 Found 8 hits in this display
Found 8 hits in this display Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
17-beta-hydroxysteroid dehydrogenase type 3
(Homo sapiens (Human)) | BDBM50350421
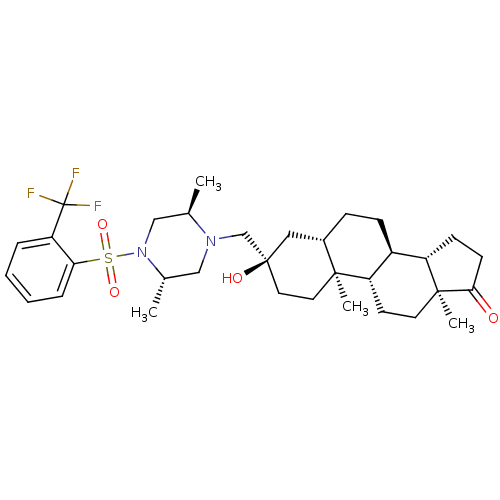
(CHEMBL1813731)Show SMILES C[C@@H]1CN([C@@H](C)CN1C[C@@]1(O)CC[C@@]2(C)[C@@H](CC[C@H]3[C@@H]4CCC(=O)[C@@]4(C)CC[C@H]23)C1)S(=O)(=O)c1ccccc1C(F)(F)F |r| Show InChI InChI=1S/C33H47F3N2O4S/c1-21-19-38(43(41,42)28-8-6-5-7-27(28)33(34,35)36)22(2)18-37(21)20-32(40)16-15-30(3)23(17-32)9-10-24-25-11-12-29(39)31(25,4)14-13-26(24)30/h5-8,21-26,40H,9-20H2,1-4H3/t21-,22+,23+,24+,25+,26+,30+,31+,32-/m1/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
CHUQ (CHUL)-Research Center
Curated by ChEMBL
| Assay Description
Inhibition of 17Beta-HSD3 expressed in intact HEK293 cells assessed as transformation of [14C]-4-androstene-3,17-dione into [14C]-testosterone in pre... |
Bioorg Med Chem 19: 4652-68 (2011)
Article DOI: 10.1016/j.bmc.2011.06.003
BindingDB Entry DOI: 10.7270/Q2222V4T |
More data for this
Ligand-Target Pair | |
17-beta-hydroxysteroid dehydrogenase type 3
(Homo sapiens (Human)) | BDBM50350421

(CHEMBL1813731)Show SMILES C[C@@H]1CN([C@@H](C)CN1C[C@@]1(O)CC[C@@]2(C)[C@@H](CC[C@H]3[C@@H]4CCC(=O)[C@@]4(C)CC[C@H]23)C1)S(=O)(=O)c1ccccc1C(F)(F)F |r| Show InChI InChI=1S/C33H47F3N2O4S/c1-21-19-38(43(41,42)28-8-6-5-7-27(28)33(34,35)36)22(2)18-37(21)20-32(40)16-15-30(3)23(17-32)9-10-24-25-11-12-29(39)31(25,4)14-13-26(24)30/h5-8,21-26,40H,9-20H2,1-4H3/t21-,22+,23+,24+,25+,26+,30+,31+,32-/m1/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
CHU de Qu£bec-Research Center
Curated by ChEMBL
| Assay Description
Inhibition of 17betaHSD3 (unknown origin) transfected in HEK293 cells |
Bioorg Med Chem Lett 26: 2179-83 (2016)
Article DOI: 10.1016/j.bmcl.2016.03.069
BindingDB Entry DOI: 10.7270/Q2FT8Q2J |
More data for this
Ligand-Target Pair | |
17-beta-hydroxysteroid dehydrogenase type 3
(Rattus norvegicus) | BDBM50350421

