 Found 171 hits with Last Name = 'zech' and Initial = 'sg'
Found 171 hits with Last Name = 'zech' and Initial = 'sg' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
L-lactate dehydrogenase A chain
(Homo sapiens (Human)) | BDBM23222
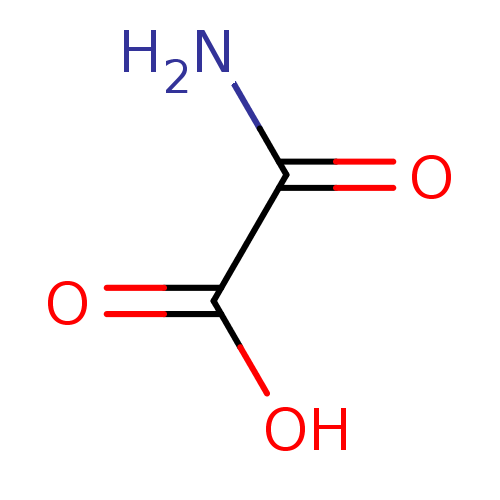
(Oxalamic acid | Oxamate | Oxamate, 3 | Oxamidic Ac...)Show InChI InChI=1S/C2H3NO3/c3-1(4)2(5)6/h(H2,3,4)(H,5,6) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
| PDB
Article
PubMed
| 2.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
ARIAD Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human LDH-A |
J Med Chem 56: 1023-40 (2013)
Article DOI: 10.1021/jm3014844
BindingDB Entry DOI: 10.7270/Q2QC04TB |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50322823

((S)-N-(4-(3-chloro-4-fluorophenylamino)-7-(tetrahy...)Show SMILES CN(C)C\C=C\C(=O)Nc1cc2c(Nc3ccc(F)c(Cl)c3)ncnc2cc1O[C@H]1CCOC1 |r| Show InChI InChI=1S/C24H25ClFN5O3/c1-31(2)8-3-4-23(32)30-21-11-17-20(12-22(21)34-16-7-9-33-13-16)27-14-28-24(17)29-15-5-6-19(26)18(25)10-15/h3-6,10-12,14,16H,7-9,13H2,1-2H3,(H,30,32)(H,27,28,29)/b4-3+/t16-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
| n/a | n/a | 3.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
GTPase KRas
(Homo sapiens (Human)) | BDBM50514386

(CHEMBL4456801)Show SMILES CC(C)c1ccccc1-n1c2cc(c(Cl)cc2c(nc1=O)N1CCN(CC1)C(=O)C=C)-c1c(C)ccc2[nH]ncc12 |(61.07,-56.45,;62.4,-57.23,;62.4,-58.77,;63.74,-56.45,;65.08,-57.23,;66.41,-56.46,;66.41,-54.91,;65.08,-54.15,;63.75,-54.91,;62.41,-54.15,;62.41,-52.62,;63.75,-51.84,;63.74,-50.29,;62.4,-49.53,;62.4,-47.99,;61.07,-50.3,;61.08,-51.84,;59.75,-52.61,;59.74,-54.14,;61.07,-54.91,;59.73,-55.67,;58.42,-51.83,;58.43,-50.29,;57.11,-49.52,;55.77,-50.27,;55.76,-51.82,;57.1,-52.6,;54.44,-49.5,;54.45,-47.96,;53.1,-50.25,;51.77,-49.48,;65.07,-49.52,;65.06,-47.99,;63.72,-47.24,;66.38,-47.22,;67.72,-47.99,;67.72,-49.52,;68.86,-50.54,;68.24,-51.94,;66.72,-51.79,;66.39,-50.29,)| Show InChI InChI=1S/C32H31ClN6O2/c1-5-29(40)37-12-14-38(15-13-37)31-23-16-25(33)22(30-20(4)10-11-26-24(30)18-34-36-26)17-28(23)39(32(41)35-31)27-9-7-6-8-21(27)19(2)3/h5-11,16-19H,1,12-15H2,2-4H3,(H,34,36) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of KRAS in human MIAPaca2 cells assessed as decrease in EGF-stimulated ERK1/2 phosphorylation preincubated for 2 hrs followed by EGF stimu... |
J Med Chem 63: 52-65 (2020)
Article DOI: 10.1021/acs.jmedchem.9b01180
BindingDB Entry DOI: 10.7270/Q2SQ93Q2 |
More data for this
Ligand-Target Pair | |
GTPase KRas
(Homo sapiens (Human)) | BDBM50514393

(CHEMBL4539214)Show SMILES CC(C)c1nccc(C)c1-n1c2nc(c(Cl)cc2c(nc1=O)N1CCN(C[C@@H]1C)C(=O)C=C)-c1c(O)cccc1F |r,wU:27.31,(30.05,-14.2,;31.38,-14.97,;31.39,-16.5,;32.71,-14.19,;34.04,-14.96,;35.37,-14.18,;35.36,-12.64,;34.03,-11.88,;34.01,-10.35,;32.7,-12.65,;31.37,-11.89,;31.36,-10.36,;32.71,-9.58,;32.7,-8.03,;31.36,-7.26,;31.36,-5.72,;30.03,-8.03,;30.03,-9.58,;28.7,-10.35,;28.7,-11.88,;30.03,-12.65,;28.69,-13.41,;27.37,-9.57,;27.38,-8.03,;26.06,-7.25,;24.72,-8.01,;24.71,-9.56,;26.05,-10.34,;26.04,-11.88,;23.39,-7.23,;23.4,-5.69,;22.05,-7.99,;20.71,-7.21,;34.03,-7.26,;35.37,-8.02,;35.37,-9.56,;36.7,-7.25,;36.7,-5.71,;35.35,-4.94,;34.02,-5.72,;32.68,-4.95,)| Show InChI InChI=1S/C30H30ClFN6O3/c1-6-23(40)36-12-13-37(18(5)15-36)28-19-14-20(31)26(24-21(32)8-7-9-22(24)39)34-29(19)38(30(41)35-28)27-17(4)10-11-33-25(27)16(2)3/h6-11,14,16,18,39H,1,12-13,15H2,2-5H3/t18-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of KRAS in human MIAPaca2 cells assessed as decrease in EGF-stimulated ERK1/2 phosphorylation preincubated for 2 hrs followed by EGF stimu... |
J Med Chem 63: 52-65 (2020)
Article DOI: 10.1021/acs.jmedchem.9b01180
BindingDB Entry DOI: 10.7270/Q2SQ93Q2 |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50609616
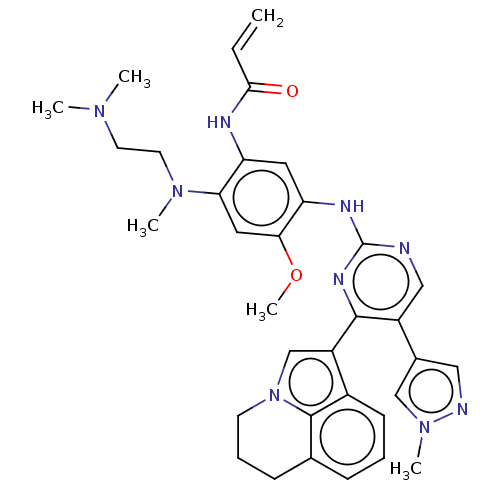
(CHEMBL5291353)Show SMILES COc1cc(N(C)CCN(C)C)c(NC(=O)C=C)cc1Nc1ncc(-c2cnn(C)c2)c(n1)-c1cn2CCCc3cccc1c23 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| UniChem
| | n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50609613
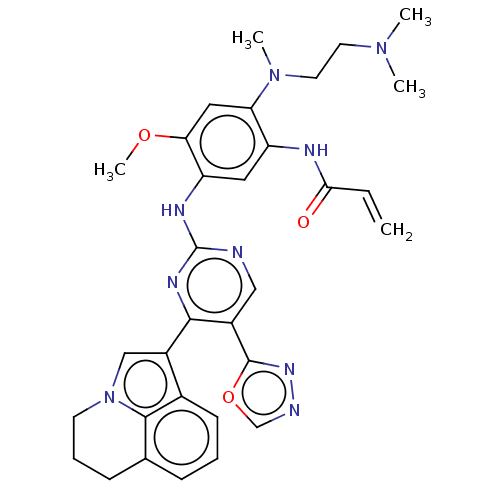
(CHEMBL5270000)Show SMILES COc1cc(N(C)CCN(C)C)c(NC(=O)C=C)cc1Nc1ncc(-c2nnco2)c(n1)-c1cn2CCCc3cccc1c23 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| UniChem
| | n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50609617

(CHEMBL5284387)Show SMILES COc1cc(N(C)CCN(C)C)c(NC(=O)C=C)cc1Nc1ncc(-c2cnn(c2)C(C)C)c(n1)-c1cn2CCCc3cccc1c23 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| UniChem
| | n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM368373
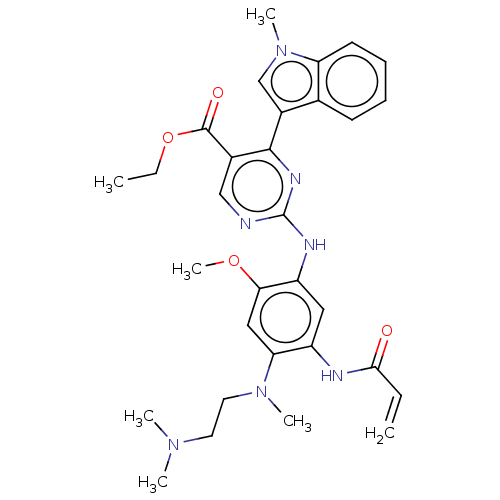
(US10227342, Example 9)Show SMILES CCOC(=O)c1cnc(Nc2cc(NC(=O)C=C)c(cc2OC)N(C)CCN(C)C)nc1-c1cn(C)c2ccccc12 Show InChI InChI=1S/C31H37N7O4/c1-8-28(39)33-23-16-24(27(41-7)17-26(23)37(5)15-14-36(3)4)34-31-32-18-21(30(40)42-9-2)29(35-31)22-19-38(6)25-13-11-10-12-20(22)25/h8,10-13,16-19H,1,9,14-15H2,2-7H3,(H,33,39)(H,32,34,35) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Choline kinase alpha
(Homo sapiens (Human)) | BDBM50146014
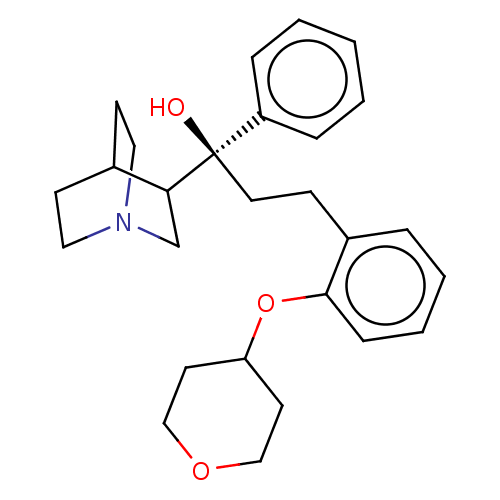
(CHEMBL3765554)Show SMILES O[C@@](CCc1ccccc1OC1CCOCC1)(C1CN2CCC1CC2)c1ccccc1 |r,wU:1.1,(.04,-1,;1.1,-1.63,;-.24,-2.39,;-.26,-3.93,;-1.6,-4.68,;-2.93,-3.9,;-4.27,-4.66,;-4.28,-6.2,;-2.96,-6.98,;-1.62,-6.22,;-.29,-7.01,;-.31,-8.55,;1.01,-9.34,;.99,-10.88,;-.36,-11.63,;-1.68,-10.84,;-1.66,-9.3,;2.43,-2.41,;2.41,-4.03,;3.81,-4.83,;5.21,-4.01,;5.21,-2.38,;3.81,-1.57,;3.26,-2.74,;4.33,-3.38,;1.11,-.09,;2.45,.67,;2.47,2.21,;1.14,2.99,;-.2,2.24,;-.22,.7,)| Show InChI InChI=1S/C27H35NO3/c29-27(23-7-2-1-3-8-23,25-20-28-16-11-21(25)12-17-28)15-10-22-6-4-5-9-26(22)31-24-13-18-30-19-14-24/h1-9,21,24-25,29H,10-20H2/t25?,27-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
ARIAD Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human ChoKalpha using choline as substrate by ultraviolet spectroscopic assay |
J Med Chem 59: 671-86 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01552
BindingDB Entry DOI: 10.7270/Q23X88HX |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM368372
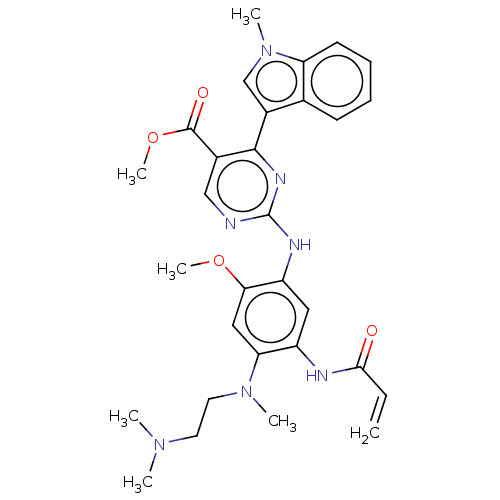
(US10227342, Example 8)Show SMILES COC(=O)c1cnc(Nc2cc(NC(=O)C=C)c(cc2OC)N(C)CCN(C)C)nc1-c1cn(C)c2ccccc12 Show InChI InChI=1S/C30H35N7O4/c1-8-27(38)32-22-15-23(26(40-6)16-25(22)36(4)14-13-35(2)3)33-30-31-17-20(29(39)41-7)28(34-30)21-18-37(5)24-12-10-9-11-19(21)24/h8-12,15-18H,1,13-14H2,2-7H3,(H,32,38)(H,31,33,34) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
GTPase KRas
(Homo sapiens (Human)) | BDBM50514400

