 Found 117 hits with Last Name = 'zuck' and Initial = 'p'
Found 117 hits with Last Name = 'zuck' and Initial = 'p' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
High affinity nerve growth factor receptor
(Homo sapiens (Human)) | BDBM50022671

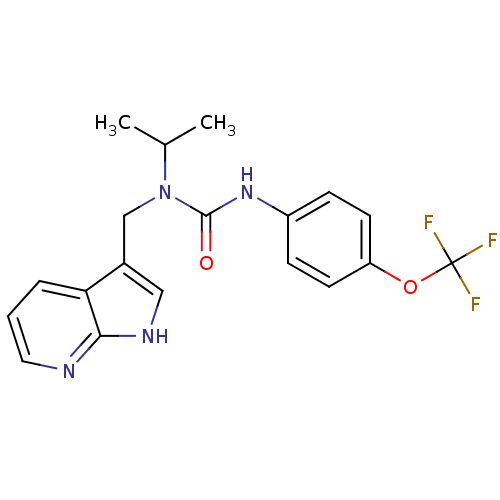
(CHEMBL3298265)Show SMILES Cc1cn2cc(CNc3ncnc4ccc(nc34)N3CCC[C@@H]3c3cc(F)ccc3F)nc2s1 |r| Show InChI InChI=1S/C24H21F2N7S/c1-14-11-32-12-16(30-24(32)34-14)10-27-23-22-19(28-13-29-23)6-7-21(31-22)33-8-2-3-20(33)17-9-15(25)4-5-18(17)26/h4-7,9,11-13,20H,2-3,8,10H2,1H3,(H,27,28,29)/t20-/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of purified TrkA cytoplasmic domain (unknown origin) by HTRF assay |
J Med Chem 57: 5800-16 (2014)
Article DOI: 10.1021/jm5006429
BindingDB Entry DOI: 10.7270/Q2BV7J69 |
More data for this
Ligand-Target Pair | |
High affinity nerve growth factor receptor
(Homo sapiens (Human)) | BDBM50446392
(CHEMBL3109645 | US9181261, 2)Show SMILES CC(C)N(Cc1c[nH]c2ncccc12)C(=O)Nc1ccc(OC(F)(F)F)cc1 Show InChI InChI=1S/C19H19F3N4O2/c1-12(2)26(11-13-10-24-17-16(13)4-3-9-23-17)18(27)25-14-5-7-15(8-6-14)28-19(20,21)22/h3-10,12H,11H2,1-2H3,(H,23,24)(H,25,27) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human TrkA expressed in human U2OS cells assessed as inhibition of NGF-induced maximum response after 1 hr by beta-galactosidase assay |
J Med Chem 57: 5800-16 (2014)
Article DOI: 10.1021/jm5006429
BindingDB Entry DOI: 10.7270/Q2BV7J69 |
More data for this
Ligand-Target Pair | |
High affinity nerve growth factor receptor
(Homo sapiens (Human)) | BDBM50022675
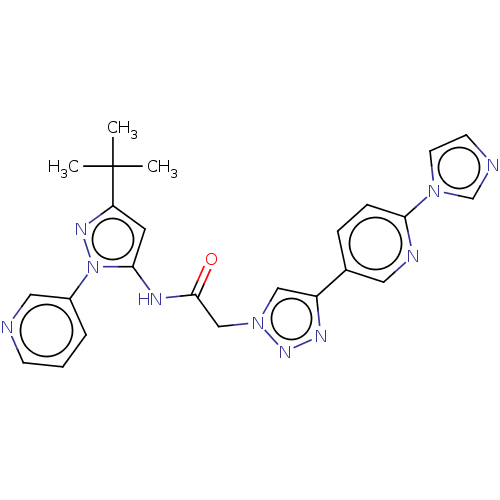
(CHEMBL3298268)Show SMILES CC(C)(C)c1cc(NC(=O)Cn2cc(nn2)-c2ccc(nc2)-n2ccnc2)n(n1)-c1cccnc1 Show InChI InChI=1S/C24H24N10O/c1-24(2,3)20-11-22(34(30-20)18-5-4-8-25-13-18)28-23(35)15-33-14-19(29-31-33)17-6-7-21(27-12-17)32-10-9-26-16-32/h4-14,16H,15H2,1-3H3,(H,28,35) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human TrkA expressed in human U2OS cells assessed as inhibition of NGF-induced maximum response after 1 hr by beta-galactosidase assay |
J Med Chem 57: 5800-16 (2014)
Article DOI: 10.1021/jm5006429
BindingDB Entry DOI: 10.7270/Q2BV7J69 |
More data for this
Ligand-Target Pair | |
High affinity nerve growth factor receptor
(Homo sapiens (Human)) | BDBM50022675

(CHEMBL3298268)Show SMILES CC(C)(C)c1cc(NC(=O)Cn2cc(nn2)-c2ccc(nc2)-n2ccnc2)n(n1)-c1cccnc1 Show InChI InChI=1S/C24H24N10O/c1-24(2,3)20-11-22(34(30-20)18-5-4-8-25-13-18)28-23(35)15-33-14-19(29-31-33)17-6-7-21(27-12-17)32-10-9-26-16-32/h4-14,16H,15H2,1-3H3,(H,28,35) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of purified TrkA cytoplasmic domain (unknown origin) by HTRF assay |
J Med Chem 57: 5800-16 (2014)
Article DOI: 10.1021/jm5006429
BindingDB Entry DOI: 10.7270/Q2BV7J69 |
More data for this
Ligand-Target Pair | |
High affinity nerve growth factor receptor
(Homo sapiens (Human)) | BDBM50022674
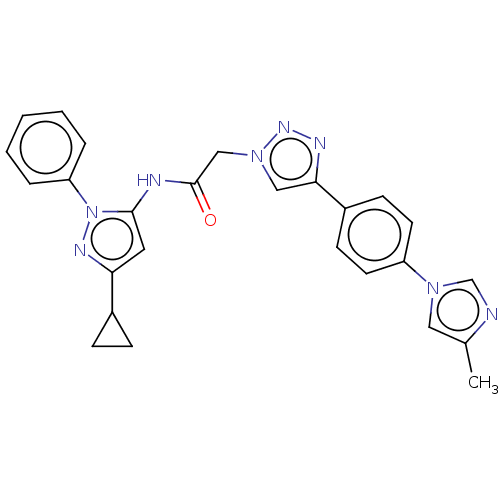
(CHEMBL3298267)Show SMILES Cc1cn(cn1)-c1ccc(cc1)-c1cn(CC(=O)Nc2cc(nn2-c2ccccc2)C2CC2)nn1 Show InChI InChI=1S/C26H24N8O/c1-18-14-32(17-27-18)21-11-9-20(10-12-21)24-15-33(31-29-24)16-26(35)28-25-13-23(19-7-8-19)30-34(25)22-5-3-2-4-6-22/h2-6,9-15,17,19H,7-8,16H2,1H3,(H,28,35) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human TrkA expressed in human U2OS cells assessed as inhibition of NGF-induced maximum response after 1 hr by beta-galactosidase assay |
J Med Chem 57: 5800-16 (2014)
Article DOI: 10.1021/jm5006429
BindingDB Entry DOI: 10.7270/Q2BV7J69 |
More data for this
Ligand-Target Pair | |
High affinity nerve growth factor receptor
(Homo sapiens (Human)) | BDBM50446392

(CHEMBL3109645 | US9181261, 2)Show SMILES CC(C)N(Cc1c[nH]c2ncccc12)C(=O)Nc1ccc(OC(F)(F)F)cc1 Show InChI InChI=1S/C19H19F3N4O2/c1-12(2)26(11-13-10-24-17-16(13)4-3-9-23-17)18(27)25-14-5-7-15(8-6-14)28-19(20,21)22/h3-10,12H,11H2,1-2H3,(H,23,24)(H,25,27) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of phsophorylated TrkA (unknown origin) assessed as inhibition of fluorescently-labelled substrate phosphorylation by CALIPER enzymatic as... |
J Med Chem 57: 5800-16 (2014)
Article DOI: 10.1021/jm5006429
BindingDB Entry DOI: 10.7270/Q2BV7J69 |
More data for this
Ligand-Target Pair | |
High affinity nerve growth factor receptor
(Homo sapiens (Human)) | BDBM50022670
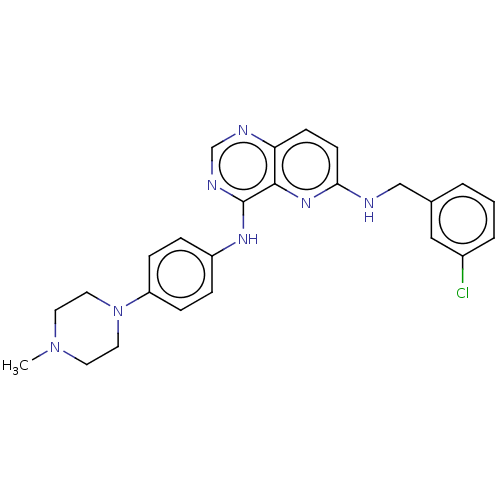
(CHEMBL3297748)Show SMILES CN1CCN(CC1)c1ccc(Nc2ncnc3ccc(NCc4cccc(Cl)c4)nc23)cc1 Show InChI InChI=1S/C25H26ClN7/c1-32-11-13-33(14-12-32)21-7-5-20(6-8-21)30-25-24-22(28-17-29-25)9-10-23(31-24)27-16-18-3-2-4-19(26)15-18/h2-10,15,17H,11-14,16H2,1H3,(H,27,31)(H,28,29,30) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of purified TrkA cytoplasmic domain (unknown origin) by HTRF assay |
J Med Chem 57: 5800-16 (2014)
Article DOI: 10.1021/jm5006429
BindingDB Entry DOI: 10.7270/Q2BV7J69 |
More data for this
Ligand-Target Pair | |
High affinity nerve growth factor receptor
(Homo sapiens (Human)) | BDBM50022671

