

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50505279 (CHEMBL4436207) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.00100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Shandong University Curated by ChEMBL | Assay Description Inhibition of HIV1 protease using RE(Edans)SGIFLETSK(Dabcyl)R as substrate by fluorescence method | J Med Chem 62: 9375-9414 (2019) Article DOI: 10.1021/acs.jmedchem.9b00359 BindingDB Entry DOI: 10.7270/Q2BR8WFX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM8125 ((3R,3aS,6aR)-hexahydrofuro[2,3-b]furan-3-yl N-[(2S...) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Shandong University Curated by ChEMBL | Assay Description Inhibition of HIV1 protease using RE(Edans)SGIFLETSK(Dabcyl)R as substrate by fluorescence method | J Med Chem 62: 9375-9414 (2019) Article DOI: 10.1021/acs.jmedchem.9b00359 BindingDB Entry DOI: 10.7270/Q2BR8WFX | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Glutamate carboxypeptidase 2 (Homo sapiens (Human)) | BDBM479434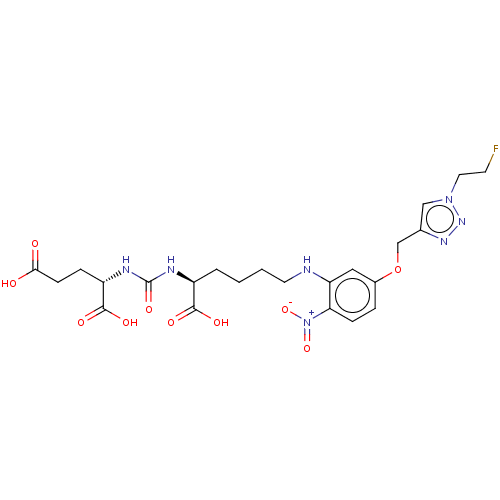 (US10894807, ID P242) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.0700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Siemens Medical Solutions USA, Inc. US Patent | Assay Description The inhibitory activity of all compounds was determined using a fluorescent assay of human PSMA activity. The enzyme used in the assay was purchased ... | US Patent US10894807 (2021) BindingDB Entry DOI: 10.7270/Q2SN0D25 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate carboxypeptidase 2 (Homo sapiens (Human)) | BDBM479437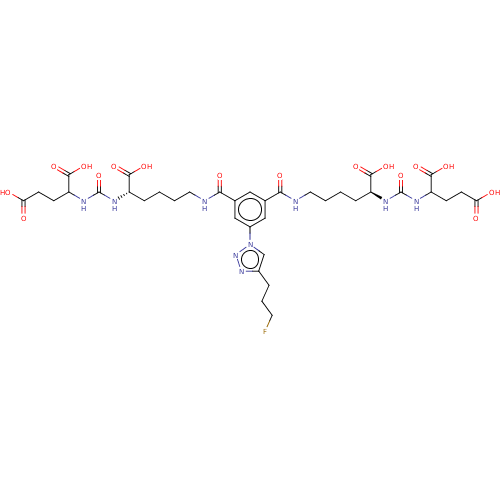 (US10894807, ID P246) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Siemens Medical Solutions USA, Inc. US Patent | Assay Description The inhibitory activity of all compounds was determined using a fluorescent assay of human PSMA activity. The enzyme used in the assay was purchased ... | US Patent US10894807 (2021) BindingDB Entry DOI: 10.7270/Q2SN0D25 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate carboxypeptidase 2 (Homo sapiens (Human)) | BDBM479458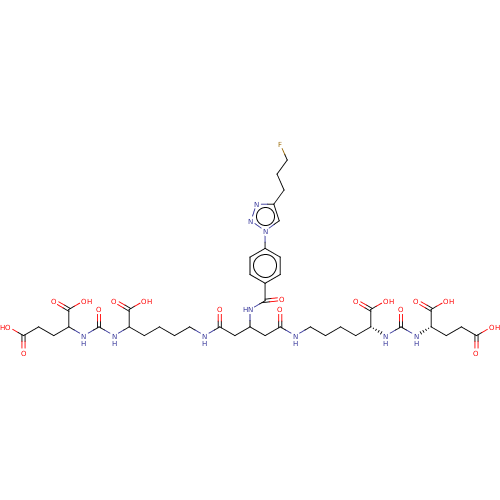 (US10894807, ID P278) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Siemens Medical Solutions USA, Inc. US Patent | Assay Description The inhibitory activity of all compounds was determined using a fluorescent assay of human PSMA activity. The enzyme used in the assay was purchased ... | US Patent US10894807 (2021) BindingDB Entry DOI: 10.7270/Q2SN0D25 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate carboxypeptidase 2 (Homo sapiens (Human)) | BDBM479453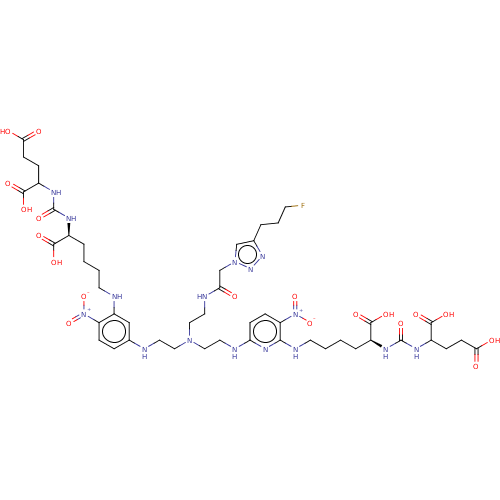 (US10894807, ID P273) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Siemens Medical Solutions USA, Inc. US Patent | Assay Description The inhibitory activity of all compounds was determined using a fluorescent assay of human PSMA activity. The enzyme used in the assay was purchased ... | US Patent US10894807 (2021) BindingDB Entry DOI: 10.7270/Q2SN0D25 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate carboxypeptidase 2 (Homo sapiens (Human)) | BDBM479450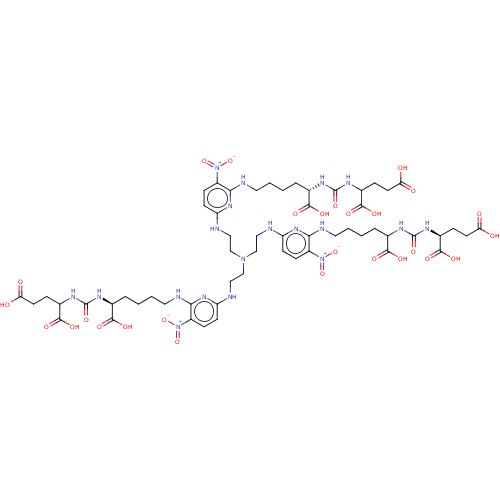 (US10894807, ID P270) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Siemens Medical Solutions USA, Inc. US Patent | Assay Description The inhibitory activity