(CHEMBL4434842)Show SMILES CC(C)c1ncnc(C(C)C)c1-n1c2nc(c(Cl)cc2c(nc1=O)N1CCN(C[C@@H]1C)C(=O)C=C)-c1c(O)cccc1F |r,wU:29.33,(40.54,-55.65,;39.21,-54.89,;39.2,-53.36,;37.88,-55.67,;39.21,-56.43,;39.23,-57.98,;37.9,-58.75,;36.56,-57.99,;36.55,-59.52,;35.21,-60.28,;37.88,-60.3,;36.56,-56.45,;35.22,-55.68,;35.21,-54.15,;36.56,-53.37,;36.55,-51.81,;35.21,-51.05,;35.21,-49.51,;33.88,-51.82,;33.88,-53.37,;32.55,-54.14,;32.54,-55.68,;33.88,-56.45,;33.87,-57.98,;31.22,-53.36,;31.23,-51.81,;29.91,-51.04,;28.56,-51.8,;28.55,-53.35,;29.89,-54.14,;29.88,-55.67,;27.23,-51.02,;27.24,-49.48,;25.88,-51.78,;24.55,-51,;37.88,-51.04,;39.22,-51.81,;40.55,-52.57,;40.56,-51.04,;40.55,-49.49,;39.2,-48.72,;37.87,-49.5,;36.53,-48.74,)| Show InChI InChI=1S/C31H33ClFN7O3/c1-7-23(42)38-11-12-39(18(6)14-38)29-19-13-20(32)27(24-21(33)9-8-10-22(24)41)36-30(19)40(31(43)37-29)28-25(16(2)3)34-15-35-26(28)17(4)5/h7-10,13,15-18,41H,1,11-12,14H2,2-6H3/t18-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 21 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of GDP bound recombinant human His-tagged KRAS G12C/C118A mutant (1 to 169 residues) assessed as reduction in SOS1-mediated GDP/GTP nucleo... |
J Med Chem 63: 52-65 (2020)
Article DOI: 10.1021/acs.jmedchem.9b01180
BindingDB Entry DOI: 10.7270/Q2SQ93Q2 |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50609607
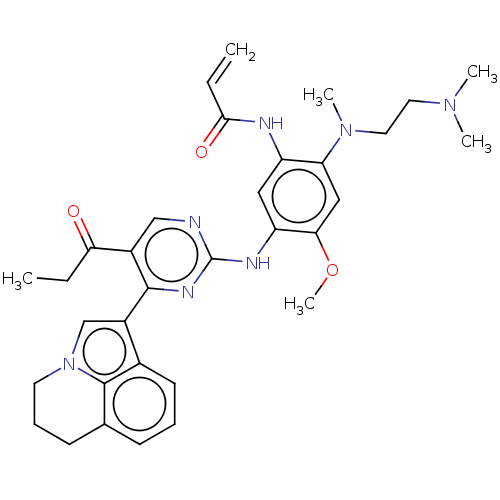
(CHEMBL5281266)Show SMILES CCC(=O)c1cnc(Nc2cc(NC(=O)C=C)c(cc2OC)N(C)CCN(C)C)nc1-c1cn2CCCc3cccc1c23 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| UniChem
| | n/a | n/a | 23 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50609615
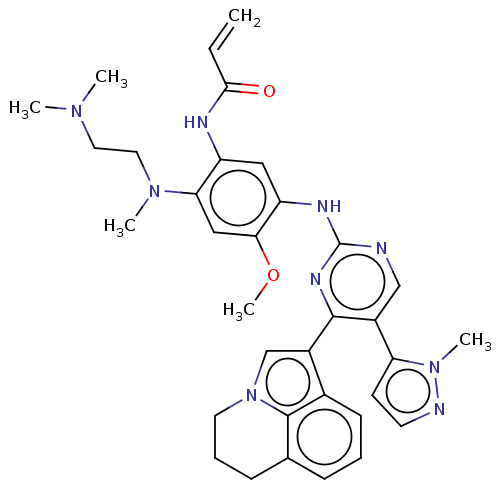
(CHEMBL5266834)Show SMILES COc1cc(N(C)CCN(C)C)c(NC(=O)C=C)cc1Nc1ncc(-c2ccnn2C)c(n1)-c1cn2CCCc3cccc1c23 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| UniChem
| | n/a | n/a | 23 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM368446
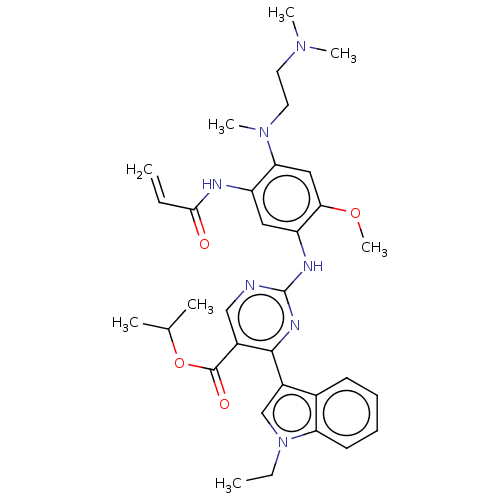
(US10227342, Example 77)Show SMILES CCn1cc(-c2nc(Nc3cc(NC(=O)C=C)c(cc3OC)N(C)CCN(C)C)ncc2C(=O)OC(C)C)c2ccccc12 Show InChI InChI=1S/C33H41N7O4/c1-9-30(41)35-25-17-26(29(43-8)18-28(25)39(7)16-15-38(5)6)36-33-34-19-23(32(42)44-21(3)4)31(37-33)24-20-40(10-2)27-14-12-11-13-22(24)27/h9,11-14,17-21H,1,10,15-16H2,2-8H3,(H,35,41)(H,34,36,37) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 24 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
GTPase KRas
(Homo sapiens (Human)) | BDBM50514372

(CHEMBL4464232)Show SMILES CCc1cccc(C)c1-n1c2nc(c(Cl)cc2c(nc1=O)N1CCN(C[C@@H]1C)C(=O)C=C)-c1c(O)cccc1F |r,wU:26.30,(12.18,-28.85,;13.51,-29.61,;14.85,-28.83,;16.18,-29.6,;17.52,-28.83,;17.51,-27.28,;16.18,-26.52,;16.18,-24.98,;14.85,-27.28,;13.52,-26.52,;13.51,-24.99,;14.85,-24.21,;14.85,-22.66,;13.51,-21.9,;13.51,-20.36,;12.18,-22.67,;12.18,-24.21,;10.86,-24.98,;10.85,-26.51,;12.18,-27.28,;10.84,-28.03,;9.53,-24.2,;9.54,-22.66,;8.22,-21.89,;6.88,-22.65,;6.87,-24.19,;8.2,-24.98,;8.2,-26.51,;5.55,-21.87,;5.56,-20.33,;4.21,-22.63,;2.88,-21.85,;16.17,-21.89,;17.51,-22.66,;17.52,-24.2,;18.84,-21.89,;18.84,-20.34,;17.49,-19.57,;16.17,-20.36,;14.83,-19.59,)| Show InChI InChI=1S/C30H29ClFN5O3/c1-5-19-10-7-9-17(3)27(19)37-29-20(15-21(31)26(33-29)25-22(32)11-8-12-23(25)38)28(34-30(37)40)36-14-13-35(16-18(36)4)24(39)6-2/h6-12,15,18,38H,2,5,13-14,16H2,1,3-4H3/t18-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of KRAS in human MIAPaca2 cells assessed as decrease in EGF-stimulated ERK1/2 phosphorylation preincubated for 2 hrs followed by EGF stimu... |
J Med Chem 63: 52-65 (2020)
Article DOI: 10.1021/acs.jmedchem.9b01180
BindingDB Entry DOI: 10.7270/Q2SQ93Q2 |
More data for this
Ligand-Target Pair | |
GTPase KRas
(Homo sapiens (Human)) | BDBM50514379
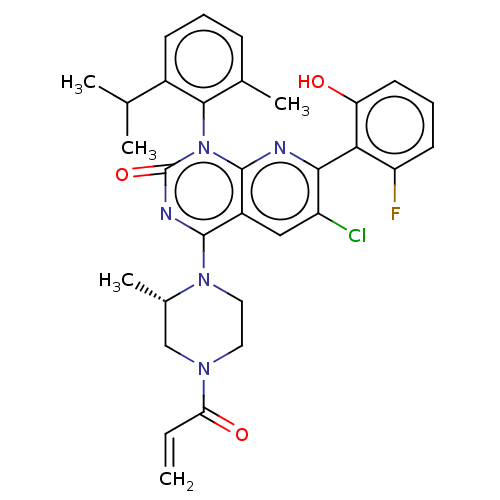
(CHEMBL4452137)Show SMILES CC(C)c1cccc(C)c1-n1c2nc(c(Cl)cc2c(nc1=O)N1CCN(C[C@@H]1C)C(=O)C=C)-c1c(O)cccc1F |r,wU:27.31,(61.09,-12.89,;62.43,-13.65,;62.44,-15.19,;63.76,-12.87,;65.09,-13.65,;66.43,-12.88,;66.43,-11.33,;65.09,-10.57,;65.09,-9.03,;63.76,-11.33,;62.43,-10.57,;62.43,-9.03,;63.77,-8.26,;63.76,-6.71,;62.42,-5.95,;62.42,-4.41,;61.09,-6.71,;61.1,-8.26,;59.77,-9.02,;59.76,-10.56,;61.09,-11.33,;59.75,-12.08,;58.44,-8.25,;58.45,-6.71,;57.13,-5.94,;55.79,-6.69,;55.78,-8.24,;57.11,-9.02,;57.11,-10.56,;54.46,-5.92,;54.47,-4.37,;53.12,-6.67,;51.79,-5.9,;65.09,-5.94,;66.43,-6.7,;66.43,-8.24,;67.76,-5.93,;67.75,-4.39,;66.41,-3.62,;65.08,-4.4,;63.74,-3.64,)| Show InChI InChI=1S/C31H31ClFN5O3/c1-6-25(40)36-13-14-37(19(5)16-36)29-21-15-22(32)27(26-23(33)11-8-12-24(26)39)34-30(21)38(31(41)35-29)28-18(4)9-7-10-20(28)17(2)3/h6-12,15,17,19,39H,1,13-14,16H2,2-5H3/t19-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of GDP bound recombinant human His-tagged KRAS G12C/C118A mutant (1 to 169 residues) assessed as reduction in SOS1-mediated GDP/GTP nucleo... |
J Med Chem 63: 52-65 (2020)
Article DOI: 10.1021/acs.jmedchem.9b01180
BindingDB Entry DOI: 10.7270/Q2SQ93Q2 |
More data for this
Ligand-Target Pair | |
GTPase KRas
(Homo sapiens (Human)) | BDBM50514400