(CHEMBL3298265)Show SMILES Cc1cn2cc(CNc3ncnc4ccc(nc34)N3CCC[C@@H]3c3cc(F)ccc3F)nc2s1 |r| Show InChI InChI=1S/C24H21F2N7S/c1-14-11-32-12-16(30-24(32)34-14)10-27-23-22-19(28-13-29-23)6-7-21(31-22)33-8-2-3-20(33)17-9-15(25)4-5-18(17)26/h4-7,9,11-13,20H,2-3,8,10H2,1H3,(H,27,28,29)/t20-/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of phsophorylated TrkA (unknown origin) assessed as inhibition of fluorescently-labelled substrate phosphorylation by CALIPER enzymatic as... |
J Med Chem 57: 5800-16 (2014)
Article DOI: 10.1021/jm5006429
BindingDB Entry DOI: 10.7270/Q2BV7J69 |
More data for this
Ligand-Target Pair | |
Beta-secretase 1
(Homo sapiens (Human)) | BDBM50360722
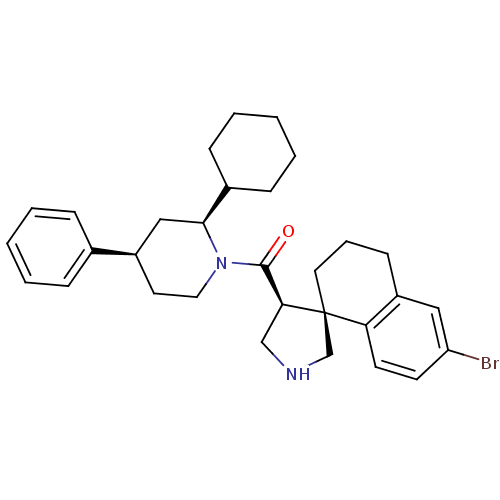
(CHEMBL1934294)Show SMILES Brc1ccc2c(CCC[C@]22CNC[C@H]2C(=O)N2CC[C@H](C[C@H]2C2CCCCC2)c2ccccc2)c1 |r| Show InChI InChI=1S/C31H39BrN2O/c32-26-13-14-27-25(18-26)12-7-16-31(27)21-33-20-28(31)30(35)34-17-15-24(22-8-3-1-4-9-22)19-29(34)23-10-5-2-6-11-23/h1,3-4,8-9,13-14,18,23-24,28-29,33H,2,5-7,10-12,15-17,19-21H2/t24-,28+,29+,31+/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 24 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of BACE1 in cell-free system |
Bioorg Med Chem Lett 22: 240-4 (2011)
Article DOI: 10.1016/j.bmcl.2011.11.024
BindingDB Entry DOI: 10.7270/Q2QR4XJK |
More data for this
Ligand-Target Pair | |
Beta-secretase 2
(Homo sapiens (Human)) | BDBM50360723

(CHEMBL1934295)Show SMILES Clc1ccc2c(CCC[C@]22CNC[C@H]2C(=O)N2CC[C@H](C[C@H]2C2CCCCC2)c2ccccc2)n1 |r| Show InChI InChI=1S/C30H38ClN3O/c31-28-14-13-24-26(33-28)12-7-16-30(24)20-32-19-25(30)29(35)34-17-15-23(21-8-3-1-4-9-21)18-27(34)22-10-5-2-6-11-22/h1,3-4,8-9,13-14,22-23,25,27,32H,2,5-7,10-12,15-20H2/t23-,25+,27+,30+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 29 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of BACE2 |
Bioorg Med Chem Lett 22: 240-4 (2011)
Article DOI: 10.1016/j.bmcl.2011.11.024
BindingDB Entry DOI: 10.7270/Q2QR4XJK |
More data for this
Ligand-Target Pair | |
Beta-secretase 1
(Homo sapiens (Human)) | BDBM50360723

(CHEMBL1934295)Show SMILES Clc1ccc2c(CCC[C@]22CNC[C@H]2C(=O)N2CC[C@H](C[C@H]2C2CCCCC2)c2ccccc2)n1 |r| Show InChI InChI=1S/C30H38ClN3O/c31-28-14-13-24-26(33-28)12-7-16-30(24)20-32-19-25(30)29(35)34-17-15-23(21-8-3-1-4-9-21)18-27(34)22-10-5-2-6-11-22/h1,3-4,8-9,13-14,22-23,25,27,32H,2,5-7,10-12,15-20H2/t23-,25+,27+,30+/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 29 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of BACE1 in cell-free system |
Bioorg Med Chem Lett 22: 240-4 (2011)
Article DOI: 10.1016/j.bmcl.2011.11.024
BindingDB Entry DOI: 10.7270/Q2QR4XJK |
More data for this
Ligand-Target Pair | |
High affinity nerve growth factor receptor
(Homo sapiens (Human)) | BDBM50022720
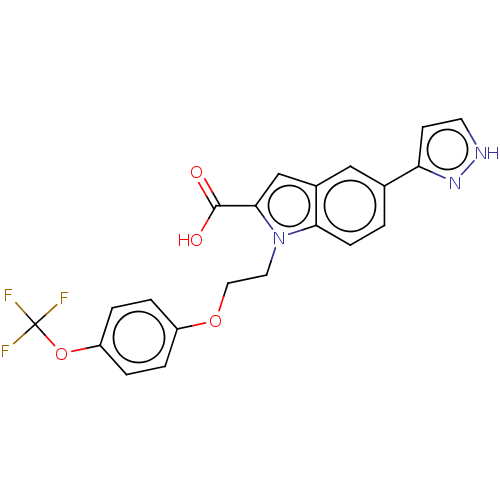
(CHEMBL3298161)Show SMILES OC(=O)c1cc2cc(ccc2n1CCOc1ccc(OC(F)(F)F)cc1)-c1cc[nH]n1 Show InChI InChI=1S/C21H16F3N3O4/c22-21(23,24)31-16-4-2-15(3-5-16)30-10-9-27-18-6-1-13(17-7-8-25-26-17)11-14(18)12-19(27)20(28)29/h1-8,11-12H,9-10H2,(H,25,26)(H,28,29) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 29 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human TrkA expressed in human U2OS cells assessed as inhibition of NGF-induced maximum response after 1 hr by beta-galactosidase assay |
J Med Chem 57: 5800-16 (2014)
Article DOI: 10.1021/jm5006429
BindingDB Entry DOI: 10.7270/Q2BV7J69 |
More data for this
Ligand-Target Pair | |
Beta-secretase 1
(Homo sapiens (Human)) | BDBM50360724
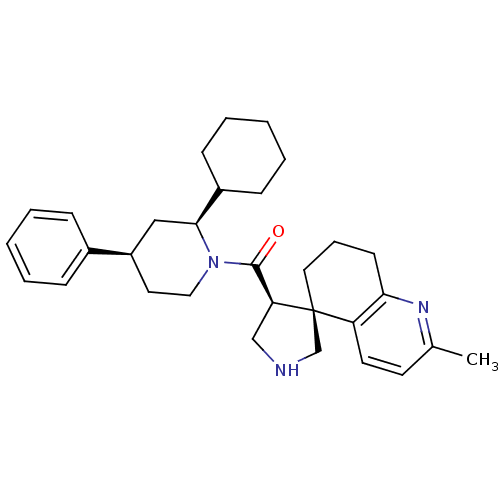
(CHEMBL1934296)Show SMILES Cc1ccc2c(CCC[C@]22CNC[C@H]2C(=O)N2CC[C@H](C[C@H]2C2CCCCC2)c2ccccc2)n1 |r| Show InChI InChI=1S/C31H41N3O/c1-22-14-15-26-28(33-22)13-8-17-31(26)21-32-20-27(31)30(35)34-18-16-25(23-9-4-2-5-10-23)19-29(34)24-11-6-3-7-12-24/h2,4-5,9-10,14-15,24-25,27,29,32H,3,6-8,11-13,16-21H2,1H3/t25-,27+,29+,31+/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 36 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of BACE1 in cell-free system |
Bioorg Med Chem Lett 22: 240-4 (2011)
Article DOI: 10.1016/j.bmcl.2011.11.024
BindingDB Entry DOI: 10.7270/Q2QR4XJK |
More data for this
Ligand-Target Pair | |
High affinity nerve growth factor receptor
(Homo sapiens (Human)) | BDBM50022672
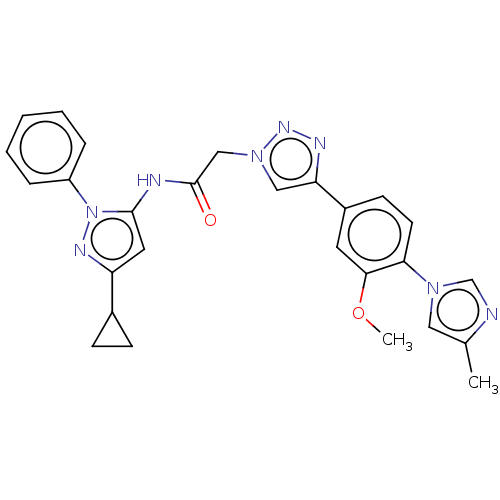
(CHEMBL3298266)Show SMILES COc1cc(ccc1-n1cnc(C)c1)-c1cn(CC(=O)Nc2cc(nn2-c2ccccc2)C2CC2)nn1 Show InChI InChI=1S/C27H26N8O2/c1-18-14-33(17-28-18)24-11-10-20(12-25(24)37-2)23-15-34(32-30-23)16-27(36)29-26-13-22(19-8-9-19)31-35(26)21-6-4-3-5-7-21/h3-7,10-15,17,19H,8-9,16H2,1-2H3,(H,29,36) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 47 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human TrkA expressed in human U2OS cells assessed as inhibition of NGF-induced maximum response after 1 hr by beta-galactosidase assay |
J Med Chem 57: 5800-16 (2014)
Article DOI: 10.1021/jm5006429
BindingDB Entry DOI: 10.7270/Q2BV7J69 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
High affinity nerve growth factor receptor
(Homo sapiens (Human)) | BDBM50022720

