,26+,27+,28-;/m1./s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.320 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Commonwealth University
Curated by ChEMBL
| Assay Description
Displacement of [3H]NLX from mu opioid receptor (unknown origin) expressed in CHO cell membranes after 1.5 hrs |
J Med Chem 56: 9156-69 (2013)
Article DOI: 10.1021/jm4012214
BindingDB Entry DOI: 10.7270/Q2JH3Q4C |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50492286
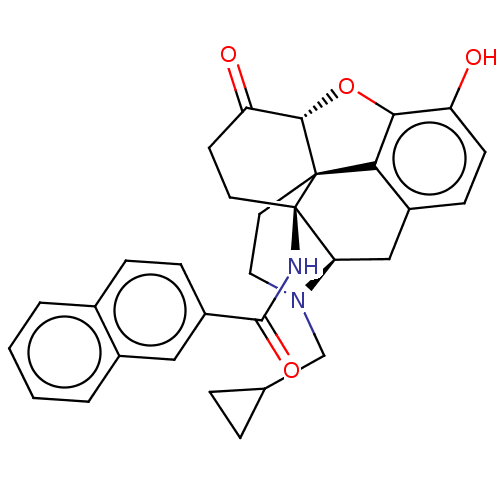
(CHEMBL2397019)Show SMILES [H][C@@]12Oc3c4c(C[C@@]5([H])N(CC6CC6)CC[C@@]14[C@]5(CCC2=O)NC(=O)c1ccc2ccccc2c1)ccc3O |r,THB:10:9:17:4.5.6| Show InChI InChI=1S/C31H30N2O4/c34-23-10-9-21-16-25-31(32-29(36)22-8-7-19-3-1-2-4-20(19)15-22)12-11-24(35)28-30(31,26(21)27(23)37-28)13-14-33(25)17-18-5-6-18/h1-4,7-10,15,18,25,28,34H,5-6,11-14,16-17H2,(H,32,36)/t25-,28+,30+,31-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.330 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Commonwealth University
Curated by ChEMBL
| Assay Description
Displacement of [3H]DPN from kappa opioid receptor (unknown origin) expressed in CHO cell membranes after 1.5 hrs |
J Med Chem 56: 9156-69 (2013)
Article DOI: 10.1021/jm4012214
BindingDB Entry DOI: 10.7270/Q2JH3Q4C |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50492290
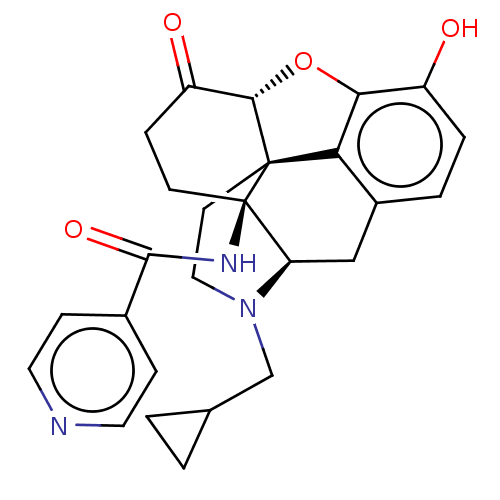
(CHEMBL2397022)Show SMILES [H][C@@]12Oc3c4c(C[C@@]5([H])N(CC6CC6)CC[C@@]14[C@]5(CCC2=O)NC(=O)c1ccncc1)ccc3O |r,THB:10:9:17:4.5.6| Show InChI InChI=1S/C26H27N3O4/c30-18-4-3-17-13-20-26(28-24(32)16-6-10-27-11-7-16)8-5-19(31)23-25(26,21(17)22(18)33-23)9-12-29(20)14-15-1-2-15/h3-4,6-7,10-11,15,20,23,30H,1-2,5,8-9,12-14H2,(H,28,32)/t20-,23+,25+,26-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.330 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Commonwealth University
Curated by ChEMBL
| Assay Description
Displacement of [3H]DPN from kappa opioid receptor (unknown origin) expressed in CHO cell membranes after 1.5 hrs |
J Med Chem 56: 9156-69 (2013)
Article DOI: 10.1021/jm4012214
BindingDB Entry DOI: 10.7270/Q2JH3Q4C |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50492290

(CHEMBL2397022)Show SMILES [H][C@@]12Oc3c4c(C[C@@]5([H])N(CC6CC6)CC[C@@]14[C@]5(CCC2=O)NC(=O)c1ccncc1)ccc3O |r,THB:10:9:17:4.5.6| Show InChI InChI=1S/C26H27N3O4/c30-18-4-3-17-13-20-26(28-24(32)16-6-10-27-11-7-16)8-5-19(31)23-25(26,21(17)22(18)33-23)9-12-29(20)14-15-1-2-15/h3-4,6-7,10-11,15,20,23,30H,1-2,5,8-9,12-14H2,(H,28,32)/t20-,23+,25+,26-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.330 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Commonwealth University