(CHEMBL4434842)Show SMILES CC(C)c1ncnc(C(C)C)c1-n1c2nc(c(Cl)cc2c(nc1=O)N1CCN(C[C@@H]1C)C(=O)C=C)-c1c(O)cccc1F |r,wU:29.33,(40.54,-55.65,;39.21,-54.89,;39.2,-53.36,;37.88,-55.67,;39.21,-56.43,;39.23,-57.98,;37.9,-58.75,;36.56,-57.99,;36.55,-59.52,;35.21,-60.28,;37.88,-60.3,;36.56,-56.45,;35.22,-55.68,;35.21,-54.15,;36.56,-53.37,;36.55,-51.81,;35.21,-51.05,;35.21,-49.51,;33.88,-51.82,;33.88,-53.37,;32.55,-54.14,;32.54,-55.68,;33.88,-56.45,;33.87,-57.98,;31.22,-53.36,;31.23,-51.81,;29.91,-51.04,;28.56,-51.8,;28.55,-53.35,;29.89,-54.14,;29.88,-55.67,;27.23,-51.02,;27.24,-49.48,;25.88,-51.78,;24.55,-51,;37.88,-51.04,;39.22,-51.81,;40.55,-52.57,;40.56,-51.04,;40.55,-49.49,;39.2,-48.72,;37.87,-49.5,;36.53,-48.74,)| Show InChI InChI=1S/C31H33ClFN7O3/c1-7-23(42)38-11-12-39(18(6)14-38)29-19-13-20(32)27(24-21(33)9-8-10-22(24)41)36-30(19)40(31(43)37-29)28-25(16(2)3)34-15-35-26(28)17(4)5/h7-10,13,15-18,41H,1,11-12,14H2,2-6H3/t18-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of KRAS in human MIAPaca2 cells assessed as decrease in EGF-stimulated ERK1/2 phosphorylation preincubated for 2 hrs followed by EGF stimu... |
J Med Chem 63: 52-65 (2020)
Article DOI: 10.1021/acs.jmedchem.9b01180
BindingDB Entry DOI: 10.7270/Q2SQ93Q2 |
More data for this
Ligand-Target Pair | |
GTPase KRas
(Homo sapiens (Human)) | BDBM50514388
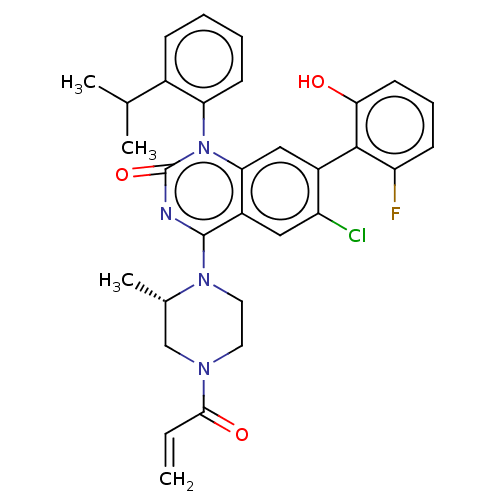
(CHEMBL4449810)Show SMILES CC(C)c1ccccc1-n1c2cc(c(Cl)cc2c(nc1=O)N1CCN(C[C@@H]1C)C(=O)C=C)-c1c(O)cccc1F |r,wU:26.30,(35.18,-28.34,;36.52,-29.12,;36.52,-30.66,;37.86,-28.35,;39.19,-29.12,;40.53,-28.35,;40.52,-26.8,;39.19,-26.04,;37.86,-26.81,;36.53,-26.04,;36.52,-24.51,;37.86,-23.73,;37.86,-22.18,;36.52,-21.42,;36.51,-19.88,;35.19,-22.19,;35.19,-23.74,;33.86,-24.5,;33.86,-26.04,;35.19,-26.8,;33.84,-27.56,;32.54,-23.73,;32.55,-22.18,;31.23,-21.41,;29.89,-22.17,;29.88,-23.71,;31.21,-24.5,;31.21,-26.03,;28.56,-21.39,;28.57,-19.85,;27.22,-22.15,;25.89,-21.37,;39.18,-21.41,;40.52,-22.18,;40.53,-23.71,;41.85,-21.41,;41.85,-19.87,;40.5,-19.1,;39.17,-19.88,;37.84,-19.12,)| Show InChI InChI=1S/C31H30ClFN4O3/c1-5-28(39)35-13-14-36(19(4)17-35)30-22-15-23(32)21(29-24(33)10-8-12-27(29)38)16-26(22)37(31(40)34-30)25-11-7-6-9-20(25)18(2)3/h5-12,15-16,18-19,38H,1,13-14,17H2,2-4H3/t19-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 28 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of KRAS in human MIAPaca2 cells assessed as decrease in EGF-stimulated ERK1/2 phosphorylation preincubated for 2 hrs followed by EGF stimu... |
J Med Chem 63: 52-65 (2020)
Article DOI: 10.1021/acs.jmedchem.9b01180
BindingDB Entry DOI: 10.7270/Q2SQ93Q2 |
More data for this
Ligand-Target Pair | |
GTPase KRas
(Homo sapiens (Human)) | BDBM50514379

(CHEMBL4452137)Show SMILES CC(C)c1cccc(C)c1-n1c2nc(c(Cl)cc2c(nc1=O)N1CCN(C[C@@H]1C)C(=O)C=C)-c1c(O)cccc1F |r,wU:27.31,(61.09,-12.89,;62.43,-13.65,;62.44,-15.19,;63.76,-12.87,;65.09,-13.65,;66.43,-12.88,;66.43,-11.33,;65.09,-10.57,;65.09,-9.03,;63.76,-11.33,;62.43,-10.57,;62.43,-9.03,;63.77,-8.26,;63.76,-6.71,;62.42,-5.95,;62.42,-4.41,;61.09,-6.71,;61.1,-8.26,;59.77,-9.02,;59.76,-10.56,;61.09,-11.33,;59.75,-12.08,;58.44,-8.25,;58.45,-6.71,;57.13,-5.94,;55.79,-6.69,;55.78,-8.24,;57.11,-9.02,;57.11,-10.56,;54.46,-5.92,;54.47,-4.37,;53.12,-6.67,;51.79,-5.9,;65.09,-5.94,;66.43,-6.7,;66.43,-8.24,;67.76,-5.93,;67.75,-4.39,;66.41,-3.62,;65.08,-4.4,;63.74,-3.64,)| Show InChI InChI=1S/C31H31ClFN5O3/c1-6-25(40)36-13-14-37(19(5)16-36)29-21-15-22(32)27(26-23(33)11-8-12-24(26)39)34-30(21)38(31(41)35-29)28-18(4)9-7-10-20(28)17(2)3/h6-12,15,17,19,39H,1,13-14,16H2,2-5H3/t19-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 28 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of KRAS in human MIAPaca2 cells assessed as decrease in EGF-stimulated ERK1/2 phosphorylation preincubated for 2 hrs followed by EGF stimu... |
J Med Chem 63: 52-65 (2020)
Article DOI: 10.1021/acs.jmedchem.9b01180
BindingDB Entry DOI: 10.7270/Q2SQ93Q2 |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM368374
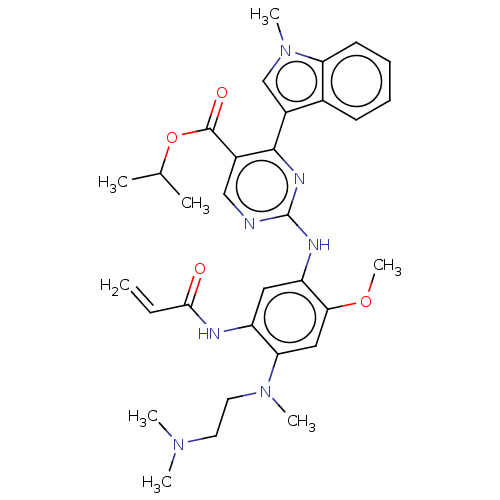
(US10227342, Example 10)Show SMILES COc1cc(N(C)CCN(C)C)c(NC(=O)C=C)cc1Nc1ncc(C(=O)OC(C)C)c(n1)-c1cn(C)c2ccccc12 Show InChI InChI=1S/C32H39N7O4/c1-9-29(40)34-24-16-25(28(42-8)17-27(24)38(6)15-14-37(4)5)35-32-33-18-22(31(41)43-20(2)3)30(36-32)23-19-39(7)26-13-11-10-12-21(23)26/h9-13,16-20H,1,14-15H2,2-8H3,(H,34,40)(H,33,35,36) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| | n/a | n/a | 31 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50609609