(CHEMBL3298161)Show SMILES OC(=O)c1cc2cc(ccc2n1CCOc1ccc(OC(F)(F)F)cc1)-c1cc[nH]n1 Show InChI InChI=1S/C21H16F3N3O4/c22-21(23,24)31-16-4-2-15(3-5-16)30-10-9-27-18-6-1-13(17-7-8-25-26-17)11-14(18)12-19(27)20(28)29/h1-8,11-12H,9-10H2,(H,25,26)(H,28,29) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 51 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of phsophorylated TrkA (unknown origin) assessed as inhibition of fluorescently-labelled substrate phosphorylation by CALIPER enzymatic as... |
J Med Chem 57: 5800-16 (2014)
Article DOI: 10.1021/jm5006429
BindingDB Entry DOI: 10.7270/Q2BV7J69 |
More data for this
Ligand-Target Pair | |
High affinity nerve growth factor receptor
(Homo sapiens (Human)) | BDBM50022677
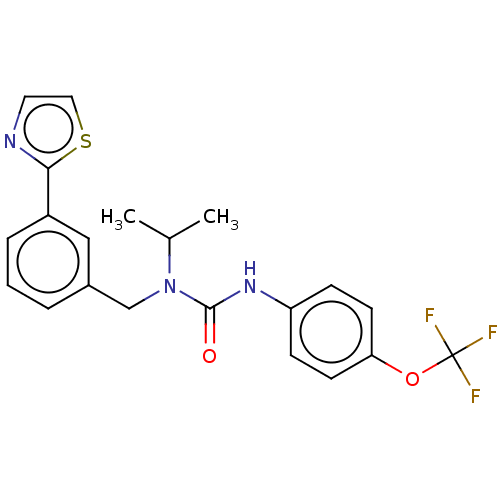
(CHEMBL3298270 | US9181261, 17)Show SMILES CC(C)N(Cc1cccc(c1)-c1nccs1)C(=O)Nc1ccc(OC(F)(F)F)cc1 Show InChI InChI=1S/C21H20F3N3O2S/c1-14(2)27(13-15-4-3-5-16(12-15)19-25-10-11-30-19)20(28)26-17-6-8-18(9-7-17)29-21(22,23)24/h3-12,14H,13H2,1-2H3,(H,26,28) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 56 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human TrkA expressed in human U2OS cells assessed as inhibition of NGF-induced maximum response after 1 hr by beta-galactosidase assay |
J Med Chem 57: 5800-16 (2014)
Article DOI: 10.1021/jm5006429
BindingDB Entry DOI: 10.7270/Q2BV7J69 |
More data for this
Ligand-Target Pair | |
High affinity nerve growth factor receptor
(Homo sapiens (Human)) | BDBM50022679
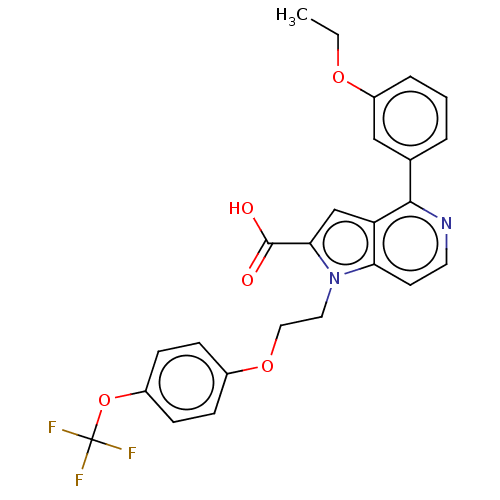
(CHEMBL3298365)Show SMILES CCOc1cccc(c1)-c1nccc2n(CCOc3ccc(OC(F)(F)F)cc3)c(cc12)C(O)=O Show InChI InChI=1S/C25H21F3N2O5/c1-2-33-19-5-3-4-16(14-19)23-20-15-22(24(31)32)30(21(20)10-11-29-23)12-13-34-17-6-8-18(9-7-17)35-25(26,27)28/h3-11,14-15H,2,12-13H2,1H3,(H,31,32) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 59 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human TrkA expressed in human U2OS cells assessed as inhibition of NGF-induced maximum response after 1 hr by beta-galactosidase assay |
J Med Chem 57: 5800-16 (2014)
Article DOI: 10.1021/jm5006429
BindingDB Entry DOI: 10.7270/Q2BV7J69 |
More data for this
Ligand-Target Pair | |
High affinity nerve growth factor receptor
(Homo sapiens (Human)) | BDBM50022674

(CHEMBL3298267)Show SMILES Cc1cn(cn1)-c1ccc(cc1)-c1cn(CC(=O)Nc2cc(nn2-c2ccccc2)C2CC2)nn1 Show InChI InChI=1S/C26H24N8O/c1-18-14-32(17-27-18)21-11-9-20(10-12-21)24-15-33(31-29-24)16-26(35)28-25-13-23(19-7-8-19)30-34(25)22-5-3-2-4-6-22/h2-6,9-15,17,19H,7-8,16H2,1H3,(H,28,35) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 60 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of purified TrkA cytoplasmic domain (unknown origin) by HTRF assay |
J Med Chem 57: 5800-16 (2014)
Article DOI: 10.1021/jm5006429
BindingDB Entry DOI: 10.7270/Q2BV7J69 |
More data for this
Ligand-Target Pair | |
Beta-secretase 1
(Homo sapiens (Human)) | BDBM50360721
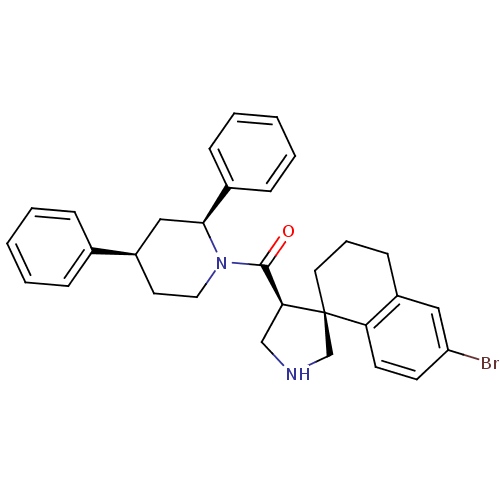
(CHEMBL1934293)Show SMILES Brc1ccc2c(CCC[C@]22CNC[C@H]2C(=O)N2CC[C@H](C[C@H]2c2ccccc2)c2ccccc2)c1 |r| Show InChI InChI=1S/C31H33BrN2O/c32-26-13-14-27-25(18-26)12-7-16-31(27)21-33-20-28(31)30(35)34-17-15-24(22-8-3-1-4-9-22)19-29(34)23-10-5-2-6-11-23/h1-6,8-11,13-14,18,24,28-29,33H,7,12,15-17,19-21H2/t24-,28+,29+,31+/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 70 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of BACE1 in cell-free system |
Bioorg Med Chem Lett 22: 240-4 (2011)
Article DOI: 10.1016/j.bmcl.2011.11.024
BindingDB Entry DOI: 10.7270/Q2QR4XJK |
More data for this
Ligand-Target Pair | |
Beta-secretase 1
(Homo sapiens (Human)) | BDBM50360724

