 Found 8 hits in this display
Found 8 hits in this display Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Macrophage metalloelastase
(Homo sapiens (Human)) | BDBM120170
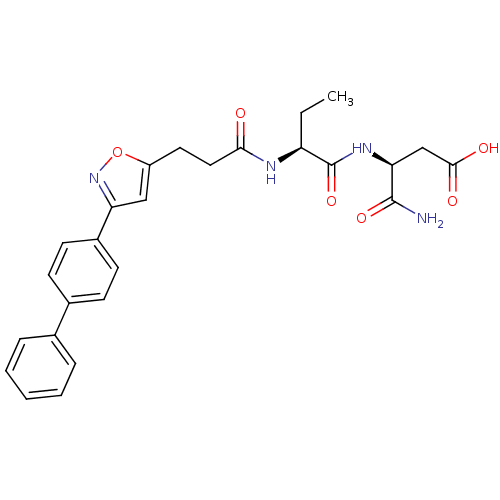
(US8691753, 18)Show SMILES CC[C@H](NC(=O)CCc1cc(no1)-c1ccc(cc1)-c1ccccc1)C(=O)N[C@@H](CC(O)=O)C(N)=O |r| Show InChI InChI=1S/C26H28N4O6/c1-2-20(26(35)29-22(25(27)34)15-24(32)33)28-23(31)13-12-19-14-21(30-36-19)18-10-8-17(9-11-18)16-6-4-3-5-7-16/h3-11,14,20,22H,2,12-13,15H2,1H3,(H2,27,34)(H,28,31)(H,29,35)(H,32,33)/t20-,22-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| 8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Commissariat A l'Energie Atomique et Aux Energies Alternatives
US Patent
| Assay Description
The inhibition tests and the evaluation of the inhibition constants (Ki) on the various MMPs were carried out as described by Devel et al. (Devel et ... |
US Patent US8691753 (2014)
BindingDB Entry DOI: 10.7270/Q28P5Z5Q |
More data for this
Ligand-Target Pair | |
Collagenase 3
(Homo sapiens (Human)) | BDBM120170

(US8691753, 18)Show SMILES CC[C@H](NC(=O)CCc1cc(no1)-c1ccc(cc1)-c1ccccc1)C(=O)N[C@@H](CC(O)=O)C(N)=O |r| Show InChI InChI=1S/C26H28N4O6/c1-2-20(26(35)29-22(25(27)34)15-24(32)33)28-23(31)13-12-19-14-21(30-36-19)18-10-8-17(9-11-18)16-6-4-3-5-7-16/h3-11,14,20,22H,2,12-13,15H2,1H3,(H2,27,34)(H,28,31)(H,29,35)(H,32,33)/t20-,22-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| 30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Commissariat A l'Energie Atomique et Aux Energies Alternatives
US Patent
| Assay Description
The inhibition tests and the evaluation of the inhibition constants (Ki) on the various MMPs were carried out as described by Devel et al. (Devel et ... |
US Patent US8691753 (2014)
BindingDB Entry DOI: 10.7270/Q28P5Z5Q |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM120170

(US8691753, 18)Show SMILES CC[C@H](NC(=O)CCc1cc(no1)-c1ccc(cc1)-c1ccccc1)C(=O)N[C@@H](CC(O)=O)C(N)=O |r| Show InChI InChI=1S/C26H28N4O6/c1-2-20(26(35)29-22(25(27)34)15-24(32)33)28-23(31)13-12-19-14-21(30-36-19)18-10-8-17(9-11-18)16-6-4-3-5-7-16/h3-11,14,20,22H,2,12-13,15H2,1H3,(H2,27,34)(H,28,31)(H,29,35)(H,32,33)/t20-,22-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| 93 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Commissariat A l'Energie Atomique et Aux Energies Alternatives
US Patent
| Assay Description
The inhibition tests and the evaluation of the inhibition constants (Ki) on the various MMPs were carried out as described by Devel et al. (Devel et ... |
US Patent US8691753 (2014)
BindingDB Entry DOI: 10.7270/Q28P5Z5Q |
More data for this
Ligand-Target Pair | |
Stromelysin-2
(Homo sapiens (Human)) | BDBM120170

(US8691753, 18)Show SMILES CC[C@H](NC(=O)CCc1cc(no1)-c1ccc(cc1)-c1ccccc1)C(=O)N[C@@H](CC(O)=O)C(N)=O |r| Show InChI InChI=1S/C26H28N4O6/c1-2-20(26(35)29-22(25(27)34)15-24(32)33)28-23(31)13-12-19-14-21(30-36-19)18-10-8-17(9-11-18)16-6-4-3-5-7-16/h3-11,14,20,22H,2,12-13,15H2,1H3,(H2,27,34)(H,28,31)(H,29,35)(H,32,33)/t20-,22-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| 196 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Commissariat A l'Energie Atomique et Aux Energies Alternatives
US Patent
| Assay Description
The inhibition tests and the evaluation of the inhibition constants (Ki) on the various MMPs were carried out as described by Devel et al. (Devel et ... |
US Patent US8691753 (2014)
BindingDB Entry DOI: 10.7270/Q28P5Z5Q |
More data for this
Ligand-Target Pair | |
Stromelysin-1
(Homo sapiens (Human)) | BDBM120170