Curated by ChEMBL
| Assay Description
Displacement of [3H]DPN from kappa opioid receptor (unknown origin) after 1.5 hrs |
Bioorg Med Chem Lett 23: 3719-22 (2013)
Article DOI: 10.1016/j.bmcl.2013.05.027
BindingDB Entry DOI: 10.7270/Q2HM5CD9 |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(MOUSE) | BDBM60212

((4R,4aS,7aR,12bS)-3-(cyclopropylmethyl)-4a,9-bis(o...)Show SMILES Oc1ccc2C[C@H]3N(CC4CC4)CC[C@@]45[C@@H](Oc1c24)C(=O)CC[C@@]35O Show InChI InChI=1S/C20H23NO4/c22-13-4-3-12-9-15-20(24)6-5-14(23)18-19(20,16(12)17(13)25-18)7-8-21(15)10-11-1-2-11/h3-4,11,15,18,22,24H,1-2,5-10H2/t15-,18+,19+,20-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.330 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Commonwealth University
Curated by ChEMBL
| Assay Description
Displacement of [3H]-naloxone from mouse mu opioid receptor expressed in CHO cells after 1.5 hrs |
Bioorg Med Chem Lett 23: 5045-8 (2013)
Article DOI: 10.1016/j.bmcl.2013.07.043
BindingDB Entry DOI: 10.7270/Q2H41VC9 |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(MOUSE) | BDBM60212

((4R,4aS,7aR,12bS)-3-(cyclopropylmethyl)-4a,9-bis(o...)Show SMILES Oc1ccc2C[C@H]3N(CC4CC4)CC[C@@]45[C@@H](Oc1c24)C(=O)CC[C@@]35O Show InChI InChI=1S/C20H23NO4/c22-13-4-3-12-9-15-20(24)6-5-14(23)18-19(20,16(12)17(13)25-18)7-8-21(15)10-11-1-2-11/h3-4,11,15,18,22,24H,1-2,5-10H2/t15-,18+,19+,20-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.330 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Commonwealth University
Curated by ChEMBL
| Assay Description
Displacement of [3H]naloxane from mouse MOR expressed in CHO cells after 1.5 hrs by [35S]GTPgammaS binding assay |
Bioorg Med Chem 23: 1701-15 (2015)
Article DOI: 10.1016/j.bmc.2015.02.055
BindingDB Entry DOI: 10.7270/Q2PK0HTN |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50492286

(CHEMBL2397019)Show SMILES [H][C@@]12Oc3c4c(C[C@@]5([H])N(CC6CC6)CC[C@@]14[C@]5(CCC2=O)NC(=O)c1ccc2ccccc2c1)ccc3O |r,THB:10:9:17:4.5.6| Show InChI InChI=1S/C31H30N2O4/c34-23-10-9-21-16-25-31(32-29(36)22-8-7-19-3-1-2-4-20(19)15-22)12-11-24(35)28-30(31,26(21)27(23)37-28)13-14-33(25)17-18-5-6-18/h1-4,7-10,15,18,25,28,34H,5-6,11-14,16-17H2,(H,32,36)/t25-,28+,30+,31-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.330 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Commonwealth University