(CHEMBL1934296)Show SMILES Cc1ccc2c(CCC[C@]22CNC[C@H]2C(=O)N2CC[C@H](C[C@H]2C2CCCCC2)c2ccccc2)n1 |r| Show InChI InChI=1S/C31H41N3O/c1-22-14-15-26-28(33-22)13-8-17-31(26)21-32-20-27(31)30(35)34-18-16-25(23-9-4-2-5-10-23)19-29(34)24-11-6-3-7-12-24/h2,4-5,9-10,14-15,24-25,27,29,32H,3,6-8,11-13,16-21H2,1H3/t25-,27+,29+,31+/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human BACE1 expressed in mouse fibroblast cells assessed as inhibition of soluble APPbeta_NF cleavage |
Bioorg Med Chem Lett 22: 240-4 (2011)
Article DOI: 10.1016/j.bmcl.2011.11.024
BindingDB Entry DOI: 10.7270/Q2QR4XJK |
More data for this
Ligand-Target Pair | |
High affinity nerve growth factor receptor
(Homo sapiens (Human)) | BDBM50022757
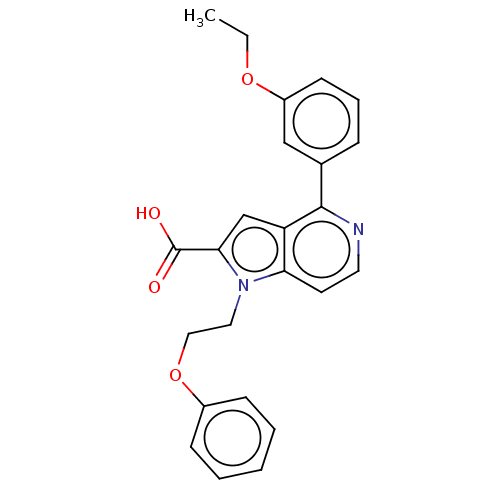
(CHEMBL3298163)Show SMILES CCOc1cccc(c1)-c1nccc2n(CCOc3ccccc3)c(cc12)C(O)=O Show InChI InChI=1S/C24H22N2O4/c1-2-29-19-10-6-7-17(15-19)23-20-16-22(24(27)28)26(21(20)11-12-25-23)13-14-30-18-8-4-3-5-9-18/h3-12,15-16H,2,13-14H2,1H3,(H,27,28) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 109 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human TrkA expressed in human U2OS cells assessed as inhibition of NGF-induced maximum response after 1 hr by beta-galactosidase assay |
J Med Chem 57: 5800-16 (2014)
Article DOI: 10.1021/jm5006429
BindingDB Entry DOI: 10.7270/Q2BV7J69 |
More data for this
Ligand-Target Pair | |
Beta-secretase 1
(Homo sapiens (Human)) | BDBM25363
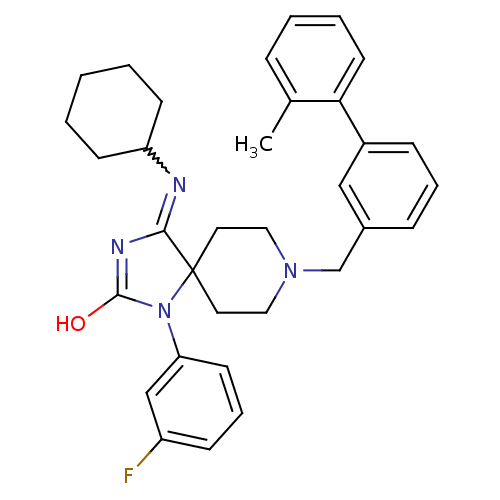
(4-(cyclohexylamino)-1-(3-fluorophenyl)-8-{[3-(2-me...)Show SMILES Cc1ccccc1-c1cccc(CN2CCC3(CC2)N(C(O)=NC3=NC2CCCCC2)c2cccc(F)c2)c1 |w:24.27,c:23| Show InChI InChI=1S/C33H37FN4O/c1-24-9-5-6-16-30(24)26-11-7-10-25(21-26)23-37-19-17-33(18-20-37)31(35-28-13-3-2-4-14-28)36-32(39)38(33)29-15-8-12-27(34)22-29/h5-12,15-16,21-22,28H,2-4,13-14,17-20,23H2,1H3,(H,35,36,39) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 110 | n/a | n/a | n/a | n/a | 4.5 | 22 |
Merck Research Laboratories
| Assay Description
The assay was performed using a 96-well format on a HPLC equipped with four 96-well plate holders. After the reaction was terminated, the solution wa... |
J Med Chem 51: 6259-62 (2008)
Article DOI: 10.1021/jm800914n
BindingDB Entry DOI: 10.7270/Q2KS6PVK |
More data for this
Ligand-Target Pair | |
High affinity nerve growth factor receptor
(Homo sapiens (Human)) | BDBM50022681
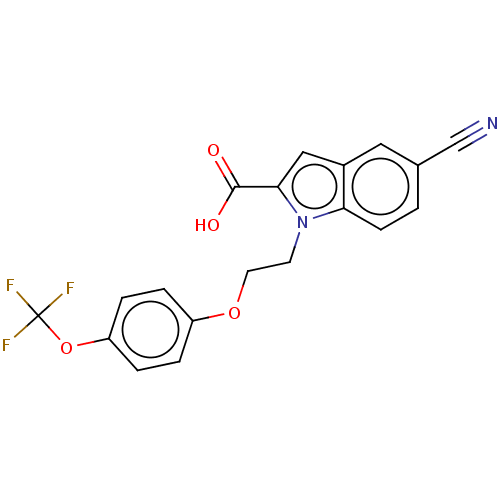
(CHEMBL3298535)Show SMILES OC(=O)c1cc2cc(ccc2n1CCOc1ccc(OC(F)(F)F)cc1)C#N Show InChI InChI=1S/C19H13F3N2O4/c20-19(21,22)28-15-4-2-14(3-5-15)27-8-7-24-16-6-1-12(11-23)9-13(16)10-17(24)18(25)26/h1-6,9-10H,7-8H2,(H,25,26) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 118 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of phsophorylated TrkA (unknown origin) assessed as inhibition of fluorescently-labelled substrate phosphorylation by CALIPER enzymatic as... |
J Med Chem 57: 5800-16 (2014)
Article DOI: 10.1021/jm5006429
BindingDB Entry DOI: 10.7270/Q2BV7J69 |
More data for this
Ligand-Target Pair | |
High affinity nerve growth factor receptor
(Homo sapiens (Human)) | BDBM50022677