(CHEMBL5275712)Show SMILES CCOC(=O)c1cnc(Nc2cc(NC(=O)C=C)c(cc2OC)N(C)CCN(C)C)nc1-c1cn2CCCc3cccc1c23 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| UniChem
| | n/a | n/a | 32 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
GTPase KRas
(Homo sapiens (Human)) | BDBM50514370
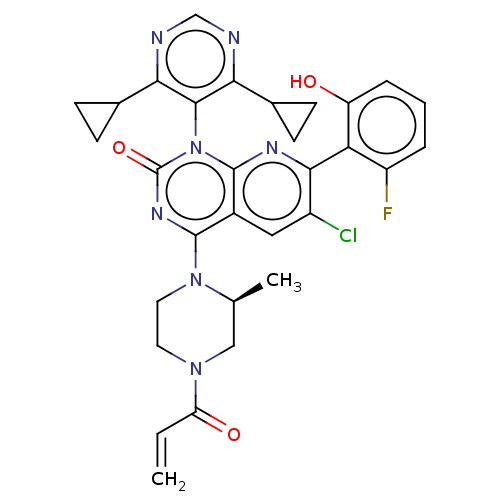
(CHEMBL4588277)Show SMILES C[C@H]1CN(CCN1c1nc(=O)n(-c2c(ncnc2C2CC2)C2CC2)c2nc(c(Cl)cc12)-c1c(O)cccc1F)C(=O)C=C |r,wU:1.0,(9.94,-54.34,;9.95,-52.81,;8.61,-52.02,;8.62,-50.47,;9.97,-49.71,;11.29,-50.49,;11.28,-52.03,;12.61,-52.81,;12.6,-54.35,;13.93,-55.12,;13.93,-56.66,;15.28,-54.36,;16.61,-55.12,;16.62,-56.66,;17.95,-57.43,;19.29,-56.65,;19.27,-55.1,;17.94,-54.35,;17.93,-52.81,;18.68,-51.48,;17.14,-51.49,;15.29,-57.44,;13.75,-57.44,;14.53,-58.77,;15.27,-52.82,;16.62,-52.04,;16.61,-50.49,;15.27,-49.72,;15.26,-48.18,;13.93,-50.49,;13.94,-52.04,;17.94,-49.71,;19.28,-50.48,;20.61,-51.25,;20.62,-49.71,;20.61,-48.16,;19.26,-47.39,;17.93,-48.18,;16.59,-47.41,;7.29,-49.69,;7.3,-48.15,;5.94,-50.45,;4.61,-49.67,)| Show InChI InChI=1S/C31H29ClFN7O3/c1-3-23(42)38-11-12-39(16(2)14-38)29-19-13-20(32)27(24-21(33)5-4-6-22(24)41)36-30(19)40(31(43)37-29)28-25(17-7-8-17)34-15-35-26(28)18-9-10-18/h3-6,13,15-18,41H,1,7-12,14H2,2H3/t16-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 34 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of GDP bound recombinant human His-tagged KRAS G12C/C118A mutant (1 to 169 residues) assessed as reduction in SOS1-mediated GDP/GTP nucleo... |
J Med Chem 63: 52-65 (2020)
Article DOI: 10.1021/acs.jmedchem.9b01180
BindingDB Entry DOI: 10.7270/Q2SQ93Q2 |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50609605

(CHEMBL5283216)Show SMILES COc1cc(N(C)CCN(C)C)c(NC(=O)C=C)cc1Nc1ncc(C#N)c(n1)-c1cn2CCCc3cccc1c23 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| UniChem
| | n/a | n/a | 35 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM368374

(US10227342, Example 10)Show SMILES COc1cc(N(C)CCN(C)C)c(NC(=O)C=C)cc1Nc1ncc(C(=O)OC(C)C)c(n1)-c1cn(C)c2ccccc12 Show InChI InChI=1S/C32H39N7O4/c1-9-29(40)34-24-16-25(28(42-8)17-27(24)38(6)15-14-37(4)5)35-32-33-18-22(31(41)43-20(2)3)30(36-32)23-19-39(7)26-13-11-10-12-21(23)26/h9-13,16-20H,1,14-15H2,2-8H3,(H,34,40)(H,33,35,36) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| | n/a | n/a | 35 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50609602
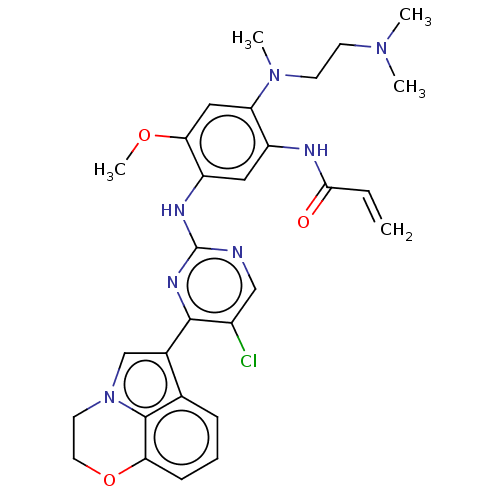
(CHEMBL5284255)Show SMILES COc1cc(N(C)CCN(C)C)c(NC(=O)C=C)cc1Nc1ncc(Cl)c(n1)-c1cn2CCOc3cccc1c23 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| UniChem
| | n/a | n/a | 36 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
GTPase KRas
(Homo sapiens (Human)) | BDBM50514370

(CHEMBL4588277)Show SMILES C[C@H]1CN(CCN1c1nc(=O)n(-c2c(ncnc2C2CC2)C2CC2)c2nc(c(Cl)cc12)-c1c(O)cccc1F)C(=O)C=C |r,wU:1.0,(9.94,-54.34,;9.95,-52.81,;8.61,-52.02,;8.62,-50.47,;9.97,-49.71,;11.29,-50.49,;11.28,-52.03,;12.61,-52.81,;12.6,-54.35,;13.93,-55.12,;13.93,-56.66,;15.28,-54.36,;16.61,-55.12,;16.62,-56.66,;17.95,-57.43,;19.29,-56.65,;19.27,-55.1,;17.94,-54.35,;17.93,-52.81,;18.68,-51.48,;17.14,-51.49,;15.29,-57.44,;13.75,-57.44,;14.53,-58.77,;15.27,-52.82,;16.62,-52.04,;16.61,-50.49,;15.27,-49.72,;15.26,-48.18,;13.93,-50.49,;13.94,-52.04,;17.94,-49.71,;19.28,-50.48,;20.61,-51.25,;20.62,-49.71,;20.61,-48.16,;19.26,-47.39,;17.93,-48.18,;16.59,-47.41,;7.29,-49.69,;7.3,-48.15,;5.94,-50.45,;4.61,-49.67,)| Show InChI InChI=1S/C31H29ClFN7O3/c1-3-23(42)38-11-12-39(16(2)14-38)29-19-13-20(32)27(24-21(33)5-4-6-22(24)41)36-30(19)40(31(43)37-29)28-25(17-7-8-17)34-15-35-26(28)18-9-10-18/h3-6,13,15-18,41H,1,7-12,14H2,2H3/t16-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 36 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of KRAS in human MIAPaca2 cells assessed as decrease in EGF-stimulated ERK1/2 phosphorylation preincubated for 2 hrs followed by EGF stimu... |
J Med Chem 63: 52-65 (2020)
Article DOI: 10.1021/acs.jmedchem.9b01180
BindingDB Entry DOI: 10.7270/Q2SQ93Q2 |
More data for this
Ligand-Target Pair | |
GTPase KRas
(Homo sapiens (Human)) | BDBM50514383