Curated by ChEMBL
| Assay Description
Displacement of [3H]DPN from kappa opioid receptor (unknown origin) after 1.5 hrs |
Bioorg Med Chem Lett 23: 3719-22 (2013)
Article DOI: 10.1016/j.bmcl.2013.05.027
BindingDB Entry DOI: 10.7270/Q2HM5CD9 |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM60212

((4R,4aS,7aR,12bS)-3-(cyclopropylmethyl)-4a,9-bis(o...)Show SMILES Oc1ccc2C[C@H]3N(CC4CC4)CC[C@@]45[C@@H](Oc1c24)C(=O)CC[C@@]35O Show InChI InChI=1S/C20H23NO4/c22-13-4-3-12-9-15-20(24)6-5-14(23)18-19(20,16(12)17(13)25-18)7-8-21(15)10-11-1-2-11/h3-4,11,15,18,22,24H,1-2,5-10H2/t15-,18+,19+,20-/m1/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
KEGG
PC cid
PC sid
UniChem
Similars
| DrugBank
Article
PubMed
| 0.340 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Commonwealth University
Curated by ChEMBL
| Assay Description
Displacement of [3H]naloxone from mu opioid receptor (unknown origin) after 1.5 hrs |
Bioorg Med Chem Lett 23: 3719-22 (2013)
Article DOI: 10.1016/j.bmcl.2013.05.027
BindingDB Entry DOI: 10.7270/Q2HM5CD9 |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
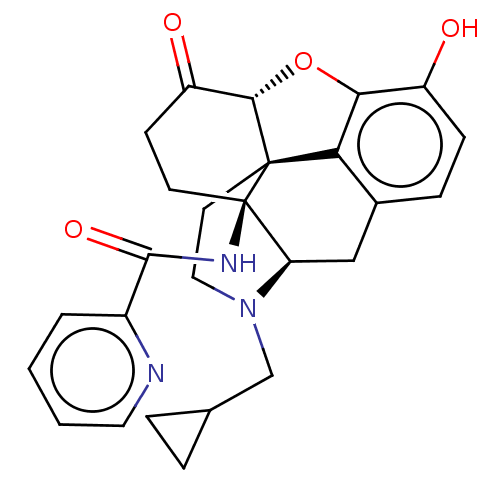
(Homo sapiens (Human)) | BDBM50492289
(CHEMBL2397020)Show SMILES [H][C@@]12Oc3c4c(C[C@@]5([H])N(CC6CC6)CC[C@@]14[C@]5(CCC2=O)NC(=O)c1ccccn1)ccc3O |r,THB:10:9:17:4.5.6| Show InChI InChI=1S/C26H27N3O4/c30-18-7-6-16-13-20-26(28-24(32)17-3-1-2-11-27-17)9-8-19(31)23-25(26,21(16)22(18)33-23)10-12-29(20)14-15-4-5-15/h1-3,6-7,11,15,20,23,30H,4-5,8-10,12-14H2,(H,28,32)/t20-,23+,25+,26-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.360 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Commonwealth University
Curated by ChEMBL
| Assay Description
Displacement of [3H]DPN from kappa opioid receptor (unknown origin) expressed in CHO cell membranes after 1.5 hrs |
J Med Chem 56: 9156-69 (2013)
Article DOI: 10.1021/jm4012214
BindingDB Entry DOI: 10.7270/Q2JH3Q4C |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50492289

(CHEMBL2397020)Show SMILES [H][C@@]12Oc3c4c(C[C@@]5([H])N(CC6CC6)CC[C@@]14[C@]5(CCC2=O)NC(=O)c1ccccn1)ccc3O |r,THB:10:9:17:4.5.6| Show InChI InChI=1S/C26H27N3O4/c30-18-7-6-16-13-20-26(28-24(32)17-3-1-2-11-27-17)9-8-19(31)23-25(26,21(16)22(18)33-23)10-12-29(20)14-15-4-5-15/h1-3,6-7,11,15,20,23,30H,4-5,8-10,12-14H2,(H,28,32)/t20-,23+,25+,26-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.360 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Commonwealth University