(CHEMBL3298270 | US9181261, 17)Show SMILES CC(C)N(Cc1cccc(c1)-c1nccs1)C(=O)Nc1ccc(OC(F)(F)F)cc1 Show InChI InChI=1S/C21H20F3N3O2S/c1-14(2)27(13-15-4-3-5-16(12-15)19-25-10-11-30-19)20(28)26-17-6-8-18(9-7-17)29-21(22,23)24/h3-12,14H,13H2,1-2H3,(H,26,28) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 132 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of phsophorylated TrkA (unknown origin) assessed as inhibition of fluorescently-labelled substrate phosphorylation by CALIPER enzymatic as... |
J Med Chem 57: 5800-16 (2014)
Article DOI: 10.1021/jm5006429
BindingDB Entry DOI: 10.7270/Q2BV7J69 |
More data for this
Ligand-Target Pair | |
Beta-secretase 1
(Homo sapiens (Human)) | BDBM50360720
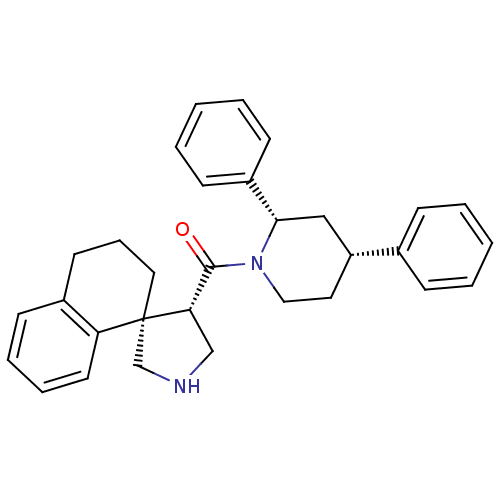
(CHEMBL1934292)Show SMILES O=C([C@@H]1CNC[C@]11CCCc2ccccc12)N1CC[C@H](C[C@H]1c1ccccc1)c1ccccc1 |r| Show InChI InChI=1S/C31H34N2O/c34-30(28-21-32-22-31(28)18-9-15-24-12-7-8-16-27(24)31)33-19-17-26(23-10-3-1-4-11-23)20-29(33)25-13-5-2-6-14-25/h1-8,10-14,16,26,28-29,32H,9,15,17-22H2/t26-,28+,29+,31+/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 150 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of BACE1 in cell-free system |
Bioorg Med Chem Lett 22: 240-4 (2011)
Article DOI: 10.1016/j.bmcl.2011.11.024
BindingDB Entry DOI: 10.7270/Q2QR4XJK |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
High affinity nerve growth factor receptor
(Homo sapiens (Human)) | BDBM50022681

(CHEMBL3298535)Show SMILES OC(=O)c1cc2cc(ccc2n1CCOc1ccc(OC(F)(F)F)cc1)C#N Show InChI InChI=1S/C19H13F3N2O4/c20-19(21,22)28-15-4-2-14(3-5-15)27-8-7-24-16-6-1-12(11-23)9-13(16)10-17(24)18(25)26/h1-6,9-10H,7-8H2,(H,25,26) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 150 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human TrkA expressed in human U2OS cells assessed as inhibition of NGF-induced maximum response after 1 hr by beta-galactosidase assay |
J Med Chem 57: 5800-16 (2014)
Article DOI: 10.1021/jm5006429
BindingDB Entry DOI: 10.7270/Q2BV7J69 |
More data for this
Ligand-Target Pair | |
High affinity nerve growth factor receptor
(Homo sapiens (Human)) | BDBM50022756
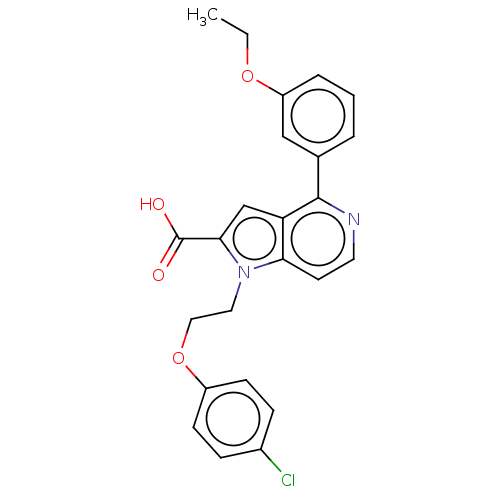
(CHEMBL3298164)Show SMILES CCOc1cccc(c1)-c1nccc2n(CCOc3ccc(Cl)cc3)c(cc12)C(O)=O Show InChI InChI=1S/C24H21ClN2O4/c1-2-30-19-5-3-4-16(14-19)23-20-15-22(24(28)29)27(21(20)10-11-26-23)12-13-31-18-8-6-17(25)7-9-18/h3-11,14-15H,2,12-13H2,1H3,(H,28,29) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human TrkA expressed in human U2OS cells assessed as inhibition of NGF-induced maximum response after 1 hr by beta-galactosidase assay |
J Med Chem 57: 5800-16 (2014)
Article DOI: 10.1021/jm5006429
BindingDB Entry DOI: 10.7270/Q2BV7J69 |
More data for this
Ligand-Target Pair | |
High affinity nerve growth factor receptor
(Homo sapiens (Human)) | BDBM50022679

(CHEMBL3298365)Show SMILES CCOc1cccc(c1)-c1nccc2n(CCOc3ccc(OC(F)(F)F)cc3)c(cc12)C(O)=O Show InChI InChI=1S/C25H21F3N2O5/c1-2-33-19-5-3-4-16(14-19)23-20-15-22(24(31)32)30(21(20)10-11-29-23)12-13-34-17-6-8-18(9-7-17)35-25(26,27)28/h3-11,14-15H,2,12-13H2,1H3,(H,31,32) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 348 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of phsophorylated TrkA (unknown origin) assessed as inhibition of fluorescently-labelled substrate phosphorylation by CALIPER enzymatic as... |
J Med Chem 57: 5800-16 (2014)
Article DOI: 10.1021/jm5006429
BindingDB Entry DOI: 10.7270/Q2BV7J69 |
More data for this
Ligand-Target Pair | |
Beta-secretase 1
(Homo sapiens (Human)) | BDBM50258280
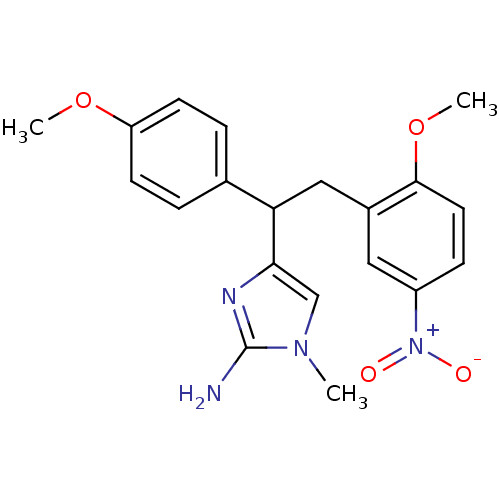
(4-(2-(2-methoxy-5-nitrophenyl)-1-(4-methoxyphenyl)...)Show SMILES COc1ccc(cc1)C(Cc1cc(ccc1OC)[N+]([O-])=O)c1cn(C)c(N)n1 Show InChI InChI=1S/C20H22N4O4/c1-23-12-18(22-20(23)21)17(13-4-7-16(27-2)8-5-13)11-14-10-15(24(25)26)6-9-19(14)28-3/h4-10,12,17H,11H2,1-3H3,(H2,21,22) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 470 | n/a | n/a | n/a | n/a | 6.5 | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of BACE1 (unknown origin) at pH 6.5 |
Bioorg Med Chem Lett 19: 2977-80 (2009)
Article DOI: 10.1016/j.bmcl.2009.04.033
BindingDB Entry DOI: 10.7270/Q2XS5V96 |
More data for this
Ligand-Target Pair | |
High affinity nerve growth factor receptor
(Homo sapiens (Human)) | BDBM50022676
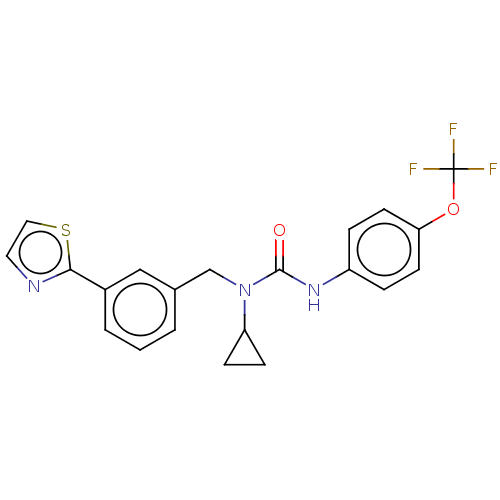
(CHEMBL3298269)Show SMILES FC(F)(F)Oc1ccc(NC(=O)N(Cc2cccc(c2)-c2nccs2)C2CC2)cc1 Show InChI InChI=1S/C21H18F3N3O2S/c22-21(23,24)29-18-8-4-16(5-9-18)26-20(28)27(17-6-7-17)13-14-2-1-3-15(12-14)19-25-10-11-30-19/h1-5,8-12,17H,6-7,13H2,(H,26,28) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 549 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human TrkA expressed in human U2OS cells assessed as inhibition of NGF-induced maximum response after 1 hr by beta-galactosidase assay |
J Med Chem 57: 5800-16 (2014)
Article DOI: 10.1021/jm5006429
BindingDB Entry DOI: 10.7270/Q2BV7J69 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Beta-secretase 1
(Homo sapiens (Human)) | BDBM50360723