of all compounds was determined using a fluorescent assay of human PSMA activity. The enzyme used in the assay was purchased ... | US Patent US10894807 (2021) BindingDB Entry DOI: 10.7270/Q2SN0D25 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate carboxypeptidase 2 (Homo sapiens (Human)) | BDBM479444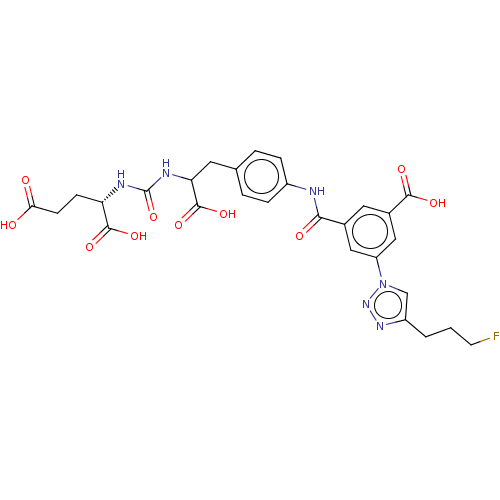 (US10894807, ID P253) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Siemens Medical Solutions USA, Inc. US Patent | Assay Description The inhibitory activity of all compounds was determined using a fluorescent assay of human PSMA activity. The enzyme used in the assay was purchased ... | US Patent US10894807 (2021) BindingDB Entry DOI: 10.7270/Q2SN0D25 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate carboxypeptidase 2 (Homo sapiens (Human)) | BDBM479431 (US10894807, ID P238) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | <0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Siemens Medical Solutions USA, Inc. US Patent | Assay Description The inhibitory activity of all compounds was determined using a fluorescent assay of human PSMA activity. The enzyme used in the assay was purchased ... | US Patent US10894807 (2021) BindingDB Entry DOI: 10.7270/Q2SN0D25 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate carboxypeptidase 2 (Homo sapiens (Human)) | BDBM479429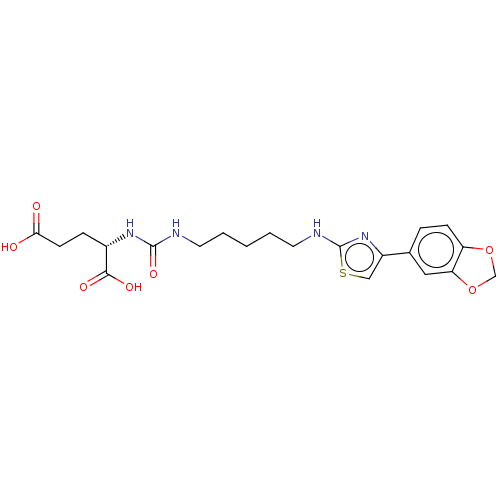 (US10894807, ID P235) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | <0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Siemens Medical Solutions USA, Inc. US Patent | Assay Description The inhibitory activity of all compounds was determined using a fluorescent assay of human PSMA activity. The enzyme used in the assay was purchased ... | US Patent US10894807 (2021) BindingDB Entry DOI: 10.7270/Q2SN0D25 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate carboxypeptidase 2 (Homo sapiens (Human)) | BDBM479415 (US10894807, ID P200) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | <0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Siemens Medical Solutions USA, Inc. US Patent | Assay Description The inhibitory activity of all compounds was determined using a fluorescent assay of human PSMA activity. The enzyme used in the assay was purchased ... | US Patent US10894807 (2021) BindingDB Entry DOI: 10.7270/Q2SN0D25 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate carboxypeptidase 2 (Homo sapiens (Human)) | BDBM479449 (US10894807, ID P266) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | US Patent | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Siemens Medical Solutions USA, Inc. US Patent | Assay Description The inhibitory activity of all compounds was determined using a fluorescent assay of human PSMA activity. The enzyme used in the assay was purchased ... | US Patent US10894807 (2021) BindingDB Entry DOI: 10.7270/Q2SN0D25 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate carboxypeptidase 2 (Homo sapiens (Human)) | BDBM479442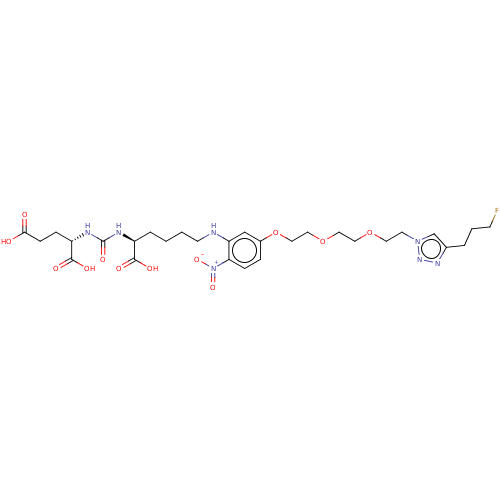 (US10894807, ID P251) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.340 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Siemens Medical Solutions USA, Inc. US Patent | Assay Description The inhibitory activity of all compounds was determined using a fluorescent assay of human PSMA