Curated by ChEMBL
| Assay Description
Displacement of [3H]DPN from kappa opioid receptor (unknown origin) after 1.5 hrs |
Bioorg Med Chem Lett 23: 3719-22 (2013)
Article DOI: 10.1016/j.bmcl.2013.05.027
BindingDB Entry DOI: 10.7270/Q2HM5CD9 |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(Homo sapiens (Human)) | BDBM50494375

(CHEMBL3086756)Show SMILES Cl.[H][C@@]12Oc3c4c(C[C@@]5([H])N(CC6CC6)CC[C@@]14[C@]5(CCC2=O)NC(=O)CNC(=O)c1ccccn1)ccc3O |r,THB:11:10:18:5.6.7| Show InChI InChI=1S/C28H30N4O5.ClH/c33-19-7-6-17-13-21-28(31-22(35)14-30-26(36)18-3-1-2-11-29-18)9-8-20(34)25-27(28,23(17)24(19)37-25)10-12-32(21)15-16-4-5-16;/h1-3,6-7,11,16,21,25,33H,4-5,8-10,12-15H2,(H,30,36)(H,31,35);1H/t21-,25+,27+,28-;/m1./s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.370 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Commonwealth University
Curated by ChEMBL
| Assay Description
Displacement of [3H]NTI from delta opioid receptor (unknown origin) expressed in CHO cell membranes after 1.5 hrs |
J Med Chem 56: 9156-69 (2013)
Article DOI: 10.1021/jm4012214
BindingDB Entry DOI: 10.7270/Q2JH3Q4C |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(MOUSE) | BDBM60212

((4R,4aS,7aR,12bS)-3-(cyclopropylmethyl)-4a,9-bis(o...)Show SMILES Oc1ccc2C[C@H]3N(CC4CC4)CC[C@@]45[C@@H](Oc1c24)C(=O)CC[C@@]35O Show InChI InChI=1S/C20H23NO4/c22-13-4-3-12-9-15-20(24)6-5-14(23)18-19(20,16(12)17(13)25-18)7-8-21(15)10-11-1-2-11/h3-4,11,15,18,22,24H,1-2,5-10H2/t15-,18+,19+,20-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.390 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]NLX from mouse MOR expressed in CHO cell membrane incubated for 90 mins by liquid scintillation spectrophotometry |
Citation and Details
Article DOI: 10.1016/j.bmc.2016.09.059
BindingDB Entry DOI: 10.7270/Q2HD80CC |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50352116
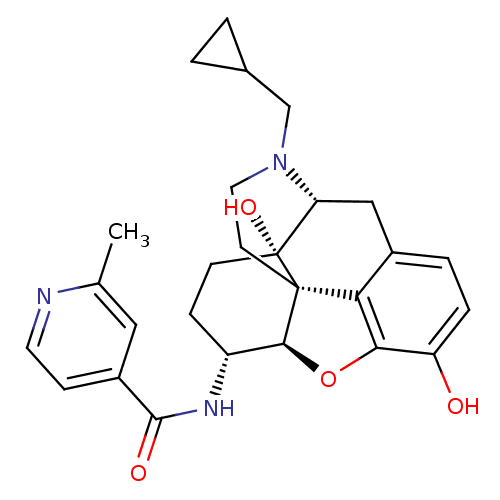
(CHEMBL1824512 | CHEMBL1852558)Show SMILES Cc1cc(ccn1)C(=O)N[C@@H]1CC[C@@]2(O)[C@H]3Cc4ccc(O)c5O[C@@H]1[C@]2(CCN3CC1CC1)c45 |r| Show InChI InChI=1S/C27H31N3O4/c1-15-12-18(7-10-28-15)25(32)29-19-6-8-27(33)21-13-17-4-5-20(31)23-22(17)26(27,24(19)34-23)9-11-30(21)14-16-2-3-16/h4-5,7,10,12,16,19,21,24,31,33H,2-3,6,8-9,11,13-14H2,1H3,(H,29,32)/t19-,21-,24+,26+,27-/m1/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.390 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Commonwealth University