(CHEMBL1934295)Show SMILES Clc1ccc2c(CCC[C@]22CNC[C@H]2C(=O)N2CC[C@H](C[C@H]2C2CCCCC2)c2ccccc2)n1 |r| Show InChI InChI=1S/C30H38ClN3O/c31-28-14-13-24-26(33-28)12-7-16-30(24)20-32-19-25(30)29(35)34-17-15-23(21-8-3-1-4-9-21)18-27(34)22-10-5-2-6-11-22/h1,3-4,8-9,13-14,22-23,25,27,32H,2,5-7,10-12,15-20H2/t23-,25+,27+,30+/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 570 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human BACE1 expressed in mouse fibroblast cells assessed as inhibition of soluble APPbeta_NF cleavage |
Bioorg Med Chem Lett 22: 240-4 (2011)
Article DOI: 10.1016/j.bmcl.2011.11.024
BindingDB Entry DOI: 10.7270/Q2QR4XJK |
More data for this
Ligand-Target Pair | |
Beta-secretase 1
(Homo sapiens (Human)) | BDBM50360717
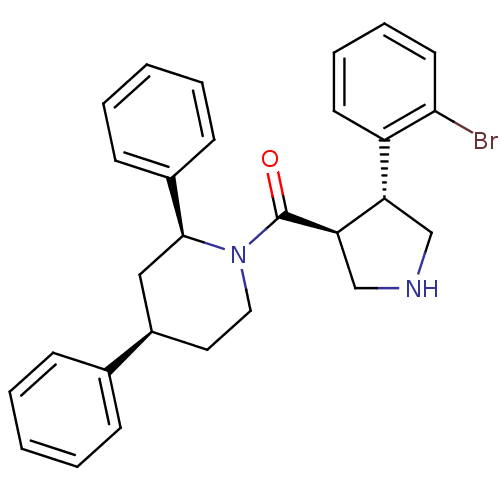
(CHEMBL1934289)Show SMILES Brc1ccccc1[C@@H]1CNC[C@H]1C(=O)N1CC[C@H](C[C@H]1c1ccccc1)c1ccccc1 |r| Show InChI InChI=1S/C28H29BrN2O/c29-26-14-8-7-13-23(26)24-18-30-19-25(24)28(32)31-16-15-22(20-9-3-1-4-10-20)17-27(31)21-11-5-2-6-12-21/h1-14,22,24-25,27,30H,15-19H2/t22-,24+,25-,27+/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 600 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of BACE1 in cell-free system |
Bioorg Med Chem Lett 22: 240-4 (2011)
Article DOI: 10.1016/j.bmcl.2011.11.024
BindingDB Entry DOI: 10.7270/Q2QR4XJK |
More data for this
Ligand-Target Pair | |
High affinity nerve growth factor receptor
(Homo sapiens (Human)) | BDBM50022756

(CHEMBL3298164)Show SMILES CCOc1cccc(c1)-c1nccc2n(CCOc3ccc(Cl)cc3)c(cc12)C(O)=O Show InChI InChI=1S/C24H21ClN2O4/c1-2-30-19-5-3-4-16(14-19)23-20-15-22(24(28)29)27(21(20)10-11-26-23)12-13-31-18-8-6-17(25)7-9-18/h3-11,14-15H,2,12-13H2,1H3,(H,28,29) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 653 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of phsophorylated TrkA (unknown origin) assessed as inhibition of fluorescently-labelled substrate phosphorylation by CALIPER enzymatic as... |
J Med Chem 57: 5800-16 (2014)
Article DOI: 10.1021/jm5006429
BindingDB Entry DOI: 10.7270/Q2BV7J69 |
More data for this
Ligand-Target Pair | |
High affinity nerve growth factor receptor
(Homo sapiens (Human)) | BDBM50022672

(CHEMBL3298266)Show SMILES COc1cc(ccc1-n1cnc(C)c1)-c1cn(CC(=O)Nc2cc(nn2-c2ccccc2)C2CC2)nn1 Show InChI InChI=1S/C27H26N8O2/c1-18-14-33(17-28-18)24-11-10-20(12-25(24)37-2)23-15-34(32-30-23)16-27(36)29-26-13-22(19-8-9-19)31-35(26)21-6-4-3-5-7-21/h3-7,10-15,17,19H,8-9,16H2,1-2H3,(H,29,36) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 662 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of purified TrkA cytoplasmic domain (unknown origin) by HTRF assay |
J Med Chem 57: 5800-16 (2014)
Article DOI: 10.1021/jm5006429
BindingDB Entry DOI: 10.7270/Q2BV7J69 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
High affinity nerve growth factor receptor
(Homo sapiens (Human)) | BDBM50022669

(CHEMBL3298264)Show SMILES C(Nc1ccc2ncnc(Nc3ccc(cc3)N3CCOCC3)c2n1)c1cccnc1 Show InChI InChI=1S/C23H23N7O/c1-2-17(14-24-9-1)15-25-21-8-7-20-22(29-21)23(27-16-26-20)28-18-3-5-19(6-4-18)30-10-12-31-13-11-30/h1-9,14,16H,10-13,15H2,(H,25,29)(H,26,27,28) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 997 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of purified TrkA cytoplasmic domain (unknown origin) by HTRF assay |
J Med Chem 57: 5800-16 (2014)
Article DOI: 10.1021/jm5006429
BindingDB Entry DOI: 10.7270/Q2BV7J69 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Beta-secretase 1
(Homo sapiens (Human)) | BDBM50258849
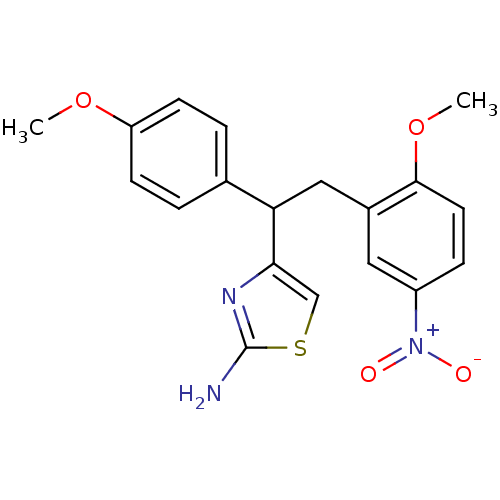
(4-(2-(2-methoxy-5-nitrophenyl)-1-(4-methoxyphenyl)...)Show SMILES COc1ccc(cc1)C(Cc1cc(ccc1OC)[N+]([O-])=O)c1csc(N)n1 Show InChI InChI=1S/C19H19N3O4S/c1-25-15-6-3-12(4-7-15)16(17-11-27-19(20)21-17)10-13-9-14(22(23)24)5-8-18(13)26-2/h3-9,11,16H,10H2,1-2H3,(H2,20,21) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.10E+3 | n/a | n/a | n/a | n/a | 4.5 | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of BACE1 (unknown origin) at pH 4.5 |
Bioorg Med Chem Lett 19: 2977-80 (2009)
Article DOI: 10.1016/j.bmcl.2009.04.033
BindingDB Entry DOI: 10.7270/Q2XS5V96 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase Chk1
(Homo sapiens (Human)) | BDBM50277001

(3-(3-chlorophenyl)-2-((R)-1-oxo-1-((S)-2,8-diazasp...)Show SMILES CCCC[C@@H](Sc1nc2ccccc2c(=O)n1-c1cccc(Cl)c1)C(=O)N1CCC[C@]2(CCCNC2)C1 |r| Show InChI InChI=1S/C29H35ClN4O2S/c1-2-3-13-25(27(36)33-17-8-15-29(20-33)14-7-16-31-19-29)37-28-32-24-12-5-4-11-23(24)26(35)34(28)22-10-6-9-21(30)18-22/h4-6,9-12,18,25,31H,2-3,7-8,13-17,19-20H2,1H3/t25-,29+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of C-terminal 6-His tagged human Chk1 by HTRF assay in presence of 0.1 mM ATP |
Bioorg Med Chem Lett 19: 1240-4 (2009)
Article DOI: 10.1016/j.bmcl.2008.12.076
BindingDB Entry DOI: 10.7270/Q2S75G53 |
More data for this
Ligand-Target Pair | |
High affinity nerve growth factor receptor
(Homo sapiens (Human)) | BDBM50022670

(CHEMBL3297748)Show SMILES CN1CCN(CC1)c1ccc(Nc2ncnc3ccc(NCc4cccc(Cl)c4)nc23)cc1 Show InChI InChI=1S/C25H26ClN7/c1-32-11-13-33(14-12-32)21-7-5-20(6-8-21)30-25-24-22(28-17-29-25)9-10-23(31-24)27-16-18-3-2-4-19(26)15-18/h2-10,15,17H,11-14,16H2,1H3,(H,27,31)(H,28,29,30) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.69E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of phsophorylated TrkA (unknown origin) assessed as inhibition of fluorescently-labelled substrate phosphorylation by CALIPER enzymatic as... |
J Med Chem 57: 5800-16 (2014)
Article DOI: 10.1021/jm5006429
BindingDB Entry DOI: 10.7270/Q2BV7J69 |
More data for this
Ligand-Target Pair | |
Beta-secretase 1
(Homo sapiens (Human)) | BDBM50360722