activity. The enzyme used in the assay was purchased ... | US Patent US10894807 (2021) BindingDB Entry DOI: 10.7270/Q2SN0D25 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate carboxypeptidase 2 (Homo sapiens (Human)) | BDBM479433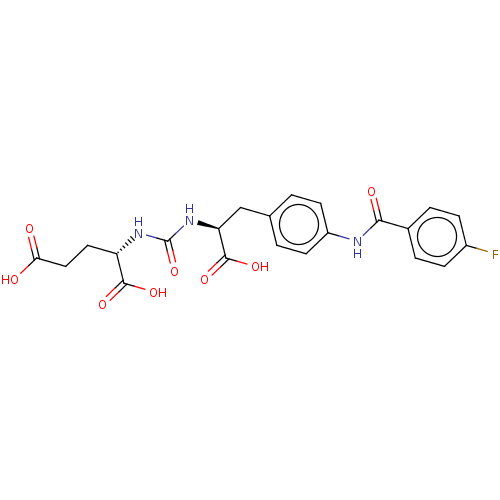 (US10894807, ID P241) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.380 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Siemens Medical Solutions USA, Inc. US Patent | Assay Description The inhibitory activity of all compounds was determined using a fluorescent assay of human PSMA activity. The enzyme used in the assay was purchased ... | US Patent US10894807 (2021) BindingDB Entry DOI: 10.7270/Q2SN0D25 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate carboxypeptidase 2 (Homo sapiens (Human)) | BDBM479435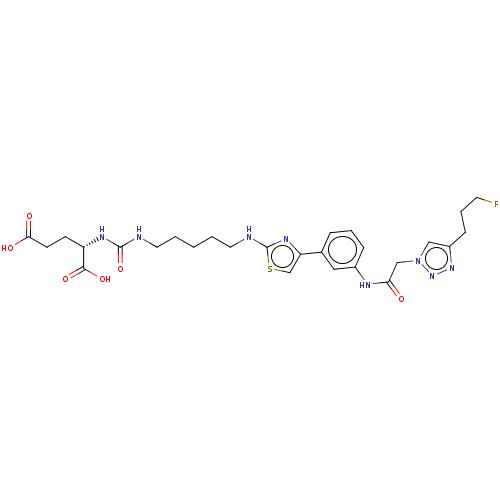 (US10894807, ID P244) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.380 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Siemens Medical Solutions USA, Inc. US Patent | Assay Description The inhibitory activity of all compounds was determined using a fluorescent assay of human PSMA activity. The enzyme used in the assay was purchased ... | US Patent US10894807 (2021) BindingDB Entry DOI: 10.7270/Q2SN0D25 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate carboxypeptidase 2 (Homo sapiens (Human)) | BDBM479445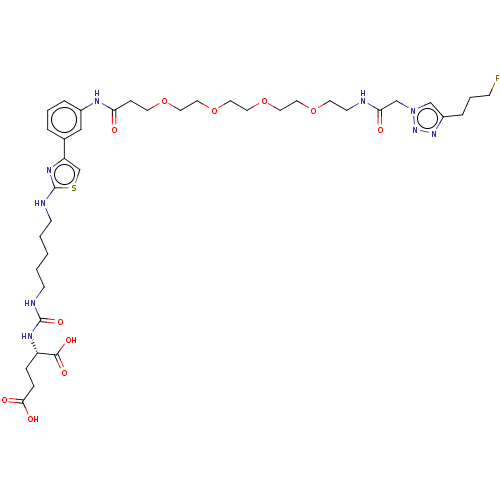 (US10894807, ID P254) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Siemens Medical Solutions USA, Inc. US Patent | Assay Description The inhibitory activity of all compounds was determined using a fluorescent assay of human PSMA activity. The enzyme used in the assay was purchased ... | US Patent US10894807 (2021) BindingDB Entry DOI: 10.7270/Q2SN0D25 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate carboxypeptidase 2 (Homo sapiens (Human)) | BDBM479457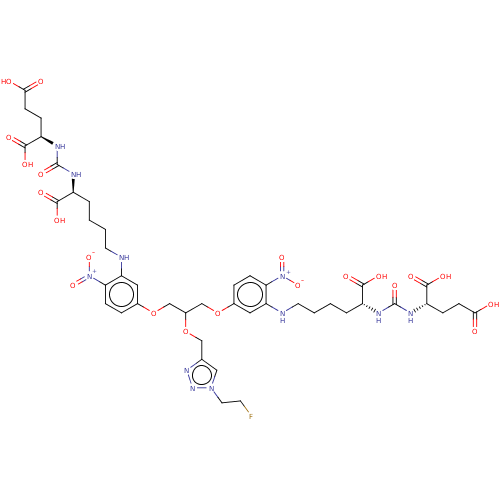 (US10894807, ID P277) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | US Patent | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Siemens Medical Solutions USA, Inc. US Patent | Assay Description The inhibitory activity of all compounds was determined using a fluorescent assay of human PSMA activity. The enzyme used in the assay was purchased ... | US Patent US10894807 (2021) BindingDB Entry DOI: 10.7270/Q2SN0D25 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| E3 ubiquitin-protein ligase Mdm2 (Homo sapiens (Human)) | BDBM50436682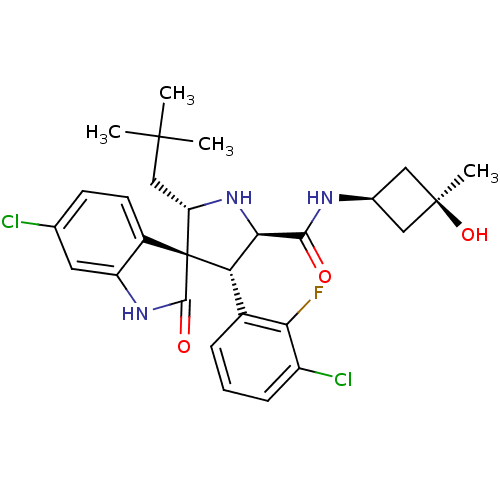 (CHEMBL2396674) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.440 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Michigan Curated by ChEMBL | Assay Description Binding affinity to recombinant human His-tagged MDM2 (1 to 118 amino acids) using p53-based PMDM6-F as probe after 15 mins by competition assay | J Med Chem 56: 5553-61 (2014) Article DOI: 10.1021/jm4005708 BindingDB Entry DOI: 10.7270/Q2SJ1N2D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate carboxypeptidase 2 (Homo sapiens (Human)) | BDBM479464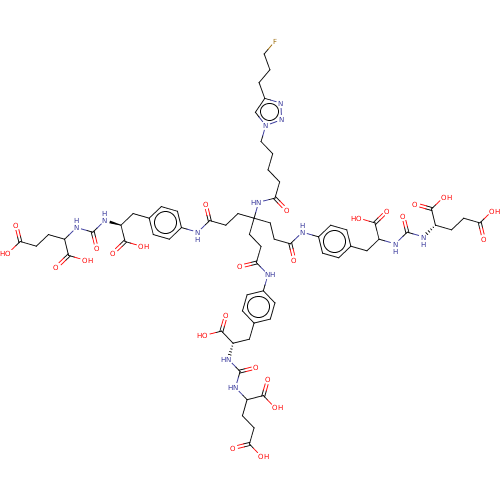 (US10894807, ID P285) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Siemens Medical Solutions USA, Inc. US Patent | Assay Description The inhibitory activity of all compounds was determined using a fluorescent assay of human PSMA