Curated by ChEMBL
| Assay Description
Displacement of [3H]Naloxone from mu opioid receptor expressed in CHO cells after 1 hr |
J Med Chem 55: 10118-29 (2012)
Article DOI: 10.1021/jm301247n
BindingDB Entry DOI: 10.7270/Q29P32SK |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50352116

(CHEMBL1824512 | CHEMBL1852558)Show SMILES Cc1cc(ccn1)C(=O)N[C@@H]1CC[C@@]2(O)[C@H]3Cc4ccc(O)c5O[C@@H]1[C@]2(CCN3CC1CC1)c45 |r| Show InChI InChI=1S/C27H31N3O4/c1-15-12-18(7-10-28-15)25(32)29-19-6-8-27(33)21-13-17-4-5-20(31)23-22(17)26(27,24(19)34-23)9-11-30(21)14-16-2-3-16/h4-5,7,10,12,16,19,21,24,31,33H,2-3,6,8-9,11,13-14H2,1H3,(H,29,32)/t19-,21-,24+,26+,27-/m1/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.390 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Commonwealth University
Curated by ChEMBL
| Assay Description
Displacement of [3H]naloxone from mu opioid receptor expressed in CHO cells after 1 hr |
Bioorg Med Chem Lett 21: 5625-9 (2011)
Article DOI: 10.1016/j.bmcl.2011.06.135
BindingDB Entry DOI: 10.7270/Q21J9B57 |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50399661
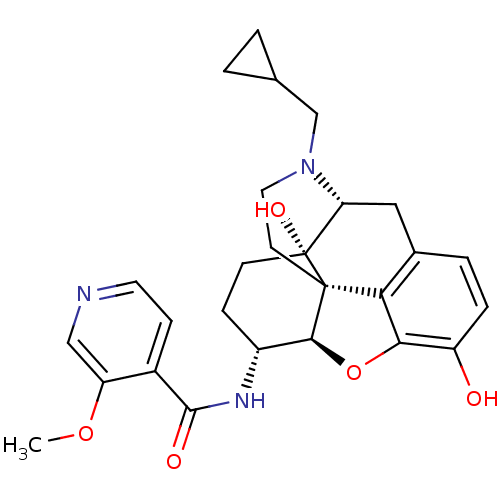
(CHEMBL2178339)Show SMILES COc1cnccc1C(=O)N[C@@H]1CC[C@@]2(O)[C@H]3Cc4ccc(O)c5O[C@@H]1[C@]2(CCN3CC1CC1)c45 |r| Show InChI InChI=1S/C27H31N3O5/c1-34-20-13-28-10-7-17(20)25(32)29-18-6-8-27(33)21-12-16-4-5-19(31)23-22(16)26(27,24(18)35-23)9-11-30(21)14-15-2-3-15/h4-5,7,10,13,15,18,21,24,31,33H,2-3,6,8-9,11-12,14H2,1H3,(H,29,32)/t18-,21-,24+,26+,27-/m1/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.430 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Commonwealth University
Curated by ChEMBL
| Assay Description
Displacement of [3H]Naloxone from mu opioid receptor expressed in CHO cells after 1 hr |
J Med Chem 55: 10118-29 (2012)
Article DOI: 10.1021/jm301247n
BindingDB Entry DOI: 10.7270/Q29P32SK |
More data for this
Ligand-Target Pair | |
E3 ubiquitin-protein ligase Mdm2
(Homo sapiens (Human)) | BDBM50513204

(CHEMBL4537250)Show SMILES CC(C)(C)C[C@H]1NC([C@H](c2cccc(Cl)c2F)[C@@]11C(=O)Nc2cc(Cl)ccc12)C(=O)N[C@H]1C[C@@](C)(O)C1 |r,wU:33.38,17.29,31.34,8.8,wD:5.4,(55.7,-19.72,;54.25,-19.21,;53.96,-17.7,;55.33,-18.11,;53.08,-20.22,;51.63,-19.71,;51.18,-18.23,;49.64,-18.2,;49.13,-19.66,;47.62,-19.35,;47.13,-17.89,;45.63,-17.58,;44.6,-18.74,;45.1,-20.2,;44.09,-21.36,;46.61,-20.5,;45.83,-21.82,;50.36,-20.59,;51.61,-21.5,;53.08,-21.03,;51.13,-22.97,;49.59,-22.97,;48.57,-24.1,;47.07,-23.79,;46.05,-24.94,;46.59,-22.32,;47.62,-21.19,;49.12,-21.51,;49.2,-16.72,;47.7,-16.36,;50.26,-15.6,;51.76,-15.96,;53.06,-15.15,;53.86,-16.46,;54.95,-15.36,;55.36,-16.85,;52.56,-17.27,)| Show