(US8691753, 18)Show SMILES CC[C@H](NC(=O)CCc1cc(no1)-c1ccc(cc1)-c1ccccc1)C(=O)N[C@@H](CC(O)=O)C(N)=O |r| Show InChI InChI=1S/C26H28N4O6/c1-2-20(26(35)29-22(25(27)34)15-24(32)33)28-23(31)13-12-19-14-21(30-36-19)18-10-8-17(9-11-18)16-6-4-3-5-7-16/h3-11,14,20,22H,2,12-13,15H2,1H3,(H2,27,34)(H,28,31)(H,29,35)(H,32,33)/t20-,22-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| 440 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Commissariat A l'Energie Atomique et Aux Energies Alternatives
US Patent
| Assay Description
The inhibition tests and the evaluation of the inhibition constants (Ki) on the various MMPs were carried out as described by Devel et al. (Devel et ... |
US Patent US8691753 (2014)
BindingDB Entry DOI: 10.7270/Q28P5Z5Q |
More data for this
Ligand-Target Pair | |
Neutrophil collagenase
(Homo sapiens (Human)) | BDBM120170

(US8691753, 18)Show SMILES CC[C@H](NC(=O)CCc1cc(no1)-c1ccc(cc1)-c1ccccc1)C(=O)N[C@@H](CC(O)=O)C(N)=O |r| Show InChI InChI=1S/C26H28N4O6/c1-2-20(26(35)29-22(25(27)34)15-24(32)33)28-23(31)13-12-19-14-21(30-36-19)18-10-8-17(9-11-18)16-6-4-3-5-7-16/h3-11,14,20,22H,2,12-13,15H2,1H3,(H2,27,34)(H,28,31)(H,29,35)(H,32,33)/t20-,22-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| 832 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Commissariat A l'Energie Atomique et Aux Energies Alternatives
US Patent
| Assay Description
The inhibition tests and the evaluation of the inhibition constants (Ki) on the various MMPs were carried out as described by Devel et al. (Devel et ... |
US Patent US8691753 (2014)
BindingDB Entry DOI: 10.7270/Q28P5Z5Q |
More data for this
Ligand-Target Pair | |
Matrix metalloproteinase-14 [1-20,P8S]
(Homo sapiens (Human)) | BDBM120170

(US8691753, 18)Show SMILES CC[C@H](NC(=O)CCc1cc(no1)-c1ccc(cc1)-c1ccccc1)C(=O)N[C@@H](CC(O)=O)C(N)=O |r| Show InChI InChI=1S/C26H28N4O6/c1-2-20(26(35)29-22(25(27)34)15-24(32)33)28-23(31)13-12-19-14-21(30-36-19)18-10-8-17(9-11-18)16-6-4-3-5-7-16/h3-11,14,20,22H,2,12-13,15H2,1H3,(H2,27,34)(H,28,31)(H,29,35)(H,32,33)/t20-,22-/m0/s1 | MMDB
Reactome pathway
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Commissariat A l'Energie Atomique et Aux Energies Alternatives
US Patent
| Assay Description
The inhibition tests and the evaluation of the inhibition constants (Ki) on the various MMPs were carried out as described by Devel et al. (Devel et ... |
US Patent US8691753 (2014)
BindingDB Entry DOI: 10.7270/Q28P5Z5Q |
More data for this
Ligand-Target Pair | |
Matrix metalloproteinase-9
(Homo sapiens (Human)) | BDBM120170

(US8691753, 18)Show SMILES CC[C@H](NC(=O)CCc1cc(no1)-c1ccc(cc1)-c1ccccc1)C(=O)N[C@@H](CC(O)=O)C(N)=O |r| Show InChI InChI=1S/C26H28N4O6/c1-2-20(26(35)29-22(25(27)34)15-24(32)33)28-23(31)13-12-19-14-21(30-36-19)18-10-8-17(9-11-18)16-6-4-3-5-7-16/h3-11,14,20,22H,2,12-13,15H2,1H3,(H2,27,34)(H,28,31)(H,29,35)(H,32,33)/t20-,22-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Commissariat A l'Energie Atomique et Aux Energies Alternatives
US Patent
| Assay Description
The inhibition tests and the evaluation of the inhibition constants (Ki) on the various MMPs were carried out as described by Devel et al. (Devel et ... |
US Patent US8691753 (2014)
BindingDB Entry DOI: 10.7270/Q28P5Z5Q |
More data for this
Ligand-Target Pair | |