(CHEMBL4554946)Show SMILES CCc1cccc(CC)c1-n1c2nc(c(Cl)cc2c(nc1=O)N1CCN(C[C@@H]1C)C(=O)C=C)-c1c(O)cccc1F |r,wU:27.31,(54.65,-47.19,;54.64,-45.66,;55.97,-44.88,;57.31,-45.65,;58.64,-44.87,;58.62,-43.32,;57.29,-42.57,;57.28,-41.03,;58.6,-40.25,;55.97,-43.34,;54.63,-42.58,;54.63,-41.04,;55.97,-40.27,;55.96,-38.71,;54.62,-37.95,;54.62,-36.41,;53.29,-38.72,;53.29,-40.27,;51.97,-41.03,;51.96,-42.57,;53.29,-43.34,;53.29,-44.88,;50.64,-40.26,;50.65,-38.71,;49.33,-37.94,;47.98,-38.7,;47.97,-40.24,;49.31,-41.03,;49.3,-42.57,;46.65,-37.92,;46.66,-36.38,;45.31,-38.68,;43.97,-37.9,;57.29,-37.94,;58.63,-38.71,;59.96,-39.47,;59.97,-37.94,;59.96,-36.39,;58.61,-35.62,;57.28,-36.41,;55.94,-35.64,)| Show InChI InChI=1S/C31H31ClFN5O3/c1-5-19-10-8-11-20(6-2)28(19)38-30-21(16-22(32)27(34-30)26-23(33)12-9-13-24(26)39)29(35-31(38)41)37-15-14-36(17-18(37)4)25(40)7-3/h7-13,16,18,39H,3,5-6,14-15,17H2,1-2,4H3/t18-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 36 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of KRAS in human MIAPaca2 cells assessed as decrease in EGF-stimulated ERK1/2 phosphorylation preincubated for 2 hrs followed by EGF stimu... |
J Med Chem 63: 52-65 (2020)
Article DOI: 10.1021/acs.jmedchem.9b01180
BindingDB Entry DOI: 10.7270/Q2SQ93Q2 |
More data for this
Ligand-Target Pair | |
GTPase KRas
(Homo sapiens (Human)) | BDBM50514395
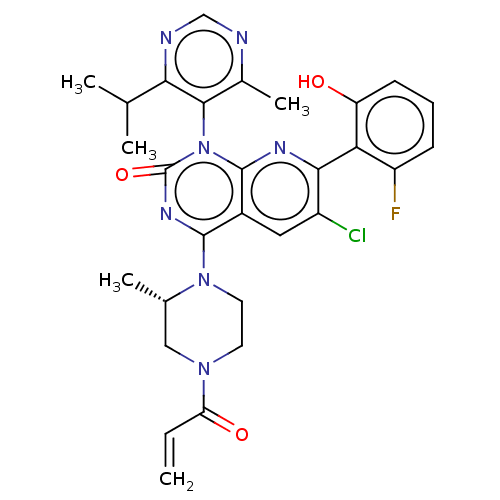
(CHEMBL4527861)Show SMILES CC(C)c1ncnc(C)c1-n1c2nc(c(Cl)cc2c(nc1=O)N1CCN(C[C@@H]1C)C(=O)C=C)-c1c(O)cccc1F |r,wU:27.31,(67.26,-14.35,;68.59,-15.11,;68.6,-16.65,;69.92,-14.34,;71.25,-15.1,;72.58,-14.33,;72.57,-12.78,;71.24,-12.03,;71.23,-10.49,;69.91,-12.8,;68.58,-12.04,;68.58,-10.5,;69.92,-9.73,;69.91,-8.17,;68.57,-7.41,;68.57,-5.87,;67.24,-8.18,;67.24,-9.73,;65.91,-10.49,;65.91,-12.03,;67.24,-12.8,;65.9,-13.55,;64.59,-9.72,;64.6,-8.17,;63.28,-7.4,;61.93,-8.16,;61.92,-9.7,;63.26,-10.49,;63.25,-12.03,;60.6,-7.38,;60.61,-5.84,;59.26,-8.14,;57.93,-7.36,;71.24,-7.4,;72.58,-8.17,;72.58,-9.71,;73.91,-7.4,;73.91,-5.85,;72.56,-5.08,;71.23,-5.87,;69.89,-5.1,)| Show InChI InChI=1S/C29H29ClFN7O3/c1-6-22(40)36-10-11-37(16(4)13-36)27-18-12-19(30)25(23-20(31)8-7-9-21(23)39)34-28(18)38(29(41)35-27)26-17(5)32-14-33-24(26)15(2)3/h6-9,12,14-16,39H,1,10-11,13H2,2-5H3/t16-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 38 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of KRAS in human MIAPaca2 cells assessed as decrease in EGF-stimulated ERK1/2 phosphorylation preincubated for 2 hrs followed by EGF stimu... |
J Med Chem 63: 52-65 (2020)
Article DOI: 10.1021/acs.jmedchem.9b01180
BindingDB Entry DOI: 10.7270/Q2SQ93Q2 |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50609610
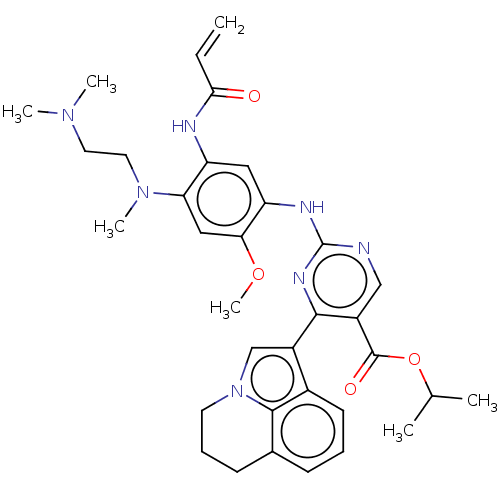
(CHEMBL5279794)Show SMILES COc1cc(N(C)CCN(C)C)c(NC(=O)C=C)cc1Nc1ncc(C(=O)OC(C)C)c(n1)-c1cn2CCCc3cccc1c23 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| UniChem
| | n/a | n/a | 39 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50609608
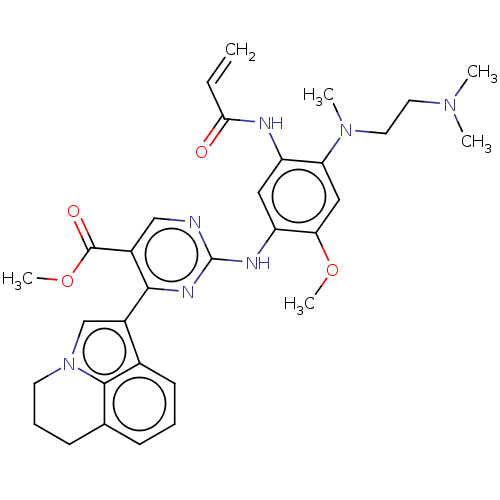
(CHEMBL5286091)Show SMILES COC(=O)c1cnc(Nc2cc(NC(=O)C=C)c(cc2OC)N(C)CCN(C)C)nc1-c1cn2CCCc3cccc1c23 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| UniChem
| | n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50609606
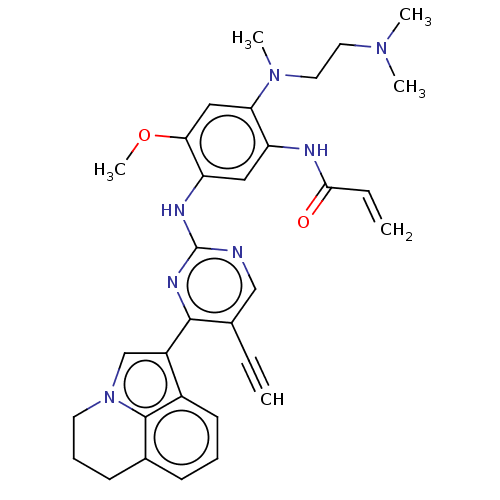
(CHEMBL5279491)Show SMILES COc1cc(N(C)CCN(C)C)c(NC(=O)C=C)cc1Nc1ncc(C#C)c(n1)-c1cn2CCCc3cccc1c23 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| UniChem
| | n/a | n/a | 42 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM368402
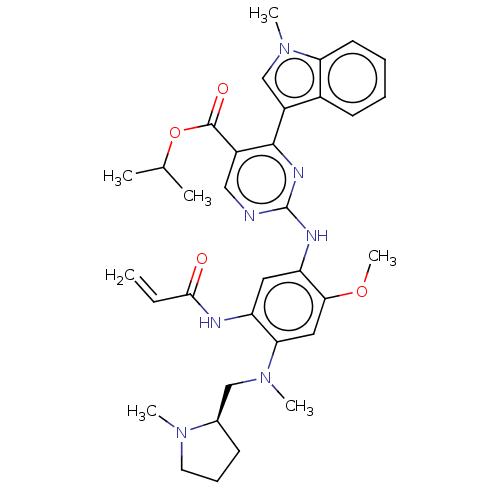
(US10227342, Example 36)Show SMILES COc1cc(N(C)C[C@H]2CCCN2C)c(NC(=O)C=C)cc1Nc1ncc(C(=O)OC(C)C)c(n1)-c1cn(C)c2ccccc12 |r| Show InChI InChI=1S/C34H41N7O4/c1-8-31(42)36-26-16-27(30(44-7)17-29(26)40(5)19-22-12-11-15-39(22)4)37-34-35-18-24(33(43)45-21(2)3)32(38-34)25-20-41(6)28-14-10-9-13-23(25)28/h8-10,13-14,16-18,20-22H,1,11-12,15,19H2,2-7H3,(H,36,42)(H,35,37,38)/t22-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 43 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
GTPase KRas
(Homo sapiens (Human)) | BDBM50514378
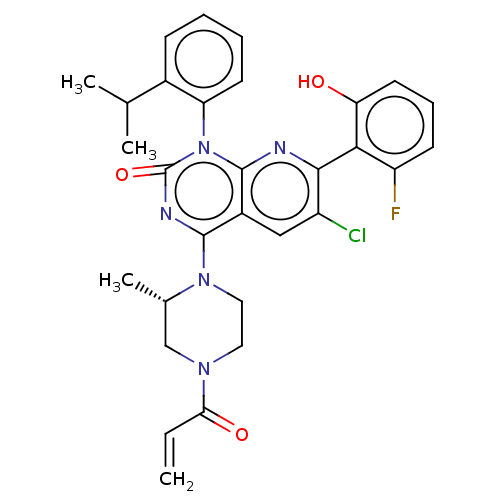
(CHEMBL4450657)Show SMILES CC(C)c1ccccc1-n1c2nc(c(Cl)cc2c(nc1=O)N1CCN(C[C@@H]1C)C(=O)C=C)-c1c(O)cccc1F |r,wU:26.30,(27.94,-14.35,;26.62,-15.13,;25.28,-15.89,;26.63,-16.66,;27.96,-17.42,;27.97,-18.97,;26.64,-19.74,;25.31,-18.98,;25.31,-17.44,;23.97,-16.68,;23.97,-15.14,;25.31,-14.36,;25.3,-12.81,;23.96,-12.05,;23.96,-10.51,;22.63,-12.82,;22.63,-14.36,;21.31,-15.13,;21.3,-16.67,;22.63,-17.44,;22.63,-18.97,;19.98,-14.36,;19.99,-12.81,;18.67,-12.04,;17.32,-12.8,;17.31,-14.34,;18.65,-15.13,;18.64,-16.66,;15.99,-12.02,;16,-10.47,;14.65,-12.78,;13.32,-12,;26.63,-12.04,;27.97,-12.81,;26.63,-13.56,;29.31,-12.03,;29.3,-10.49,;27.95,-9.72,;26.62,-10.5,;25.28,-9.74,)| Show InChI InChI=1S/C30H29ClFN5O3/c1-5-25(39)35-13-14-36(18(4)16-35)28-20-15-21(31)27(26-22(32)10-8-12-24(26)38)33-29(20)37(30(40)34-28)23-11-7-6-9-19(23)17(2)3/h5-12,15,17-18,38H,1,13-14,16H2,2-4H3/t18-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 44 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of KRAS in human MIAPaca2 cells assessed as decrease in EGF-stimulated ERK1/2 phosphorylation preincubated for 2 hrs followed by EGF stimu... |
J Med Chem 63: 52-65 (2020)
Article DOI: 10.1021/acs.jmedchem.9b01180
BindingDB Entry DOI: 10.7270/Q2SQ93Q2 |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50609612
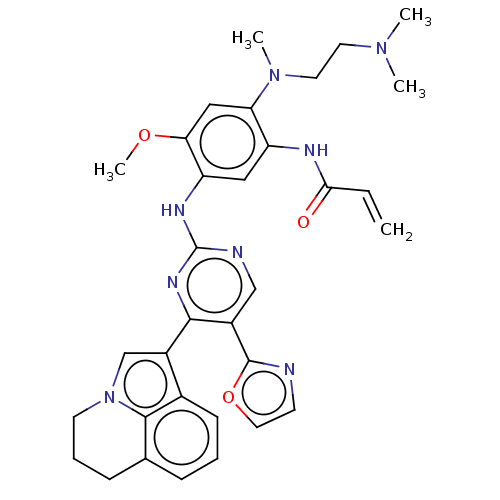
(CHEMBL5271640)Show SMILES COc1cc(N(C)CCN(C)C)c(NC(=O)C=C)cc1Nc1ncc(-c2ncco2)c(n1)-c1cn2CCCc3cccc1c23 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| UniChem
| | n/a | n/a | 45 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
GTPase KRas
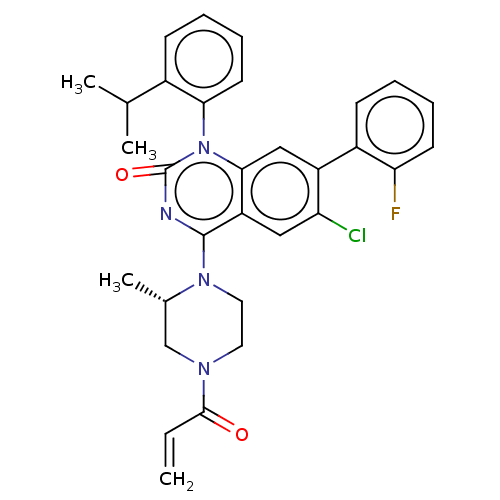
(Homo sapiens (Human)) | BDBM50514387
(CHEMBL4465551)Show SMILES CC(C)c1ccccc1-n1c2cc(c(Cl)cc2c(nc1=O)N1CCN(C[C@@H]1C)C(=O)C=C)-c1ccccc1F |r,wU:26.30,(33,-13.63,;34.34,-14.41,;34.33,-15.95,;35.67,-13.64,;37.01,-14.41,;38.35,-13.64,;38.34,-12.09,;37.01,-11.33,;35.68,-12.09,;34.34,-11.33,;34.34,-9.8,;35.68,-9.02,;35.67,-7.47,;34.34,-6.71,;34.33,-5.17,;33.01,-7.48,;33.01,-9.02,;31.68,-9.79,;31.68,-11.33,;33.01,-12.09,;31.66,-12.85,;30.36,-9.02,;30.37,-7.47,;29.05,-6.7,;27.71,-7.46,;27.7,-9,;29.03,-9.79,;29.02,-11.32,;26.38,-6.68,;26.39,-5.14,;25.03,-7.44,;23.71,-6.66,;37,-6.7,;36.99,-5.17,;38.32,-4.39,;39.67,-5.16,;39.67,-6.7,;38.34,-7.47,;38.34,-9,)| Show InChI InChI=1S/C31H30ClFN4O2/c1-5-29(38)35-14-15-36(20(4)18-35)30-24-16-25(32)23(22-11-6-8-12-26(22)33)17-28(24)37(31(39)34-30)27-13-9-7-10-21(27)19(2)3/h5-13,16-17,19-20H,1,14-15,18H2,2-4H3/t20-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 47 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of KRAS in human MIAPaca2 cells assessed as decrease in EGF-stimulated ERK1/2 phosphorylation preincubated for 2 hrs followed by EGF stimu... |
J Med Chem 63: 52-65 (2020)
Article DOI: 10.1021/acs.jmedchem.9b01180
BindingDB Entry DOI: 10.7270/Q2SQ93Q2 |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50609614
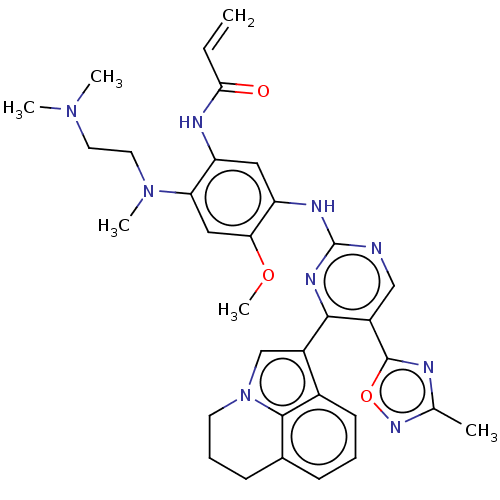
(CHEMBL5271718)Show SMILES COc1cc(N(C)CCN(C)C)c(NC(=O)C=C)cc1Nc1ncc(-c2nc(C)no2)c(n1)-c1cn2CCCc3cccc1c23 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| UniChem
| | n/a | n/a | 49 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
GTPase KRas
(Homo sapiens (Human)) | BDBM50514378