activity. The enzyme used in the assay was purchased ... | US Patent US10894807 (2021) BindingDB Entry DOI: 10.7270/Q2SN0D25 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain-containing protein 4 (Homo sapiens (Human)) | BDBM50453769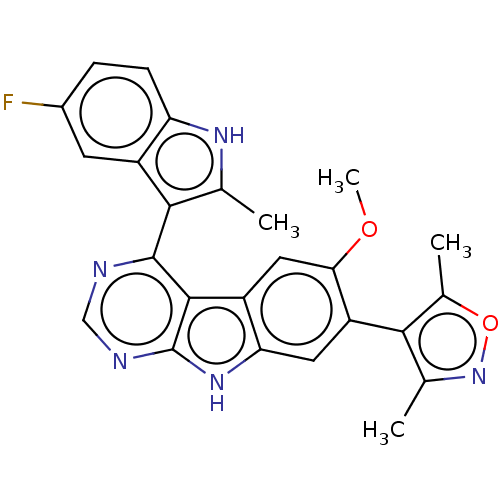 (CHEMBL4214978) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | <0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Michigan Curated by ChEMBL | Assay Description Inhibition of FAM-labeled ZBA248 binding to recombinant human N-terminal His6-tagged BRD4 bromodomain 1 (44 to 168 residues) expressed in Rosetta2 DE... | J Med Chem 60: 3887-3901 (2017) Article DOI: 10.1021/acs.jmedchem.7b00193 BindingDB Entry DOI: 10.7270/Q28W3GWN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain-containing protein 4 (Homo sapiens (Human)) | BDBM50366704 (CHEMBL4166630) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of FAM-labeled ZBA248 binding to recombinant human BRD4 bromodomain 1 (44 to 168 residues) expressed in Rosetta2 Escherichia coli DE3 cell... | J Med Chem 61: 6110-6120 (2018) Article DOI: 10.1021/acs.jmedchem.8b00483 BindingDB Entry DOI: 10.7270/Q26M39B1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain-containing protein 4 (Homo sapiens (Human)) | BDBM179480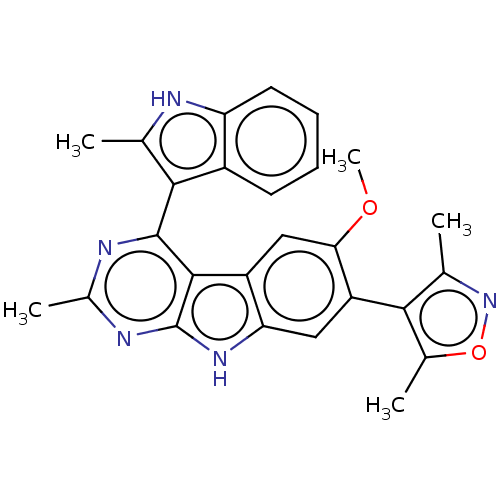 (US9675697, Cpd. No. 68) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | <0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Michigan Curated by ChEMBL | Assay Description Inhibition of FAM-labeled ZBA248 binding to recombinant human N-terminal His6-tagged BRD4 bromodomain 2 (333 to 460 residues) expressed in Rosetta2 D... | J Med Chem 60: 3887-3901 (2017) Article DOI: 10.1021/acs.jmedchem.7b00193 BindingDB Entry DOI: 10.7270/Q28W3GWN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain-containing protein 4 (Homo sapiens (Human)) | BDBM50453810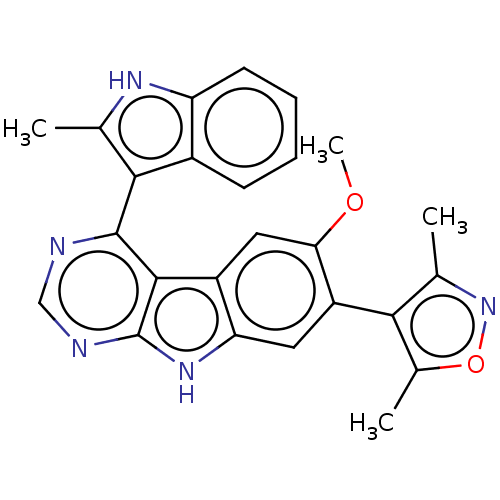 (CHEMBL4208405) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | <0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Michigan Curated by ChEMBL | Assay Description Inhibition of FAM-labeled ZBA248 binding to recombinant human N-terminal His6-tagged BRD4 bromodomain 1 (44 to 168 residues) expressed in Rosetta2 DE... | J Med Chem 60: 3887-3901 (2017) Article DOI: 10.1021/acs.jmedchem.7b00193 BindingDB Entry DOI: 10.7270/Q28W3GWN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain-containing protein 4 (Homo sapiens (Human)) | BDBM50453807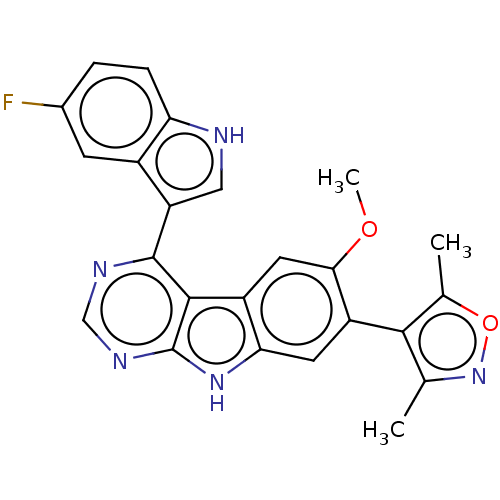 (CHEMBL4211497) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | <0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Michigan Curated by ChEMBL | Assay Description Inhibition of FAM-labeled ZBA248 binding to recombinant human N-terminal His6-tagged BRD4 bromodomain 1 (44 to 168 residues) expressed in Rosetta2 DE... | J Med Chem 60: 3887-3901 (2017) Article DOI: 10.1021/acs.jmedchem.7b00193 BindingDB Entry DOI: 10.7270/Q28W3GWN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate carboxypeptidase 2 (Homo sapiens (Human)) | BDBM479426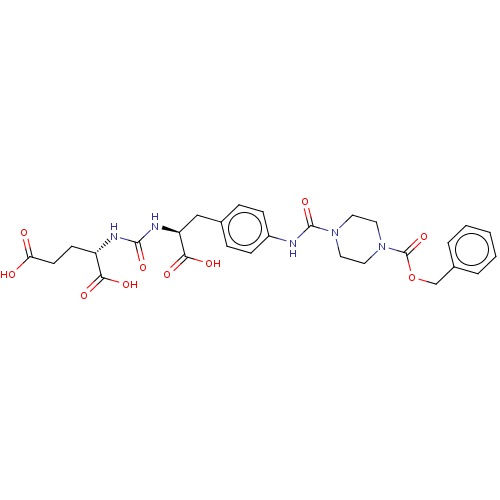 (US10894807, ID P222) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.560 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Siemens Medical Solutions USA, Inc. US Patent | Assay Description The inhibitory activity of all compounds