InChI InChI=1S/C28H32Cl2FN3O3/c1-26(2,3)13-20-28(17-9-8-14(29)10-19(17)33-25(28)36)21(16-6-5-7-18(30)22(16)31)23(34-20)24(35)32-15-11-27(4,37)12-15/h5-10,15,20-21,23,34,37H,11-13H2,1-4H3,(H,32,35)(H,33,36)/t15-,20-,21+,23?,27+,28+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.440 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sichuan University
Curated by ChEMBL
| Assay Description
Inhibition of p53 protein binding to MDM2 (unknown origin) by western blot analysis |
Eur J Med Chem 176: 92-104 (2019)
Article DOI: 10.1016/j.ejmech.2019.05.018
BindingDB Entry DOI: 10.7270/Q2V12851 |
More data for this
Ligand-Target Pair | |
E3 ubiquitin-protein ligase Mdm2
(Homo sapiens (Human)) | BDBM50513204

(CHEMBL4537250)Show SMILES CC(C)(C)C[C@H]1NC([C@H](c2cccc(Cl)c2F)[C@@]11C(=O)Nc2cc(Cl)ccc12)C(=O)N[C@H]1C[C@@](C)(O)C1 |r,wU:33.38,17.29,31.34,8.8,wD:5.4,(55.7,-19.72,;54.25,-19.21,;53.96,-17.7,;55.33,-18.11,;53.08,-20.22,;51.63,-19.71,;51.18,-18.23,;49.64,-18.2,;49.13,-19.66,;47.62,-19.35,;47.13,-17.89,;45.63,-17.58,;44.6,-18.74,;45.1,-20.2,;44.09,-21.36,;46.61,-20.5,;45.83,-21.82,;50.36,-20.59,;51.61,-21.5,;53.08,-21.03,;51.13,-22.97,;49.59,-22.97,;48.57,-24.1,;47.07,-23.79,;46.05,-24.94,;46.59,-22.32,;47.62,-21.19,;49.12,-21.51,;49.2,-16.72,;47.7,-16.36,;50.26,-15.6,;51.76,-15.96,;53.06,-15.15,;53.86,-16.46,;54.95,-15.36,;55.36,-16.85,;52.56,-17.27,)| Show InChI InChI=1S/C28H32Cl2FN3O3/c1-26(2,3)13-20-28(17-9-8-14(29)10-19(17)33-25(28)36)21(16-6-5-7-18(30)22(16)31)23(34-20)24(35)32-15-11-27(4,37)12-15/h5-10,15,20-21,23,34,37H,11-13H2,1-4H3,(H,32,35)(H,33,36)/t15-,20-,21+,23?,27+,28+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.440 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sichuan University
Curated by ChEMBL
| Assay Description
Inhibition of wild type p53 protein binding to MDM2 in human HCT116 cells by immunoblot analysis |
Eur J Med Chem 176: 92-104 (2019)
Article DOI: 10.1016/j.ejmech.2019.05.018
BindingDB Entry DOI: 10.7270/Q2V12851 |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50352118
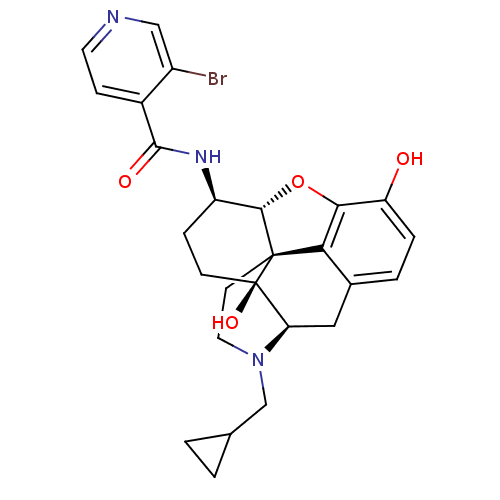