(CHEMBL4450657)Show SMILES CC(C)c1ccccc1-n1c2nc(c(Cl)cc2c(nc1=O)N1CCN(C[C@@H]1C)C(=O)C=C)-c1c(O)cccc1F |r,wU:26.30,(27.94,-14.35,;26.62,-15.13,;25.28,-15.89,;26.63,-16.66,;27.96,-17.42,;27.97,-18.97,;26.64,-19.74,;25.31,-18.98,;25.31,-17.44,;23.97,-16.68,;23.97,-15.14,;25.31,-14.36,;25.3,-12.81,;23.96,-12.05,;23.96,-10.51,;22.63,-12.82,;22.63,-14.36,;21.31,-15.13,;21.3,-16.67,;22.63,-17.44,;22.63,-18.97,;19.98,-14.36,;19.99,-12.81,;18.67,-12.04,;17.32,-12.8,;17.31,-14.34,;18.65,-15.13,;18.64,-16.66,;15.99,-12.02,;16,-10.47,;14.65,-12.78,;13.32,-12,;26.63,-12.04,;27.97,-12.81,;26.63,-13.56,;29.31,-12.03,;29.3,-10.49,;27.95,-9.72,;26.62,-10.5,;25.28,-9.74,)| Show InChI InChI=1S/C30H29ClFN5O3/c1-5-25(39)35-13-14-36(18(4)16-35)28-20-15-21(31)27(26-22(32)10-8-12-24(26)38)33-29(20)37(30(40)34-28)23-11-7-6-9-19(23)17(2)3/h5-12,15,17-18,38H,1,13-14,16H2,2-4H3/t18-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 51 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of GDP bound recombinant human His-tagged KRAS G12C/C118A mutant (1 to 169 residues) assessed as reduction in SOS1-mediated GDP/GTP nucleo... |
J Med Chem 63: 52-65 (2020)
Article DOI: 10.1021/acs.jmedchem.9b01180
BindingDB Entry DOI: 10.7270/Q2SQ93Q2 |
More data for this
Ligand-Target Pair | |
GTPase KRas
(Homo sapiens (Human)) | BDBM50514369

(CHEMBL4467413)Show SMILES C[C@H]1CN(CCN1c1nc(=O)n(-c2ccccc2C(C)(C)C)c2nc(c(Cl)cc12)-c1c(O)cccc1F)C(=O)C=C |r,wU:1.0,(33.78,-12.93,;33.79,-11.4,;32.46,-10.61,;32.46,-9.07,;33.81,-8.31,;35.13,-9.09,;35.12,-10.63,;36.44,-11.4,;36.44,-12.94,;37.77,-13.71,;36.43,-14.46,;39.1,-12.95,;40.44,-13.71,;41.77,-12.94,;43.1,-13.7,;43.11,-15.25,;41.77,-16.03,;40.43,-15.25,;39.1,-16.03,;37.77,-15.27,;39.11,-17.56,;37.77,-16.78,;39.1,-11.41,;40.44,-10.64,;40.43,-9.08,;39.1,-8.32,;39.09,-6.78,;37.77,-9.09,;37.77,-10.64,;41.76,-8.32,;43.1,-9.08,;43.1,-10.62,;44.43,-8.31,;44.43,-6.77,;43.08,-6,;41.75,-6.78,;40.41,-6.02,;31.13,-8.29,;31.15,-6.75,;29.79,-9.05,;28.46,-8.27,)| Show InChI InChI=1S/C31H31ClFN5O3/c1-6-25(40)36-14-15-37(18(2)17-36)28-19-16-21(32)27(26-22(33)11-9-13-24(26)39)34-29(19)38(30(41)35-28)23-12-8-7-10-20(23)31(3,4)5/h6-13,16,18,39H,1,14-15,17H2,2-5H3/t18-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 51 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of KRAS in human MIAPaca2 cells assessed as decrease in EGF-stimulated ERK1/2 phosphorylation preincubated for 2 hrs followed by EGF stimu... |
J Med Chem 63: 52-65 (2020)
Article DOI: 10.1021/acs.jmedchem.9b01180
BindingDB Entry DOI: 10.7270/Q2SQ93Q2 |
More data for this
Ligand-Target Pair | |
GTPase KRas
(Homo sapiens (Human)) | BDBM50514399
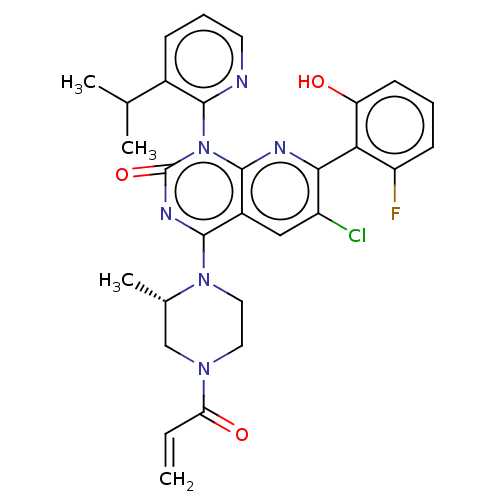
(CHEMBL4467518)Show SMILES CC(C)c1cccnc1-n1c2nc(c(Cl)cc2c(nc1=O)N1CCN(C[C@@H]1C)C(=O)C=C)-c1c(O)cccc1F |r,wU:26.30,(30.46,-30,;31.79,-30.76,;31.8,-32.29,;33.12,-29.98,;34.46,-30.75,;35.79,-29.98,;35.79,-28.43,;34.46,-27.67,;33.13,-28.44,;31.79,-27.67,;31.79,-26.14,;33.13,-25.37,;33.12,-23.81,;31.79,-23.05,;31.78,-21.51,;30.45,-23.82,;30.46,-25.37,;29.13,-26.13,;29.12,-27.67,;30.45,-28.43,;29.12,-29.19,;27.8,-25.36,;27.81,-23.81,;26.5,-23.04,;25.15,-23.8,;25.14,-25.34,;26.48,-26.13,;26.47,-27.66,;23.82,-23.02,;23.83,-21.48,;22.48,-23.78,;21.15,-23,;34.45,-23.04,;35.79,-23.81,;35.79,-25.35,;37.12,-23.04,;37.12,-21.5,;35.77,-20.73,;34.44,-21.51,;33.1,-20.74,)| Show InChI InChI=1S/C29H28ClFN6O3/c1-5-23(39)35-12-13-36(17(4)15-35)27-19-14-20(30)25(24-21(31)9-6-10-22(24)38)33-28(19)37(29(40)34-27)26-18(16(2)3)8-7-11-32-26/h5-11,14,16-17,38H,1,12-13,15H2,2-4H3/t17-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 53 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of KRAS in human MIAPaca2 cells assessed as decrease in EGF-stimulated ERK1/2 phosphorylation preincubated for 2 hrs followed by EGF stimu... |
J Med Chem 63: 52-65 (2020)
Article DOI: 10.1021/acs.jmedchem.9b01180
BindingDB Entry DOI: 10.7270/Q2SQ93Q2 |
More data for this
Ligand-Target Pair | |
GTPase KRas
(Homo sapiens (Human)) | BDBM50514381