was determined using a fluorescent assay of human PSMA activity. The enzyme used in the assay was purchased ... | US Patent US10894807 (2021) BindingDB Entry DOI: 10.7270/Q2SN0D25 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate carboxypeptidase 2 (Homo sapiens (Human)) | BDBM479425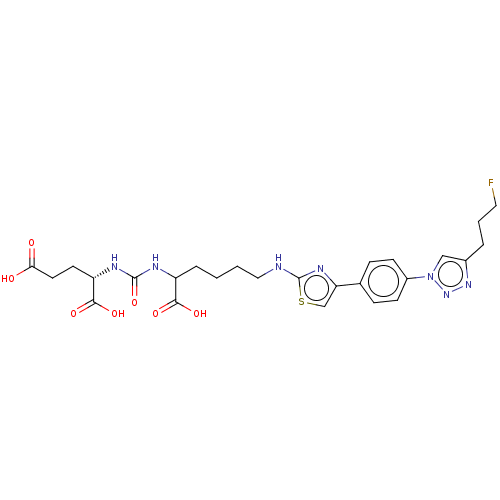 (US10894807, ID P218) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Siemens Medical Solutions USA, Inc. US Patent | Assay Description The inhibitory activity of all compounds was determined using a fluorescent assay of human PSMA activity. The enzyme used in the assay was purchased ... | US Patent US10894807 (2021) BindingDB Entry DOI: 10.7270/Q2SN0D25 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| E3 ubiquitin-protein ligase Mdm2 (Homo sapiens (Human)) | BDBM50436688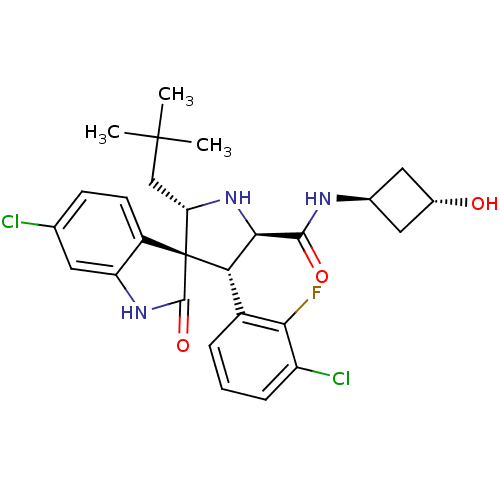 (CHEMBL2398473) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.610 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Michigan Curated by ChEMBL | Assay Description Binding affinity to recombinant human His-tagged MDM2 (1 to 118 amino acids) using p53-based PMDM6-F as probe after 15 mins by competition assay | J Med Chem 56: 5553-61 (2014) Article DOI: 10.1021/jm4005708 BindingDB Entry DOI: 10.7270/Q2SJ1N2D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| E3 ubiquitin-protein ligase Mdm2 (Homo sapiens (Human)) | BDBM50436685 (CHEMBL2398476) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.620 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Michigan Curated by ChEMBL | Assay Description Binding affinity to recombinant human His-tagged MDM2 (1 to 118 amino acids) using p53-based PMDM6-F as probe after 15 mins by competition assay | J Med Chem 56: 5553-61 (2014) Article DOI: 10.1021/jm4005708 BindingDB Entry DOI: 10.7270/Q2SJ1N2D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| E3 ubiquitin-protein ligase Mdm2 (Homo sapiens (Human)) | BDBM50436681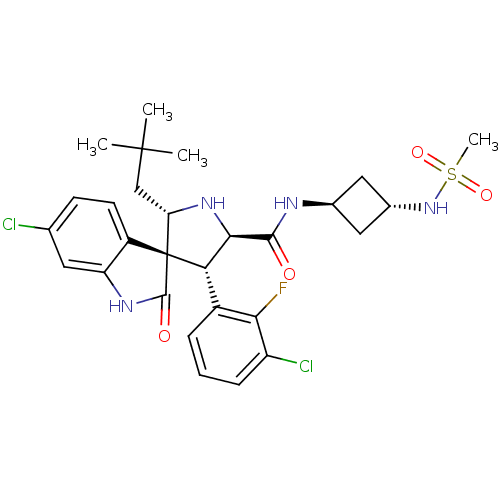 (CHEMBL2398479) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.620 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Michigan Curated by ChEMBL | Assay Description Binding affinity to recombinant human His-tagged MDM2 (1 to 118 amino acids) using p53-based PMDM6-F as probe after 15 mins by competition assay | J Med Chem 56: 5553-61 (2014) Article DOI: 10.1021/jm4005708 BindingDB Entry DOI: 10.7270/Q2SJ1N2D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate carboxypeptidase 2 (Homo sapiens (Human)) | BDBM479438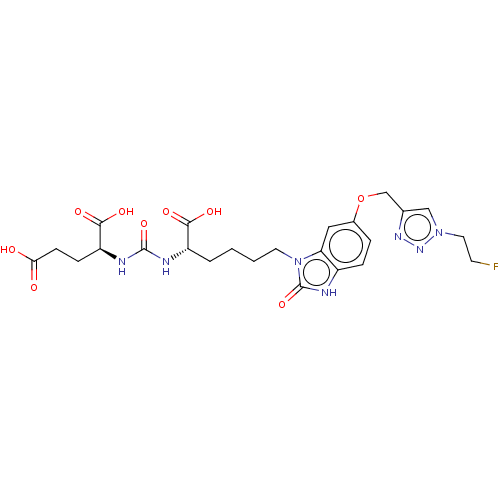 (US10894807, ID P247) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.630 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Siemens Medical Solutions USA, Inc. US Patent | Assay Description The inhibitory activity of all compounds was determined using a fluorescent assay of human PSMA activity. The enzyme used in the assay was purchased ... | US Patent US10894807 (2021) BindingDB Entry DOI: 10.7270/Q2SN0D25 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain-containing protein 4 (Homo sapiens (Human)) | BDBM50366716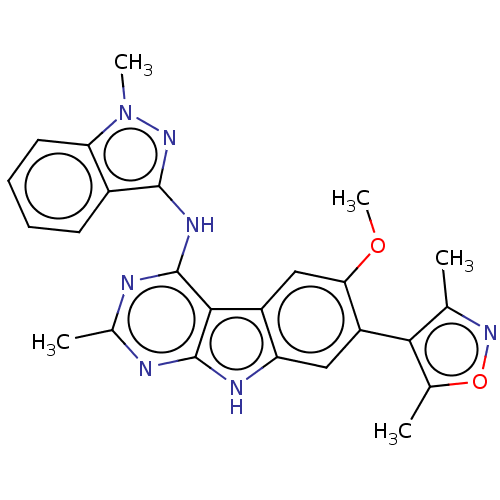 (CHEMBL4174669) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of FAM-labeled ZBA248 binding to recombinant human BRD4 bromodomain 1 (44 to 168 residues) expressed in Rosetta2 Escherichia coli