(CHEMBL1824513 | CHEMBL1852602)Show SMILES Oc1ccc2C[C@H]3N(CC4CC4)CC[C@@]45[C@@H](Oc1c24)[C@@H](CC[C@@]35O)NC(=O)c1ccncc1Br |r| Show InChI InChI=1S/C26H28BrN3O4/c27-17-12-28-9-6-16(17)24(32)29-18-5-7-26(33)20-11-15-3-4-19(31)22-21(15)25(26,23(18)34-22)8-10-30(20)13-14-1-2-14/h3-4,6,9,12,14,18,20,23,31,33H,1-2,5,7-8,10-11,13H2,(H,29,32)/t18-,20-,23+,25+,26-/m1/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.450 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Commonwealth University
Curated by ChEMBL
| Assay Description
Displacement of [3H]Naloxone from mu opioid receptor expressed in CHO cells after 1 hr |
J Med Chem 55: 10118-29 (2012)
Article DOI: 10.1021/jm301247n
BindingDB Entry DOI: 10.7270/Q29P32SK |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(MOUSE) | BDBM50066282
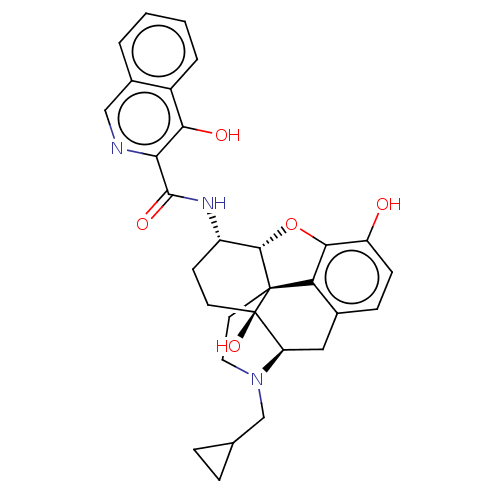
(CHEMBL2417568)Show SMILES [H][C@@]12Oc3c4c(C[C@@]5([H])N(CC6CC6)CC[C@@]14[C@@]5(O)CC[C@@H]2NC(=O)c1ncc2ccccc2c1O)ccc3O |r,THB:10:9:17:4.5.6| Show InChI InChI=1S/C30H31N3O5/c34-21-8-7-17-13-22-30(37)10-9-20(32-28(36)24-25(35)19-4-2-1-3-18(19)14-31-24)27-29(30,23(17)26(21)38-27)11-12-33(22)15-16-5-6-16/h1-4,7-8,14,16,20,22,27,34-35,37H,5-6,9-13,15H2,(H,32,36)/t20-,22+,27-,29-,30+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.450 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Commonwealth University
Curated by ChEMBL
| Assay Description
Displacement of [3H]-naloxone from mouse mu opioid receptor expressed in CHO cells after 1.5 hrs |
Bioorg Med Chem Lett 23: 5045-8 (2013)
Article DOI: 10.1016/j.bmcl.2013.07.043
BindingDB Entry DOI: 10.7270/Q2H41VC9 |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(MOUSE) | BDBM50066282

(CHEMBL2417568)Show SMILES [H][C@@]12Oc3c4c(C[C@@]5([H])N(CC6CC6)CC[C@@]14[C@@]5(O)CC[C@@H]2NC(=O)c1ncc2ccccc2c1O)ccc3O |r,THB:10:9:17:4.5.6| Show InChI InChI=1S/C30H31N3O5/c34-21-8-7-17-13-22-30(37)10-9-20(32-28(36)24-25(35)19-4-2-1-3-18(19)14-31-24)27-29(30,23(17)26(21)38-27)11-12-33(22)15-16-5-6-16/h1-4,7-8,14,16,20,22,27,34-35,37H,5-6,9-13,15H2,(H,32,36)/t20-,22+,27-,29-,30+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.450 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Commonwealth University
Curated by ChEMBL
| Assay Description
Displacement of [3H]naloxane from mouse MOR expressed in CHO cells after 1.5 hrs by [35S]GTPgammaS binding assay |
Bioorg Med Chem 23: 1701-15 (2015)
Article DOI: 10.1016/j.bmc.2015.02.055
BindingDB Entry DOI: 10.7270/Q2PK0HTN |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50352118