(CHEMBL4516179)Show SMILES CC(C)n1ncc(C)c1-n1c2nc(c(Cl)cc2c(nc1=O)N1CCN(C[C@@H]1C)C(=O)C=C)-c1c(O)cccc1F |r,wU:26.30,(13.29,-42.25,;14.61,-43.03,;14.6,-44.56,;15.95,-42.27,;17.45,-42.58,;18.22,-41.25,;17.18,-40.11,;17.49,-38.61,;15.77,-40.75,;14.44,-39.99,;14.43,-38.45,;15.77,-37.68,;15.77,-36.12,;14.43,-35.36,;14.42,-33.82,;13.1,-36.13,;13.1,-37.68,;11.77,-38.44,;11.77,-39.98,;13.1,-40.75,;11.76,-41.5,;10.44,-37.67,;10.45,-36.12,;9.14,-35.35,;7.79,-36.11,;7.78,-37.65,;9.12,-38.44,;9.11,-39.98,;6.46,-35.33,;6.47,-33.79,;5.12,-36.09,;3.79,-35.31,;17.1,-35.35,;18.44,-36.12,;18.44,-37.66,;19.77,-35.35,;19.76,-33.8,;18.42,-33.03,;17.09,-33.82,;15.75,-33.05,)| Show InChI InChI=1S/C28H29ClFN7O3/c1-6-22(39)34-10-11-35(17(5)14-34)25-18-12-19(29)24(23-20(30)8-7-9-21(23)38)32-26(18)36(28(40)33-25)27-16(4)13-31-37(27)15(2)3/h6-9,12-13,15,17,38H,1,10-11,14H2,2-5H3/t17-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 53 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of GDP bound recombinant human His-tagged KRAS G12C/C118A mutant (1 to 169 residues) assessed as reduction in SOS1-mediated GDP/GTP nucleo... |
J Med Chem 63: 52-65 (2020)
Article DOI: 10.1021/acs.jmedchem.9b01180
BindingDB Entry DOI: 10.7270/Q2SQ93Q2 |
More data for this
Ligand-Target Pair | |
GTPase KRas
(Homo sapiens (Human)) | BDBM50514404
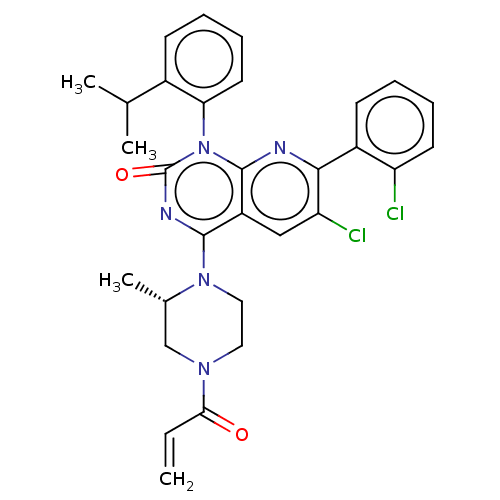
(CHEMBL4591772)Show SMILES CC(C)c1ccccc1-n1c2nc(c(Cl)cc2c(nc1=O)N1CCN(C[C@@H]1C)C(=O)C=C)-c1ccccc1Cl |r,wU:26.30,(59.39,-43.96,;60.73,-44.74,;60.73,-46.28,;62.07,-43.97,;63.4,-44.74,;64.74,-43.97,;64.73,-42.42,;63.4,-41.66,;62.07,-42.43,;60.74,-41.66,;60.73,-40.13,;62.07,-39.36,;62.07,-37.8,;60.73,-37.04,;60.72,-35.5,;59.4,-37.81,;59.4,-39.36,;58.07,-40.12,;58.07,-41.66,;59.4,-42.43,;58.05,-43.19,;56.75,-39.35,;56.76,-37.81,;55.44,-37.03,;54.1,-37.79,;54.09,-39.33,;55.42,-40.12,;55.42,-41.65,;52.77,-37.01,;52.78,-35.47,;51.43,-37.77,;50.1,-36.99,;63.39,-37.04,;63.38,-35.5,;64.71,-34.72,;66.06,-35.49,;66.06,-37.03,;64.73,-37.8,;64.74,-39.33,)| Show InChI InChI=1S/C30H29Cl2N5O2/c1-5-26(38)35-14-15-36(19(4)17-35)28-22-16-24(32)27(21-11-6-8-12-23(21)31)33-29(22)37(30(39)34-28)25-13-9-7-10-20(25)18(2)3/h5-13,16,18-19H,1,14-15,17H2,2-4H3/t19-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 56 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of KRAS in human MIAPaca2 cells assessed as decrease in EGF-stimulated ERK1/2 phosphorylation preincubated for 2 hrs followed by EGF stimu... |
J Med Chem 63: 52-65 (2020)
Article DOI: 10.1021/acs.jmedchem.9b01180
BindingDB Entry DOI: 10.7270/Q2SQ93Q2 |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM368401
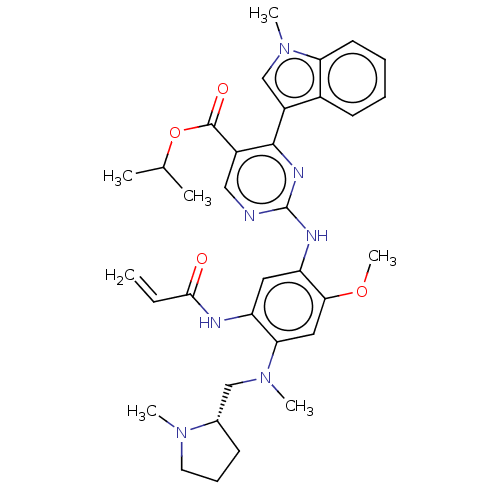
(US10227342, Example 35)Show SMILES COc1cc(N(C)C[C@@H]2CCCN2C)c(NC(=O)C=C)cc1Nc1ncc(C(=O)OC(C)C)c(n1)-c1cn(C)c2ccccc12 |r| Show InChI InChI=1S/C34H41N7O4/c1-8-31(42)36-26-16-27(30(44-7)17-29(26)40(5)19-22-12-11-15-39(22)4)37-34-35-18-24(33(43)45-21(2)3)32(38-34)25-20-41(6)28-14-10-9-13-23(25)28/h8-10,13-14,16-18,20-22H,1,11-12,15,19H2,2-7H3,(H,36,42)(H,35,37,38)/t22-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 57 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
GTPase KRas
(Homo sapiens (Human)) | BDBM50514392

(CHEMBL4573279)Show SMILES CC(C)c1ncsc1-n1c2nc(c(Cl)cc2c(nc1=O)N1CCN(C[C@@H]1C)C(=O)C=C)-c1c(O)cccc1F |r,wU:25.29,(68.93,-30.64,;70.25,-31.42,;70.24,-32.95,;71.59,-30.66,;73.09,-30.98,;73.85,-29.64,;72.82,-28.5,;71.41,-29.14,;70.08,-28.38,;70.07,-26.84,;71.41,-26.07,;71.41,-24.51,;70.07,-23.75,;70.06,-22.21,;68.74,-24.52,;68.74,-26.07,;67.41,-26.83,;67.4,-28.37,;68.74,-29.14,;67.4,-29.89,;66.08,-26.06,;66.09,-24.51,;64.77,-23.74,;63.43,-24.5,;63.42,-26.04,;64.76,-26.83,;64.75,-28.37,;62.1,-23.72,;62.11,-22.18,;60.76,-24.48,;59.42,-23.7,;72.74,-23.74,;74.08,-24.51,;74.08,-26.05,;75.41,-23.74,;75.4,-22.19,;74.06,-21.42,;72.73,-22.21,;71.39,-21.44,)| Show InChI InChI=1S/C27H26ClFN6O3S/c1-5-20(37)33-9-10-34(15(4)12-33)24-16-11-17(28)23(21-18(29)7-6-8-19(21)36)31-25(16)35(27(38)32-24)26-22(14(2)3)30-13-39-26/h5-8,11,13-15,36H,1,9-10,12H2,2-4H3/t15-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 63 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of KRAS in human MIAPaca2 cells assessed as decrease in EGF-stimulated ERK1/2 phosphorylation preincubated for 2 hrs followed by EGF stimu... |
J Med Chem 63: 52-65 (2020)
Article DOI: 10.1021/acs.jmedchem.9b01180
BindingDB Entry DOI: 10.7270/Q2SQ93Q2 |
More data for this
Ligand-Target Pair | |
GTPase KRas
(Homo sapiens (Human)) | BDBM50514394
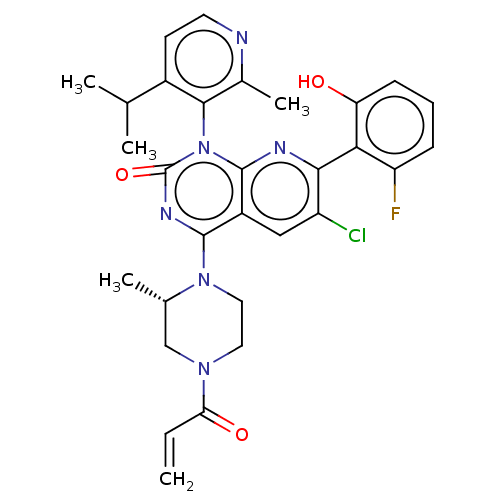
(CHEMBL4578214)Show SMILES CC(C)c1ccnc(C)c1-n1c2nc(c(Cl)cc2c(nc1=O)N1CCN(C[C@@H]1C)C(=O)C=C)-c1c(O)cccc1F |r,wU:27.31,(49.04,-14.69,;50.37,-15.46,;50.38,-16.99,;51.7,-14.69,;53.03,-15.45,;54.36,-14.67,;54.35,-13.13,;53.01,-12.37,;53,-10.84,;51.69,-13.14,;50.36,-12.38,;50.35,-10.85,;51.69,-10.07,;51.69,-8.52,;50.35,-7.75,;50.35,-6.21,;49.02,-8.52,;49.02,-10.07,;47.69,-10.84,;47.68,-12.37,;49.02,-13.14,;47.68,-13.9,;46.36,-10.06,;46.37,-8.52,;45.05,-7.74,;43.71,-8.5,;43.7,-10.05,;45.03,-10.83,;45.03,-12.37,;42.38,-7.72,;42.39,-6.18,;41.03,-8.48,;39.7,-7.7,;53.02,-7.75,;54.36,-8.51,;54.36,-10.05,;55.69,-7.74,;55.69,-6.2,;54.34,-5.43,;53.01,-6.21,;51.67,-5.44,)| Show InChI InChI=1S/C30H30ClFN6O3/c1-6-24(40)36-12-13-37(17(4)15-36)28-20-14-21(31)26(25-22(32)8-7-9-23(25)39)34-29(20)38(30(41)35-28)27-18(5)33-11-10-19(27)16(2)3/h6-11,14,16-17,39H,1,12-13,15H2,2-5H3/t17-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 65 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of KRAS in human MIAPaca2 cells assessed as decrease in EGF-stimulated ERK1/2 phosphorylation preincubated for 2 hrs followed by EGF stimu... |
J Med Chem 63: 52-65 (2020)
Article DOI: 10.1021/acs.jmedchem.9b01180
BindingDB Entry DOI: 10.7270/Q2SQ93Q2 |
More data for this
Ligand-Target Pair | |
Choline kinase alpha
(Homo sapiens (Human)) | BDBM50145944
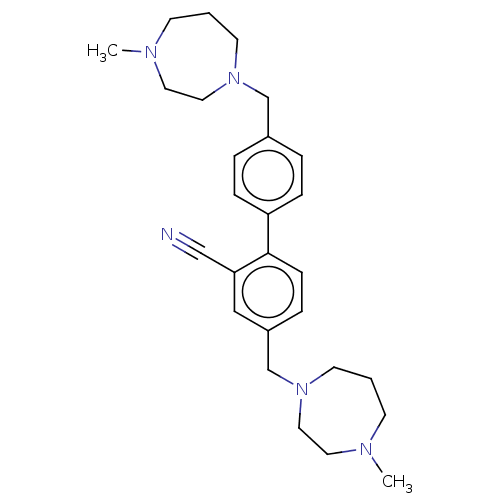
(CHEMBL3763540)Show SMILES CN1CCCN(Cc2ccc(cc2)-c2ccc(CN3CCCN(C)CC3)cc2C#N)CC1 Show InChI InChI=1S/C27H37N5/c1-29-11-3-13-31(17-15-29)21-23-5-8-25(9-6-23)27-10-7-24(19-26(27)20-28)22-32-14-4-12-30(2)16-18-32/h5-10,19H,3-4,11-18,21-22H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 66 | n/a | n/a | n/a | n/a | n/a | n/a |
ARIAD Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human N-terminal truncated ChoKalpha1 (75 to 457 residues) using choline chloride as substrate measured over 10 to 30 mins by coupled A... |
J Med Chem 59: 671-86 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01552
BindingDB Entry DOI: 10.7270/Q23X88HX |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
GTPase KRas
(Homo sapiens (Human)) | BDBM50514390
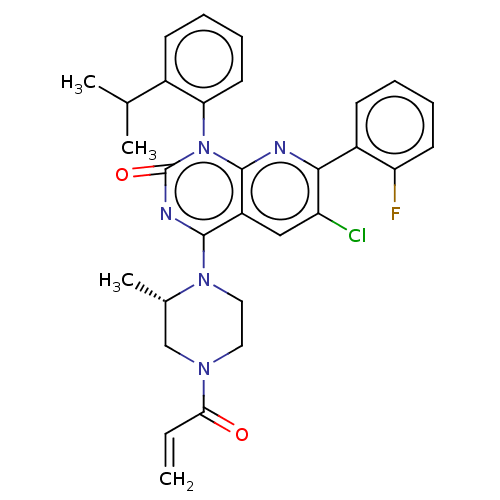
(CHEMBL4450519)Show SMILES CC(C)c1ccccc1-n1c2nc(c(Cl)cc2c(nc1=O)N1CCN(C[C@@H]1C)C(=O)C=C)-c1ccccc1F |r,wU:26.30,(37.46,-44.6,;38.79,-45.38,;38.79,-46.92,;40.13,-44.61,;41.46,-45.38,;42.8,-44.61,;42.8,-43.06,;41.46,-42.3,;40.13,-43.06,;38.8,-42.3,;38.79,-40.77,;40.13,-39.99,;40.13,-38.44,;38.79,-37.68,;38.79,-36.14,;37.46,-38.45,;37.46,-39.99,;36.14,-40.76,;36.13,-42.29,;37.46,-43.06,;36.12,-43.82,;34.81,-39.98,;34.82,-38.44,;33.5,-37.67,;32.16,-38.43,;32.15,-39.97,;33.48,-40.76,;33.48,-42.29,;30.83,-37.65,;30.84,-36.11,;29.49,-38.41,;28.16,-37.63,;41.46,-37.67,;41.45,-36.14,;42.77,-35.35,;44.12,-36.12,;44.13,-37.67,;42.79,-38.44,;42.8,-39.97,)| Show InChI InChI=1S/C30H29ClFN5O2/c1-5-26(38)35-14-15-36(19(4)17-35)28-22-16-23(31)27(21-11-6-8-12-24(21)32)33-29(22)37(30(39)34-28)25-13-9-7-10-20(25)18(2)3/h5-13,16,18-19H,1,14-15,17H2,2-4H3/t19-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 66 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of KRAS in human MIAPaca2 cells assessed as decrease in EGF-stimulated ERK1/2 phosphorylation preincubated for 2 hrs followed by EGF stimu... |
J Med Chem 63: 52-65 (2020)
Article DOI: 10.1021/acs.jmedchem.9b01180
BindingDB Entry DOI: 10.7270/Q2SQ93Q2 |
More data for this
Ligand-Target Pair | |
GTPase KRas
(Homo sapiens (Human)) | BDBM50514383