(CHEMBL1934294)Show SMILES Brc1ccc2c(CCC[C@]22CNC[C@H]2C(=O)N2CC[C@H](C[C@H]2C2CCCCC2)c2ccccc2)c1 |r| Show InChI InChI=1S/C31H39BrN2O/c32-26-13-14-27-25(18-26)12-7-16-31(27)21-33-20-28(31)30(35)34-17-15-24(22-8-3-1-4-9-22)19-29(34)23-10-5-2-6-11-23/h1,3-4,8-9,13-14,18,23-24,28-29,33H,2,5-7,10-12,15-17,19-21H2/t24-,28+,29+,31+/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human BACE1 expressed in mouse fibroblast cells assessed as inhibition of soluble APPbeta_NF cleavage |
Bioorg Med Chem Lett 22: 240-4 (2011)
Article DOI: 10.1016/j.bmcl.2011.11.024
BindingDB Entry DOI: 10.7270/Q2QR4XJK |
More data for this
Ligand-Target Pair | |
Beta-secretase 1
(Homo sapiens (Human)) | BDBM50258280

(4-(2-(2-methoxy-5-nitrophenyl)-1-(4-methoxyphenyl)...)Show SMILES COc1ccc(cc1)C(Cc1cc(ccc1OC)[N+]([O-])=O)c1cn(C)c(N)n1 Show InChI InChI=1S/C20H22N4O4/c1-23-12-18(22-20(23)21)17(13-4-7-16(27-2)8-5-13)11-14-10-15(24(25)26)6-9-19(14)28-3/h4-10,12,17H,11H2,1-3H3,(H2,21,22) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of BACE1 (unknown origin) by sAPP_NF cell based assay |
Bioorg Med Chem Lett 19: 2977-80 (2009)
Article DOI: 10.1016/j.bmcl.2009.04.033
BindingDB Entry DOI: 10.7270/Q2XS5V96 |
More data for this
Ligand-Target Pair | |
High affinity nerve growth factor receptor
(Homo sapiens (Human)) | BDBM50022757

(CHEMBL3298163)Show SMILES CCOc1cccc(c1)-c1nccc2n(CCOc3ccccc3)c(cc12)C(O)=O Show InChI InChI=1S/C24H22N2O4/c1-2-29-19-10-6-7-17(15-19)23-20-16-22(24(27)28)26(21(20)11-12-25-23)13-14-30-18-8-4-3-5-9-18/h3-12,15-16H,2,13-14H2,1H3,(H,27,28) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.07E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of phsophorylated TrkA (unknown origin) assessed as inhibition of fluorescently-labelled substrate phosphorylation by CALIPER enzymatic as... |
J Med Chem 57: 5800-16 (2014)
Article DOI: 10.1021/jm5006429
BindingDB Entry DOI: 10.7270/Q2BV7J69 |
More data for this
Ligand-Target Pair | |
High affinity nerve growth factor receptor
(Homo sapiens (Human)) | BDBM50022755
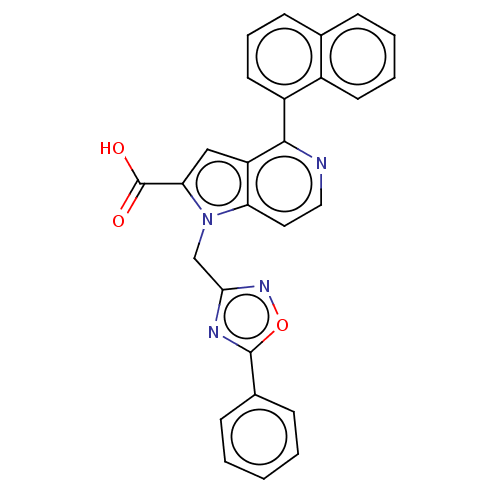
(CHEMBL3298162)Show SMILES OC(=O)c1cc2c(nccc2n1Cc1noc(n1)-c1ccccc1)-c1cccc2ccccc12 Show InChI InChI=1S/C27H18N4O3/c32-27(33)23-15-21-22(31(23)16-24-29-26(34-30-24)18-8-2-1-3-9-18)13-14-28-25(21)20-12-6-10-17-7-4-5-11-19(17)20/h1-15H,16H2,(H,32,33) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 2.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of non-phosphorylated TrkA (unknown origin) assessed as inhibition of fluorescently-labelled substrate phosphorylation by CALIPER enzymati... |
J Med Chem 57: 5800-16 (2014)
Article DOI: 10.1021/jm5006429
BindingDB Entry DOI: 10.7270/Q2BV7J69 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Beta-secretase 1
(Homo sapiens (Human)) | BDBM25361
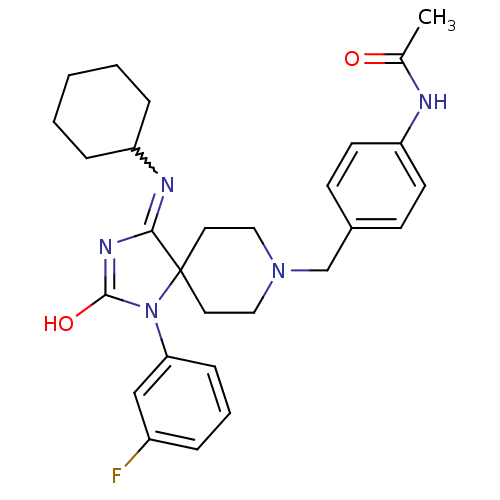
(N-(4-{[4-(cyclohexylamino)-1-(3-fluorophenyl)-2-ox...)Show SMILES CC(=O)Nc1ccc(CN2CCC3(CC2)N(C(O)=NC3=NC2CCCCC2)c2cccc(F)c2)cc1 |w:20.22,c:18| Show InChI InChI=1S/C28H34FN5O2/c1-20(35)30-24-12-10-21(11-13-24)19-33-16-14-28(15-17-33)26(31-23-7-3-2-4-8-23)32-27(36)34(28)25-9-5-6-22(29)18-25/h5-6,9-13,18,23H,2-4,7-8,14-17,19H2,1H3,(H,30,35)(H,31,32,36) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 2.80E+3 | n/a | n/a | n/a | n/a | 4.5 | 22 |
Merck Research Laboratories
| Assay Description
The assay was performed using a 96-well format on a HPLC equipped with four 96-well plate holders. After the reaction was terminated, the solution wa... |
J Med Chem 51: 6259-62 (2008)
Article DOI: 10.1021/jm800914n
BindingDB Entry DOI: 10.7270/Q2KS6PVK |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Serine/threonine-protein kinase Chk1
(Homo sapiens (Human)) | BDBM50276996

(1-(2-(3-(3-chlorophenyl)-4-oxo-3,4-dihydroquinazol...)Show SMILES CC(C)CC(Sc1nc2ccccc2c(=O)n1-c1cccc(Cl)c1)C(=O)N1CCC(CC1)C(N)=O Show InChI InChI=1S/C26H29ClN4O3S/c1-16(2)14-22(25(34)30-12-10-17(11-13-30)23(28)32)35-26-29-21-9-4-3-8-20(21)24(33)31(26)19-7-5-6-18(27)15-19/h3-9,15-17,22H,10-14H2,1-2H3,(H2,28,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of C-terminal 6-His tagged human Chk1 by HTRF assay in presence of 0.1 mM ATP |
Bioorg Med Chem Lett 19: 1240-4 (2009)
Article DOI: 10.1016/j.bmcl.2008.12.076
BindingDB Entry DOI: 10.7270/Q2S75G53 |
More data for this
Ligand-Target Pair | |
Beta-secretase 1
(Homo sapiens (Human)) | BDBM50258279