(CHEMBL1813731)Show SMILES C[C@@H]1CN([C@@H](C)CN1C[C@@]1(O)CC[C@@]2(C)[C@@H](CC[C@H]3[C@@H]4CCC(=O)[C@@]4(C)CC[C@H]23)C1)S(=O)(=O)c1ccccc1C(F)(F)F |r| Show InChI InChI=1S/C33H47F3N2O4S/c1-21-19-38(43(41,42)28-8-6-5-7-27(28)33(34,35)36)22(2)18-37(21)20-32(40)16-15-30(3)23(17-32)9-10-24-25-11-12-29(39)31(25,4)14-13-26(24)30/h5-8,21-26,40H,9-20H2,1-4H3/t21-,22+,23+,24+,25+,26+,30+,31+,32-/m1/s1 | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
CHU de Qu£bec-Research Center (CHUL)
Curated by ChEMBL
| Assay Description
Inhibition of 17beta-HSD3 in rat testes microsomes using [14C]-4-androstene-3,17-dione as substrate after 2 hrs |
Bioorg Med Chem 23: 5433-51 (2015)
Article DOI: 10.1016/j.bmc.2015.07.049
BindingDB Entry DOI: 10.7270/Q20C4XHT |
More data for this
Ligand-Target Pair | |
17-beta-hydroxysteroid dehydrogenase type 3
(Rattus norvegicus) | BDBM50443289

(CHEMBL3088217)Show SMILES CC1CN(C(C)CN1C[C@@]1(O)CC[C@@]2(C)[C@@H](CC[C@H]3[C@@H]4CCC(=O)[C@@]4(C)CC[C@H]23)C1)S(=O)(=O)c1ccccc1C(F)(F)F |r| Show InChI InChI=1S/C33H47F3N2O4S/c1-21-19-38(43(41,42)28-8-6-5-7-27(28)33(34,35)36)22(2)18-37(21)20-32(40)16-15-30(3)23(17-32)9-10-24-25-11-12-29(39)31(25,4)14-13-26(24)30/h5-8,21-26,40H,9-20H2,1-4H3/t21?,22?,23-,24-,25-,26-,30-,31-,32+/m0/s1 | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
Laval University
Curated by ChEMBL
| Assay Description
Inhibition of 17beta-HSD3 in rat testes microsomal fraction using [14C]-4-androstene-3,17-dione as substrate assessed as testosterone formation after... |
Bioorg Med Chem Lett 23: 6360-2 (2013)
Article DOI: 10.1016/j.bmcl.2013.09.072
BindingDB Entry DOI: 10.7270/Q2KP83M4 |
More data for this
Ligand-Target Pair | |
17-beta-hydroxysteroid dehydrogenase type 3
(Rattus norvegicus) | BDBM50443289

(CHEMBL3088217)Show SMILES CC1CN(C(C)CN1C[C@@]1(O)CC[C@@]2(C)[C@@H](CC[C@H]3[C@@H]4CCC(=O)[C@@]4(C)CC[C@H]23)C1)S(=O)(=O)c1ccccc1C(F)(F)F |r| Show InChI InChI=1S/C33H47F3N2O4S/c1-21-19-38(43(41,42)28-8-6-5-7-27(28)33(34,35)36)22(2)18-37(21)20-32(40)16-15-30(3)23(17-32)9-10-24-25-11-12-29(39)31(25,4)14-13-26(24)30/h5-8,21-26,40H,9-20H2,1-4H3/t21?,22?,23-,24-,25-,26-,30-,31-,32+/m0/s1 | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 80 | n/a | n/a | n/a | n/a | n/a | n/a |
CHU de Queb£c-Research Center
Curated by ChEMBL
| Assay Description
Inhibition of 17beta-HSD3 in Sprague-Dawley rat testes microsomal fraction assessed as reduction in [14C]-testosterone formation from [14C]-4-androst... |
J Med Chem 62: 7070-7088 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00624
BindingDB Entry DOI: 10.7270/Q2B56P6P |
More data for this
Ligand-Target Pair | |
17-beta-hydroxysteroid dehydrogenase type 3
(Homo sapiens (Human)) | BDBM50350421