(CHEMBL1824513 | CHEMBL1852602)Show SMILES Oc1ccc2C[C@H]3N(CC4CC4)CC[C@@]45[C@@H](Oc1c24)[C@@H](CC[C@@]35O)NC(=O)c1ccncc1Br |r| Show InChI InChI=1S/C26H28BrN3O4/c27-17-12-28-9-6-16(17)24(32)29-18-5-7-26(33)20-11-15-3-4-19(31)22-21(15)25(26,23(18)34-22)8-10-30(20)13-14-1-2-14/h3-4,6,9,12,14,18,20,23,31,33H,1-2,5,7-8,10-11,13H2,(H,29,32)/t18-,20-,23+,25+,26-/m1/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.450 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Commonwealth University
Curated by ChEMBL
| Assay Description
Displacement of [3H]naloxone from mu opioid receptor expressed in CHO cells after 1 hr |
Bioorg Med Chem Lett 21: 5625-9 (2011)
Article DOI: 10.1016/j.bmcl.2011.06.135
BindingDB Entry DOI: 10.7270/Q21J9B57 |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50399662
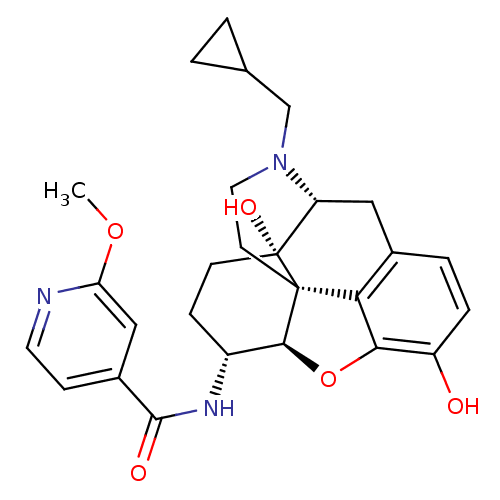
(CHEMBL2178338)Show SMILES COc1cc(ccn1)C(=O)N[C@@H]1CC[C@@]2(O)[C@H]3Cc4ccc(O)c5O[C@@H]1[C@]2(CCN3CC1CC1)c45 |r| Show InChI InChI=1S/C27H31N3O5/c1-34-21-13-17(7-10-28-21)25(32)29-18-6-8-27(33)20-12-16-4-5-19(31)23-22(16)26(27,24(18)35-23)9-11-30(20)14-15-2-3-15/h4-5,7,10,13,15,18,20,24,31,33H,2-3,6,8-9,11-12,14H2,1H3,(H,29,32)/t18-,20-,24+,26+,27-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.460 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Commonwealth University
Curated by ChEMBL
| Assay Description
Displacement of [3H]DPN from kappa opioid receptor expressed in CHO cells after 1 hr |
J Med Chem 55: 10118-29 (2012)
Article DOI: 10.1021/jm301247n
BindingDB Entry DOI: 10.7270/Q29P32SK |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(MOUSE) | BDBM50066279
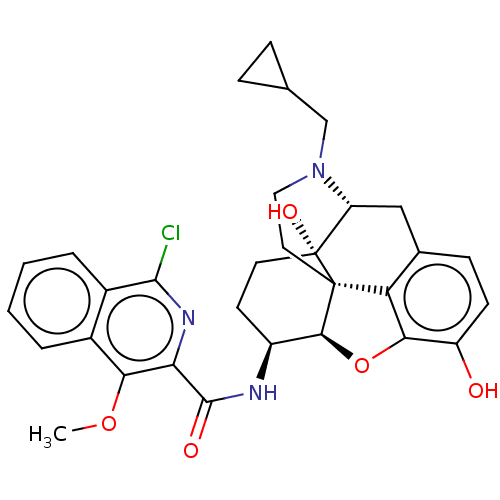
(CHEMBL2419121)Show SMILES [H][C@@]12Oc3c4c(C[C@@]5([H])N(CC6CC6)CC[C@@]14[C@@]5(O)CC[C@@H]2NC(=O)c1nc(Cl)c2ccccc2c1OC)ccc3O |r,THB:10:9:17:4.5.6| Show InChI InChI=1S/C31H32ClN3O5/c1-39-25-18-4-2-3-5-19(18)28(32)34-24(25)29(37)33-20-10-11-31(38)22-14-17-8-9-21(36)26-23(17)30(31,27(20)40-26)12-13-35(22)15-16-6-7-16/h2-5,8-9,16,20,22,27,36,38H,6-7,10-15H2,1H3,(H,33,37)/t20-,22+,27-,30-,31+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.550 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Commonwealth University
Curated by ChEMBL
| Assay Description
Displacement of [3H]naloxane from mouse MOR expressed in CHO cells after 1.5 hrs by [35S]GTPgammaS binding assay |
Bioorg Med Chem 23: 1701-15 (2015)
Article DOI: 10.1016/j.bmc.2015.02.055
BindingDB Entry DOI: 10.7270/Q2PK0HTN |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data