(4-(1-(3-fluoro-4-methoxyphenyl)-2-(2-methoxy-5-nit...)Show SMILES COc1ccc(cc1F)C(Cc1cc(ccc1OC)[N+]([O-])=O)c1cnc(N)[nH]1 Show InChI InChI=1S/C19H19FN4O4/c1-27-17-6-4-13(24(25)26)7-12(17)8-14(16-10-22-19(21)23-16)11-3-5-18(28-2)15(20)9-11/h3-7,9-10,14H,8H2,1-2H3,(H3,21,22,23) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3.20E+3 | n/a | n/a | n/a | n/a | 6.4 | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of BACE1 at pH 6.4 |
Bioorg Med Chem Lett 19: 2977-80 (2009)
Article DOI: 10.1016/j.bmcl.2009.04.033
BindingDB Entry DOI: 10.7270/Q2XS5V96 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase Chk1
(Homo sapiens (Human)) | BDBM50277026
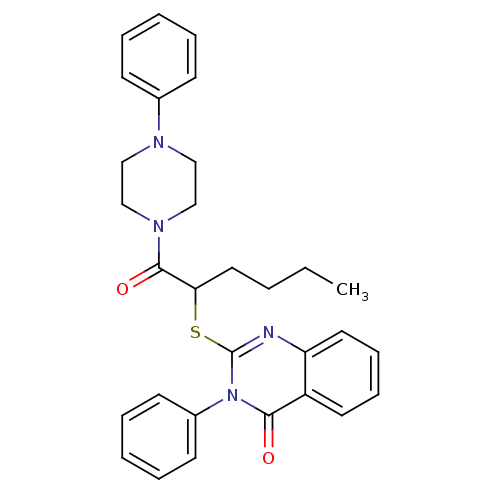
(2-(1-oxo-1-(4-phenylpiperazin-1-yl)hexan-2-ylthio)...)Show SMILES CCCCC(Sc1nc2ccccc2c(=O)n1-c1ccccc1)C(=O)N1CCN(CC1)c1ccccc1 Show InChI InChI=1S/C30H32N4O2S/c1-2-3-18-27(29(36)33-21-19-32(20-22-33)23-12-6-4-7-13-23)37-30-31-26-17-11-10-16-25(26)28(35)34(30)24-14-8-5-9-15-24/h4-17,27H,2-3,18-22H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of C-terminal 6-His tagged human Chk1 by HTRF assay in presence of 0.1 mM ATP |
Bioorg Med Chem Lett 19: 1240-4 (2009)
Article DOI: 10.1016/j.bmcl.2008.12.076
BindingDB Entry DOI: 10.7270/Q2S75G53 |
More data for this
Ligand-Target Pair | |
Beta-secretase 1
(Homo sapiens (Human)) | BDBM50258278
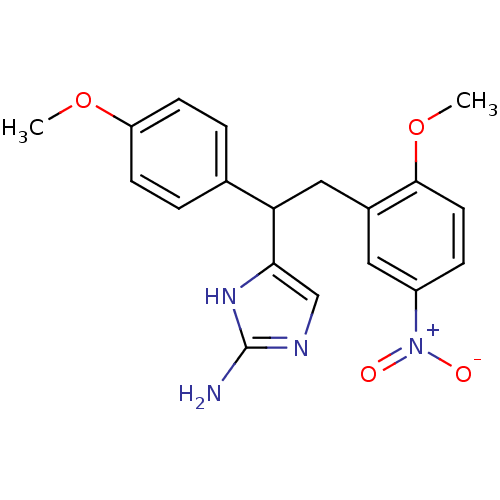
(4-(2-(2-methoxy-5-nitrophenyl)-1-(4-methoxyphenyl)...)Show SMILES COc1ccc(cc1)C(Cc1cc(ccc1OC)[N+]([O-])=O)c1cnc(N)[nH]1 Show InChI InChI=1S/C19H20N4O4/c1-26-15-6-3-12(4-7-15)16(17-11-21-19(20)22-17)10-13-9-14(23(24)25)5-8-18(13)27-2/h3-9,11,16H,10H2,1-2H3,(H3,20,21,22) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3.40E+3 | n/a | n/a | n/a | n/a | 6.5 | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of BACE1 (unknown origin) at pH 6.5 |
Bioorg Med Chem Lett 19: 2977-80 (2009)
Article DOI: 10.1016/j.bmcl.2009.04.033
BindingDB Entry DOI: 10.7270/Q2XS5V96 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase Chk1
(Homo sapiens (Human)) | BDBM50277028
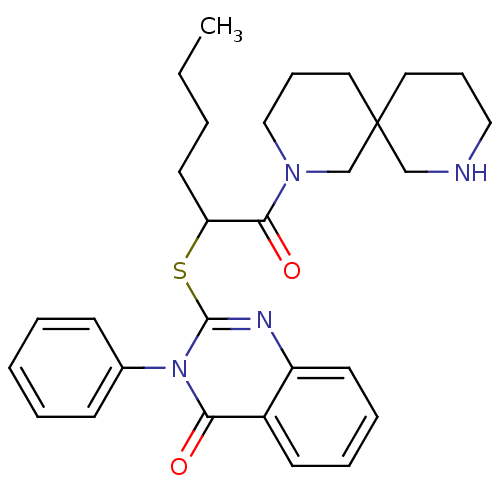
(2-[1-(2,8-Diaza-spiro[5.5]undecane-2-carbonyl)-pen...)Show SMILES CCCCC(Sc1nc2ccccc2c(=O)n1-c1ccccc1)C(=O)N1CCCC2(CCCNC2)C1 Show InChI InChI=1S/C29H36N4O2S/c1-2-3-15-25(27(35)32-19-10-17-29(21-32)16-9-18-30-20-29)36-28-31-24-14-8-7-13-23(24)26(34)33(28)22-11-5-4-6-12-22/h4-8,11-14,25,30H,2-3,9-10,15-21H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of C-terminal 6-His tagged human Chk1 by HTRF assay in presence of 0.1 mM ATP |
Bioorg Med Chem Lett 19: 1240-4 (2009)
Article DOI: 10.1016/j.bmcl.2008.12.076
BindingDB Entry DOI: 10.7270/Q2S75G53 |
More data for this
Ligand-Target Pair | |
Beta-secretase 1
(Homo sapiens (Human)) | BDBM50360719
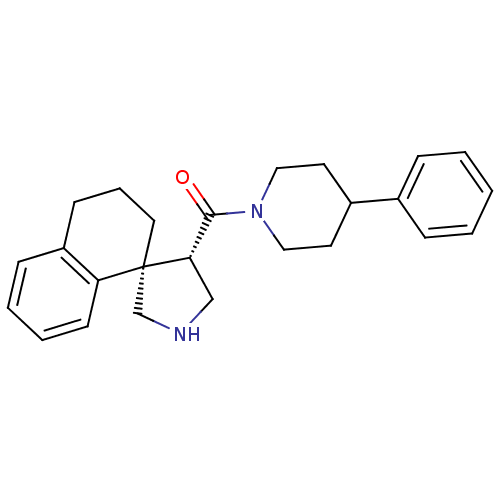
(CHEMBL1934291)Show SMILES O=C([C@@H]1CNC[C@]11CCCc2ccccc12)N1CCC(CC1)c1ccccc1 |r| Show InChI InChI=1S/C25H30N2O/c28-24(27-15-12-20(13-16-27)19-7-2-1-3-8-19)23-17-26-18-25(23)14-6-10-21-9-4-5-11-22(21)25/h1-5,7-9,11,20,23,26H,6,10,12-18H2/t23-,25-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 4.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of BACE1 in cell-free system |
Bioorg Med Chem Lett 22: 240-4 (2011)
Article DOI: 10.1016/j.bmcl.2011.11.024
BindingDB Entry DOI: 10.7270/Q2QR4XJK |
More data for this
Ligand-Target Pair | |
Beta-secretase 1
(Homo sapiens (Human)) | BDBM50360714
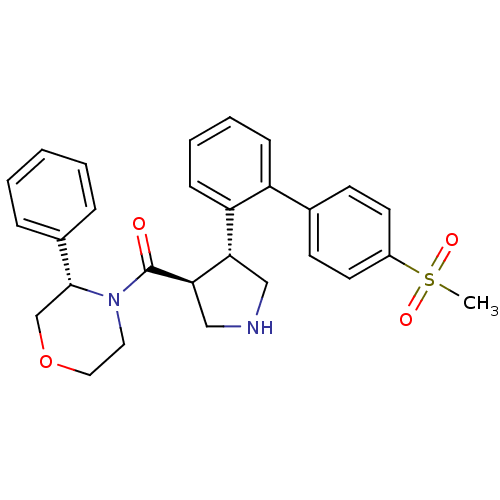
(CHEMBL1934286)Show SMILES CS(=O)(=O)c1ccc(cc1)-c1ccccc1[C@@H]1CNC[C@H]1C(=O)N1CCOC[C@@H]1c1ccccc1 |r| Show InChI InChI=1S/C28H30N2O4S/c1-35(32,33)22-13-11-20(12-14-22)23-9-5-6-10-24(23)25-17-29-18-26(25)28(31)30-15-16-34-19-27(30)21-7-3-2-4-8-21/h2-14,25-27,29H,15-19H2,1H3/t25-,26+,27+/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 4.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of BACE1 in cell-free system |
Bioorg Med Chem Lett 22: 240-4 (2011)
Article DOI: 10.1016/j.bmcl.2011.11.024
BindingDB Entry DOI: 10.7270/Q2QR4XJK |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data