DE3 cell... | J Med Chem 61: 6110-6120 (2018) Article DOI: 10.1021/acs.jmedchem.8b00483 BindingDB Entry DOI: 10.7270/Q26M39B1 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Glutamate carboxypeptidase 2 (Homo sapiens (Human)) | BDBM479469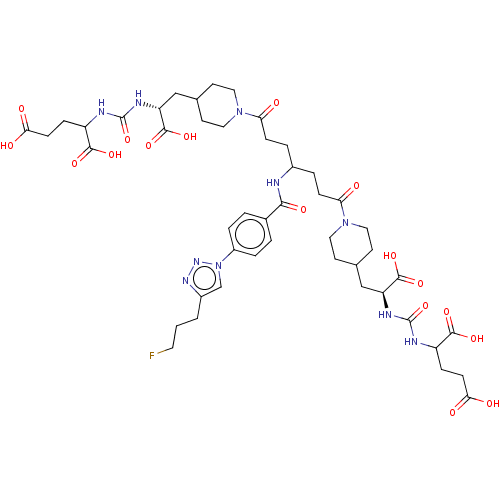 (US10894807, ID P292) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Siemens Medical Solutions USA, Inc. US Patent | Assay Description The inhibitory activity of all compounds was determined using a fluorescent assay of human PSMA activity. The enzyme used in the assay was purchased ... | US Patent US10894807 (2021) BindingDB Entry DOI: 10.7270/Q2SN0D25 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain-containing protein 4 (Homo sapiens (Human)) | BDBM50453810 (CHEMBL4208405) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Michigan Curated by ChEMBL | Assay Description Inhibition of FAM-labeled ZBA248 binding to recombinant human N-terminal His6-tagged BRD4 bromodomain 2 (333 to 460 residues) expressed in Rosetta2 D... | J Med Chem 60: 3887-3901 (2017) Article DOI: 10.1021/acs.jmedchem.7b00193 BindingDB Entry DOI: 10.7270/Q28W3GWN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate carboxypeptidase 2 (Homo sapiens (Human)) | BDBM479375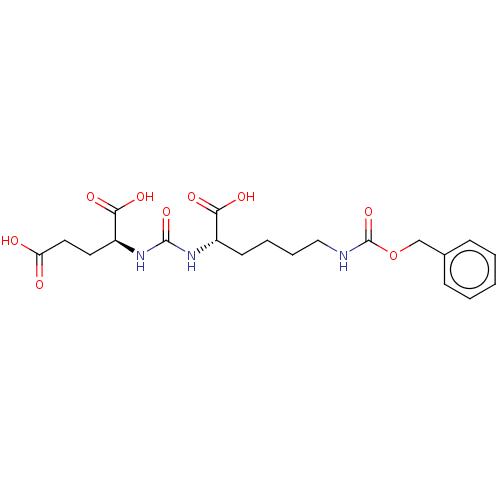 (US10894807, ID P033) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.780 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Siemens Medical Solutions USA, Inc. US Patent | Assay Description The inhibitory activity of all compounds was determined using a fluorescent assay of human PSMA activity. The enzyme used in the assay was purchased ... | US Patent US10894807 (2021) BindingDB Entry DOI: 10.7270/Q2SN0D25 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate carboxypeptidase 2 (Homo sapiens (Human)) | BDBM479451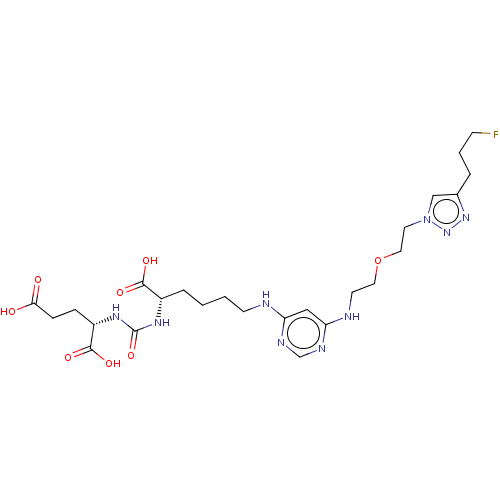 (US10894807, ID P271) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Siemens Medical Solutions USA, Inc. US Patent | Assay Description The inhibitory activity of all compounds was determined using a fluorescent assay of human PSMA activity. The enzyme used in the assay was purchased ... | US Patent US10894807 (2021) BindingDB Entry DOI: 10.7270/Q2SN0D25 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain-containing protein 4 (Homo sapiens (Human)) | BDBM179480 (US9675697, Cpd. No. 68) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Michigan Curated by ChEMBL | Assay Description Inhibition of FAM-labeled ZBA248 binding to recombinant human N-terminal His6-tagged BRD4 bromodomain 1 (44 to 168 residues) expressed in Rosetta2 DE... | J Med Chem 60: 3887-3901 (2017) Article DOI: 10.1021/acs.jmedchem.7b00193 BindingDB Entry DOI: 10.7270/Q28W3GWN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain-containing protein 4 (Homo sapiens (Human)) | BDBM50366724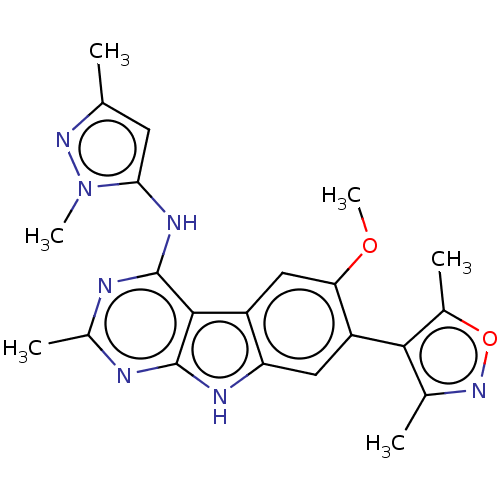 (CHEMBL4172277) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of FAM-labeled ZBA248 binding to recombinant human BRD4 bromodomain 1 (44 to 168 residues) expressed in Rosetta2 Escherichia coli DE3 cell... | J Med Chem 61: 6110-6120 (2018) Article DOI: 10.1021/acs.jmedchem.8b00483 BindingDB Entry DOI: 10.7270/Q26M39B1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain-containing protein 4 (Homo sapiens (Human)) | BDBM179480 (US9675697, Cpd. No. 68) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of FAM-labeled ZBA248 binding to recombinant human BRD4 bromodomain 1 (44 to 168 residues) expressed in Rosetta2 Escherichia coli DE3 cell... | J Med Chem 61: 6110-6120 (2018) Article DOI: 10.1021/acs.jmedchem.8b00483 BindingDB Entry DOI: 10.7270/Q26M39B1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| E3 ubiquitin-protein ligase Mdm2 (Homo sapiens (Human)) | BDBM50436689 (CHEMBL2398472) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.860 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Michigan Curated by ChEMBL | Assay Description Binding affinity to recombinant human His-tagged MDM2 (1 to 118 amino acids) using p53-based PMDM6-F as probe after 15 mins by competition assay | J Med Chem 56: 5553-61 (2014) Article DOI: 10.1021/jm4005708 BindingDB Entry DOI: 10.7270/Q2SJ1N2D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain-containing protein 4 (Homo sapiens (Human)) | BDBM50453769 (CHEMBL4214978) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Michigan Curated by ChEMBL | Assay Description Inhibition of FAM-labeled ZBA248 binding to recombinant human N-terminal His6-tagged BRD4 bromodomain 2 (333 to 460 residues) expressed in Rosetta2 D... | J Med Chem 60: 3887-3901 (2017) Article DOI: 10.1021/acs.jmedchem.7b00193 BindingDB Entry DOI: 10.7270/Q28W3GWN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate carboxypeptidase 2 (Homo sapiens (Human)) | BDBM479460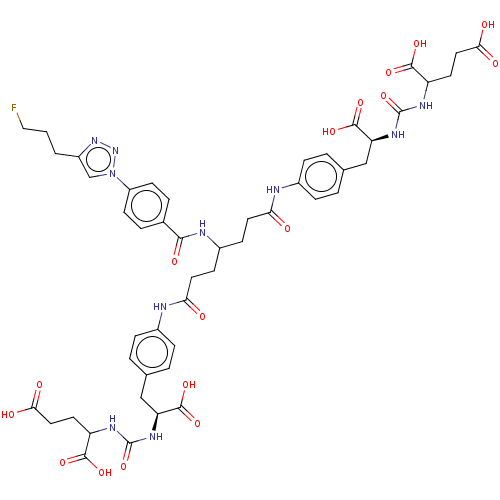 (US10894807, ID P280) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Siemens Medical Solutions USA, Inc. US Patent | Assay Description The inhibitory activity of all compounds was determined using a fluorescent assay of human PSMA activity. The enzyme used in the assay was purchased ... | US Patent US10894807 (2021) BindingDB Entry DOI: 10.7270/Q2SN0D25 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate carboxypeptidase 2 (Homo sapiens (Human)) | BDBM479463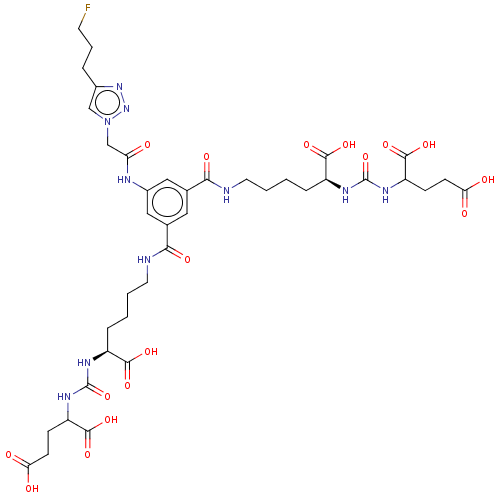 (US10894807, ID P283) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Siemens Medical Solutions USA, Inc. US Patent | Assay Description The inhibitory activity of all compounds was determined using a fluorescent assay of human PSMA activity. The enzyme used in the assay was purchased ... | US Patent US10894807 (2021) BindingDB Entry DOI: 10.7270/Q2SN0D25 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| E3 ubiquitin-protein ligase Mdm2 (Homo sapiens (Human)) | BDBM50436686 (CHEMBL2398475) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.940 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Michigan Curated by ChEMBL | Assay Description Binding affinity to recombinant human His-tagged MDM2 (1 to 118 amino acids) using p53-based PMDM6-F as probe after 15 mins by competition assay | J Med Chem 56: 5553-61 (2014) Article DOI: 10.1021/jm4005708 BindingDB Entry DOI: 10.7270/Q2SJ1N2D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| E3 ubiquitin-protein ligase Mdm2 (Homo sapiens (Human)) | BDBM50436683 (CHEMBL2398478) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.970 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Michigan Curated by ChEMBL | Assay Description Binding affinity to recombinant human His-tagged MDM2 (1 to 118 amino acids) using p53-based PMDM6-F