(CHEMBL1813731)Show SMILES C[C@@H]1CN([C@@H](C)CN1C[C@@]1(O)CC[C@@]2(C)[C@@H](CC[C@H]3[C@@H]4CCC(=O)[C@@]4(C)CC[C@H]23)C1)S(=O)(=O)c1ccccc1C(F)(F)F |r| Show InChI InChI=1S/C33H47F3N2O4S/c1-21-19-38(43(41,42)28-8-6-5-7-27(28)33(34,35)36)22(2)18-37(21)20-32(40)16-15-30(3)23(17-32)9-10-24-25-11-12-29(39)31(25,4)14-13-26(24)30/h5-8,21-26,40H,9-20H2,1-4H3/t21-,22+,23+,24+,25+,26+,30+,31+,32-/m1/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 85 | n/a | n/a | n/a | n/a | n/a | n/a |
CHU de Qu£bec-Research Center
Curated by ChEMBL
| Assay Description
Inhibition of 17betaHSD3 (unknown origin) transfected in human LNCAP cells assessed as conversion of [14C]-4-androstene-3,17-dione into [14C]-testost... |
Bioorg Med Chem Lett 26: 2179-83 (2016)
Article DOI: 10.1016/j.bmcl.2016.03.069
BindingDB Entry DOI: 10.7270/Q2FT8Q2J |
More data for this
Ligand-Target Pair | |
17-beta-hydroxysteroid dehydrogenase type 3
(Homo sapiens (Human)) | BDBM50443289

(CHEMBL3088217)Show SMILES CC1CN(C(C)CN1C[C@@]1(O)CC[C@@]2(C)[C@@H](CC[C@H]3[C@@H]4CCC(=O)[C@@]4(C)CC[C@H]23)C1)S(=O)(=O)c1ccccc1C(F)(F)F |r| Show InChI InChI=1S/C33H47F3N2O4S/c1-21-19-38(43(41,42)28-8-6-5-7-27(28)33(34,35)36)22(2)18-37(21)20-32(40)16-15-30(3)23(17-32)9-10-24-25-11-12-29(39)31(25,4)14-13-26(24)30/h5-8,21-26,40H,9-20H2,1-4H3/t21?,22?,23-,24-,25-,26-,30-,31-,32+/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 90 | n/a | n/a | n/a | n/a | n/a | n/a |
CHU de Queb£c-Research Center
Curated by ChEMBL
| Assay Description
Inhibition of 17beta-HSD3 (unknown origin) expressed in human LNCAP cells assessed as reduction in [14C]-testosterone formation from [14C]-4-androste... |
J Med Chem 62: 7070-7088 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00624
BindingDB Entry DOI: 10.7270/Q2B56P6P |
More data for this
Ligand-Target Pair | |
17-beta-hydroxysteroid dehydrogenase type 3
(Homo sapiens (Human)) | BDBM50350421

(CHEMBL1813731)Show SMILES C[C@@H]1CN([C@@H](C)CN1C[C@@]1(O)CC[C@@]2(C)[C@@H](CC[C@H]3[C@@H]4CCC(=O)[C@@]4(C)CC[C@H]23)C1)S(=O)(=O)c1ccccc1C(F)(F)F |r| Show InChI InChI=1S/C33H47F3N2O4S/c1-21-19-38(43(41,42)28-8-6-5-7-27(28)33(34,35)36)22(2)18-37(21)20-32(40)16-15-30(3)23(17-32)9-10-24-25-11-12-29(39)31(25,4)14-13-26(24)30/h5-8,21-26,40H,9-20H2,1-4H3/t21-,22+,23+,24+,25+,26+,30+,31+,32-/m1/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 110 | n/a | n/a | n/a | n/a | n/a | n/a |
CHU de Qu£bec - Research Center
Curated by ChEMBL
| Assay Description
Inhibition of 17-beta-HSD3 (unknown origin) expressed in human LNCaP cells using [14C]-4-androstene-3,17-dione as substrate assessed as reduction of ... |
Bioorg Med Chem 25: 2065-2073 (2017)
Article DOI: 10.1016/j.bmc.2017.02.008
BindingDB Entry DOI: 10.7270/Q2668GF6 |
More data for this
Ligand-Target Pair | |