(CHEMBL4554946)Show SMILES CCc1cccc(CC)c1-n1c2nc(c(Cl)cc2c(nc1=O)N1CCN(C[C@@H]1C)C(=O)C=C)-c1c(O)cccc1F |r,wU:27.31,(54.65,-47.19,;54.64,-45.66,;55.97,-44.88,;57.31,-45.65,;58.64,-44.87,;58.62,-43.32,;57.29,-42.57,;57.28,-41.03,;58.6,-40.25,;55.97,-43.34,;54.63,-42.58,;54.63,-41.04,;55.97,-40.27,;55.96,-38.71,;54.62,-37.95,;54.62,-36.41,;53.29,-38.72,;53.29,-40.27,;51.97,-41.03,;51.96,-42.57,;53.29,-43.34,;53.29,-44.88,;50.64,-40.26,;50.65,-38.71,;49.33,-37.94,;47.98,-38.7,;47.97,-40.24,;49.31,-41.03,;49.3,-42.57,;46.65,-37.92,;46.66,-36.38,;45.31,-38.68,;43.97,-37.9,;57.29,-37.94,;58.63,-38.71,;59.96,-39.47,;59.97,-37.94,;59.96,-36.39,;58.61,-35.62,;57.28,-36.41,;55.94,-35.64,)| Show InChI InChI=1S/C31H31ClFN5O3/c1-5-19-10-8-11-20(6-2)28(19)38-30-21(16-22(32)27(34-30)26-23(33)12-9-13-24(26)39)29(35-31(38)41)37-15-14-36(17-18(37)4)25(40)7-3/h7-13,16,18,39H,3,5-6,14-15,17H2,1-2,4H3/t18-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 68 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of GDP bound recombinant human His-tagged KRAS G12C/C118A mutant (1 to 169 residues) assessed as reduction in SOS1-mediated GDP/GTP nucleo... |
J Med Chem 63: 52-65 (2020)
Article DOI: 10.1021/acs.jmedchem.9b01180
BindingDB Entry DOI: 10.7270/Q2SQ93Q2 |
More data for this
Ligand-Target Pair | |
GTPase KRas
(Homo sapiens (Human)) | BDBM50514402
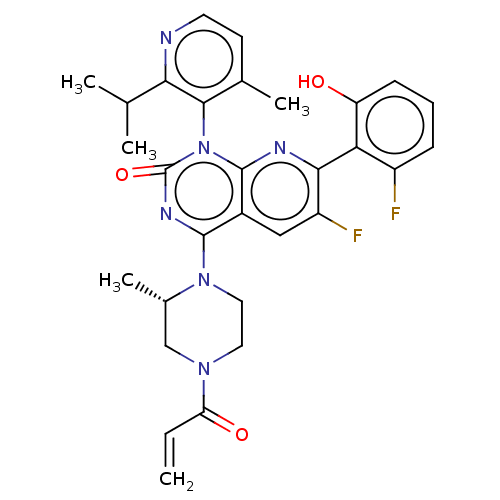
(CHEMBL4535757 | US11345701, Compound Amg-510)Show SMILES CC(C)c1nccc(C)c1-n1c2nc(c(F)cc2c(nc1=O)N1CCN(C[C@@H]1C)C(=O)C=C)-c1c(O)cccc1F |r,wU:27.31,(31.19,-31.19,;32.52,-31.96,;32.53,-33.49,;33.85,-31.18,;35.18,-31.95,;36.51,-31.17,;36.5,-29.62,;35.16,-28.87,;35.15,-27.33,;33.84,-29.64,;32.51,-28.88,;32.5,-27.35,;33.85,-26.57,;33.84,-25.02,;32.5,-24.25,;32.5,-22.71,;31.17,-25.02,;31.17,-26.57,;29.84,-27.34,;29.84,-28.87,;31.17,-29.64,;29.83,-30.4,;28.51,-26.56,;28.52,-25.02,;27.2,-24.24,;25.86,-25,;25.85,-26.54,;27.19,-27.33,;27.18,-28.87,;24.53,-24.22,;24.54,-22.68,;23.19,-24.98,;21.85,-24.2,;35.17,-24.24,;36.51,-25.01,;36.51,-26.55,;37.84,-24.24,;37.84,-22.69,;36.49,-21.92,;35.16,-22.71,;33.82,-21.94,)| Show InChI InChI=1S/C30H30F2N6O3/c1-6-23(40)36-12-13-37(18(5)15-36)28-19-14-21(32)26(24-20(31)8-7-9-22(24)39)34-29(19)38(30(41)35-28)27-17(4)10-11-33-25(27)16(2)3/h6-11,14,16,18,39H,1,12-13,15H2,2-5H3/t18-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 68 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of KRAS in human MIAPaca2 cells assessed as decrease in EGF-stimulated ERK1/2 phosphorylation preincubated for 2 hrs followed by EGF stimu... |
J Med Chem 63: 52-65 (2020)
Article DOI: 10.1021/acs.jmedchem.9b01180
BindingDB Entry DOI: 10.7270/Q2SQ93Q2 |
More data for this
Ligand-Target Pair | |
GTPase KRas
(Homo sapiens (Human)) | BDBM50514389
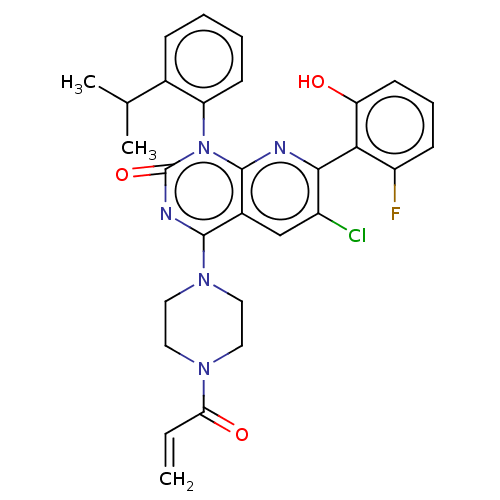
(CHEMBL4439782)Show SMILES CC(C)c1ccccc1-n1c2nc(c(Cl)cc2c(nc1=O)N1CCN(CC1)C(=O)C=C)-c1c(O)cccc1F |(53.81,-29.04,;55.14,-29.82,;55.14,-31.36,;56.48,-29.05,;57.82,-29.82,;59.15,-29.05,;59.15,-27.5,;57.82,-26.74,;56.49,-27.5,;55.15,-26.74,;55.15,-25.21,;56.49,-24.43,;56.48,-22.88,;55.15,-22.12,;55.14,-20.58,;53.81,-22.89,;53.82,-24.43,;52.49,-25.2,;52.48,-26.74,;53.81,-27.5,;52.47,-28.26,;51.16,-24.43,;51.17,-22.88,;49.86,-22.11,;48.51,-22.87,;48.5,-24.41,;49.84,-25.2,;47.18,-22.09,;47.2,-20.55,;45.84,-22.85,;44.51,-22.07,;57.81,-22.11,;59.15,-22.88,;59.15,-24.41,;60.48,-22.11,;60.48,-20.57,;59.13,-19.8,;57.8,-20.58,;56.47,-19.82,)| Show InChI InChI=1S/C29H27ClFN5O3/c1-4-24(38)34-12-14-35(15-13-34)27-19-16-20(30)26(25-21(31)9-7-11-23(25)37)32-28(19)36(29(39)33-27)22-10-6-5-8-18(22)17(2)3/h4-11,16-17,37H,1,12-15H2,2-3H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 69 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of KRAS in human MIAPaca2 cells assessed as decrease in EGF-stimulated ERK1/2 phosphorylation preincubated for 2 hrs followed by EGF stimu... |
J Med Chem 63: 52-65 (2020)
Article DOI: 10.1021/acs.jmedchem.9b01180
BindingDB Entry DOI: 10.7270/Q2SQ93Q2 |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50609601
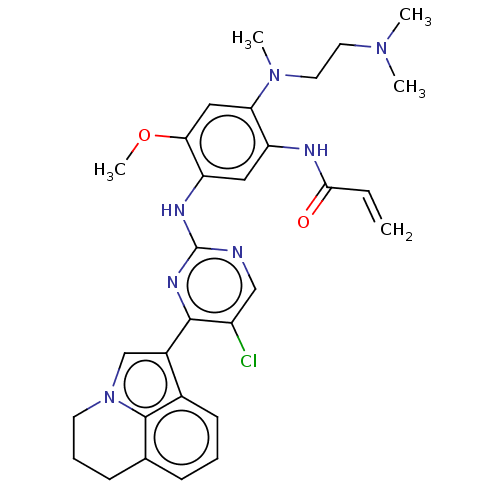
(CHEMBL5266482)Show SMILES COc1cc(N(C)CCN(C)C)c(NC(=O)C=C)cc1Nc1ncc(Cl)c(n1)-c1cn2CCCc3cccc1c23 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| UniChem
| | n/a | n/a | 71 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data