as probe after 15 mins by competition assay | J Med Chem 56: 5553-61 (2014) Article DOI: 10.1021/jm4005708 BindingDB Entry DOI: 10.7270/Q2SJ1N2D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate carboxypeptidase 2 (Homo sapiens (Human)) | BDBM479466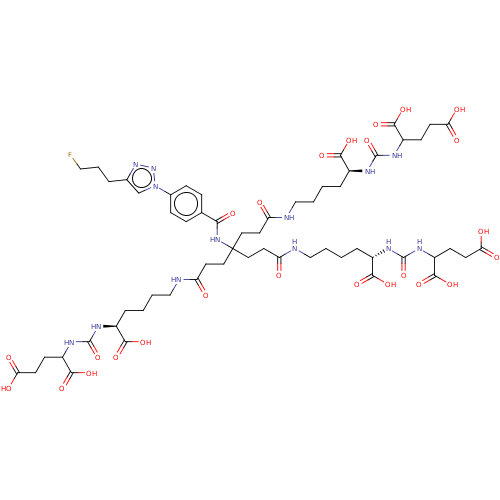 (US10894807, ID P287) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Siemens Medical Solutions USA, Inc. US Patent | Assay Description The inhibitory activity of all compounds was determined using a fluorescent assay of human PSMA activity. The enzyme used in the assay was purchased ... | US Patent US10894807 (2021) BindingDB Entry DOI: 10.7270/Q2SN0D25 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain-containing protein 4 (Homo sapiens (Human)) | BDBM50366670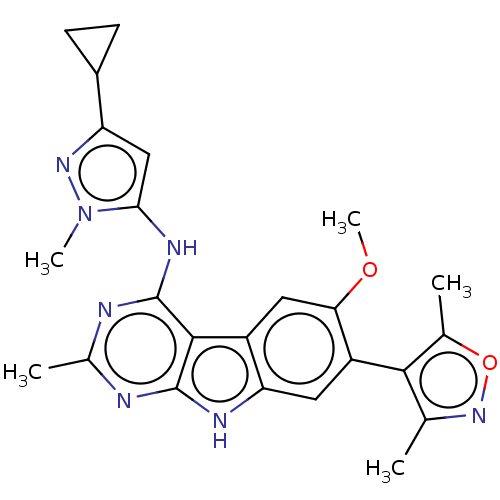 (CHEMBL4173488) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem Similars | PDB Article PubMed | <1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of FAM-labeled ZBA248 binding to recombinant human BRD4 bromodomain 1 (44 to 168 residues) expressed in Rosetta2 Escherichia coli DE3 cell... | J Med Chem 61: 6110-6120 (2018) Article DOI: 10.1021/acs.jmedchem.8b00483 BindingDB Entry DOI: 10.7270/Q26M39B1 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Bromodomain-containing protein 4 (Homo sapiens (Human)) | BDBM50366748 (CHEMBL4172447) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | <1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of FAM-labeled ZBA248 binding to recombinant human BRD4 bromodomain 1 (44 to 168 residues) expressed in Rosetta2 Escherichia coli DE3 cell... | J Med Chem 61: 6110-6120 (2018) Article DOI: 10.1021/acs.jmedchem.8b00483 BindingDB Entry DOI: 10.7270/Q26M39B1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain-containing protein 4 (Homo sapiens (Human)) | BDBM50366678 (CHEMBL4167996) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | <1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of FAM-labeled ZBA248 binding to recombinant human BRD4 bromodomain 1 (44 to 168 residues) expressed in Rosetta2 Escherichia coli DE3 cell... | J Med Chem 61: 6110-6120 (2018) Article DOI: 10.1021/acs.jmedchem.8b00483 BindingDB Entry DOI: 10.7270/Q26M39B1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain-containing protein 4 (Homo sapiens (Human)) | BDBM50366721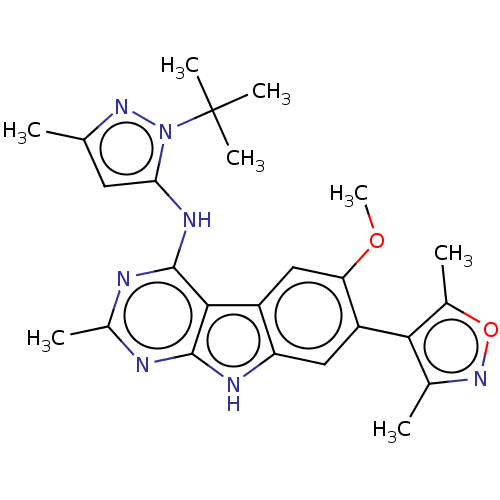 (CHEMBL4171109) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | <1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of FAM-labeled ZBA248 binding to recombinant human BRD4 bromodomain 1 (44 to 168 residues) expressed in Rosetta2 Escherichia coli DE3 cell... | J Med Chem 61: 6110-6120 (2018) Article DOI: 10.1021/acs.jmedchem.8b00483 BindingDB Entry DOI: 10.7270/Q26M39B1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain-containing protein 4 (Homo sapiens (Human)) | BDBM50366707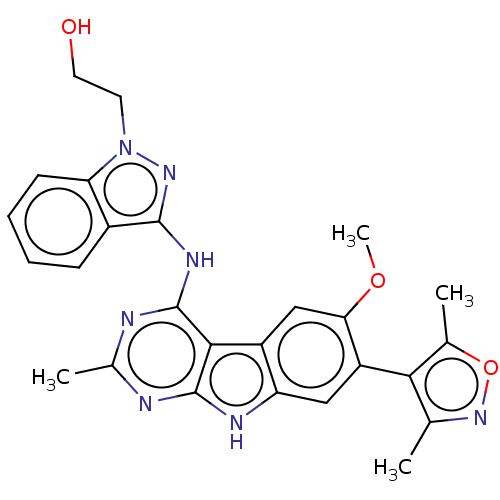 (CHEMBL4164393) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of FAM-labeled ZBA248 binding to recombinant human BRD4 bromodomain 1 (44 to 168 residues) expressed in Rosetta2 Escherichia coli DE3 cell... | J Med Chem 61: 6110-6120 (2018) Article DOI: 10.1021/acs.jmedchem.8b00483 BindingDB Entry DOI: 10.7270/Q26M39B1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 1418 total ) | Next | Last >> |