 Found 8 hits in this display
Found 8 hits in this display Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Cannabinoid receptor 1
(Rattus norvegicus (rat)) | BDBM50056486
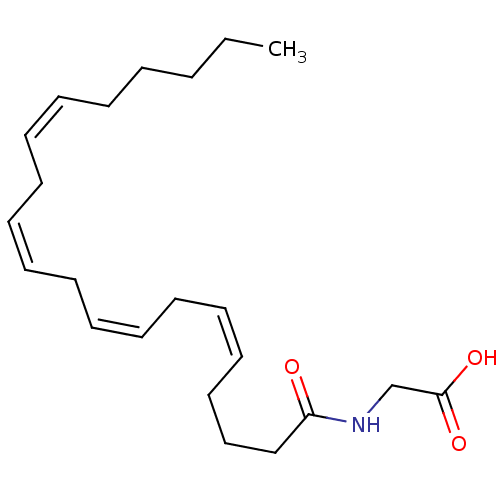
(((5Z,8Z)-Icosa-5,8,11,14-tetraenoylamino)-acetic a...)Show SMILES CCCCC\C=C/C\C=C/C\C=C/C\C=C/CCCC(=O)NCC(O)=O Show InChI InChI=1S/C22H35NO3/c1-2-3-4-5-6-7-8-9-10-11-12-13-14-15-16-17-18-19-21(24)23-20-22(25)26/h6-7,9-10,12-13,15-16H,2-5,8,11,14,17-20H2,1H3,(H,23,24)(H,25,26)/b7-6-,10-9-,13-12-,16-15- | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hebrew University
Curated by ChEMBL
| Assay Description
In vitro binding affinity was determined against rat brain Cannabinoid receptor 1 |
J Med Chem 40: 659-67 (1997)
Article DOI: 10.1021/jm960752x
BindingDB Entry DOI: 10.7270/Q2JS9R3C |
More data for this
Ligand-Target Pair | |
Sodium- and chloride-dependent glycine transporter 2
(Homo sapiens (Human)) | BDBM50056486

(((5Z,8Z)-Icosa-5,8,11,14-tetraenoylamino)-acetic a...)Show SMILES CCCCC\C=C/C\C=C/C\C=C/C\C=C/CCCC(=O)NCC(O)=O Show InChI InChI=1S/C22H35NO3/c1-2-3-4-5-6-7-8-9-10-11-12-13-14-15-16-17-18-19-21(24)23-20-22(25)26/h6-7,9-10,12-13,15-16H,2-5,8,11,14,17-20H2,1H3,(H,23,24)(H,25,26)/b7-6-,10-9-,13-12-,16-15- | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Albany College of Pharmacy and Health Sciences
Curated by ChEMBL
| Assay Description
Inhibition of GlyT-2 (unknown origin) |
J Med Chem 61: 2652-2679 (2018)
Article DOI: 10.1021/acs.jmedchem.7b00956
BindingDB Entry DOI: 10.7270/Q23B62RZ |
More data for this
Ligand-Target Pair | |
Fatty-acid amide hydrolase 1
(Homo sapiens (Human)) | BDBM50056486

(((5Z,8Z)-Icosa-5,8,11,14-tetraenoylamino)-acetic a...)Show SMILES CCCCC\C=C/C\C=C/C\C=C/C\C=C/CCCC(=O)NCC(O)=O Show InChI InChI=1S/C22H35NO3/c1-2-3-4-5-6-7-8-9-10-11-12-13-14-15-16-17-18-19-21(24)23-20-22(25)26/h6-7,9-10,12-13,15-16H,2-5,8,11,14,17-20H2,1H3,(H,23,24)(H,25,26)/b7-6-,10-9-,13-12-,16-15- | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 7.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ Catholique de Louvain
Curated by ChEMBL
| Assay Description
Inhibition of fatty acid amide hydrolase; range = 4.1-7 uM |
J Med Chem 48: 5059-87 (2005)
Article DOI: 10.1021/jm058183t
BindingDB Entry DOI: 10.7270/Q2J96753 |
More data for this
Ligand-Target Pair | |
Sodium- and chloride-dependent glycine transporter 2
(Homo sapiens (Human)) | BDBM50056486

(((5Z,8Z)-Icosa-5,8,11,14-tetraenoylamino)-acetic a...)Show SMILES CCCCC\C=C/C\C=C/C\C=C/C\C=C/CCCC(=O)NCC(O)=O Show InChI InChI=1S/C22H35NO3/c1-2-3-4-5-6-7-8-9-10-11-12-13-14-15-16-17-18-19-21(24)23-20-22(25)26/h6-7,9-10,12-13,15-16H,2-5,8,11,14,17-20H2,1H3,(H,23,24)(H,25,26)/b7-6-,10-9-,13-12-,16-15- | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 9.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Sydney
Curated by ChEMBL
| Assay Description
Reversible non-competitive inhibition of human GlyT2a expressed in Xenopus laevis oocytes by two-electrode voltage clamp electrophysiology |
J Med Chem 62: 2466-2484 (2019)
Article DOI: 10.1021/acs.jmedchem.8b01775
BindingDB Entry DOI: 10.7270/Q21R6V1K |
More data for this
Ligand-Target Pair | |
N-arachidonyl glycine receptor
(Homo sapiens (Human)) | BDBM50056486

(((5Z,8Z)-Icosa-5,8,11,14-tetraenoylamino)-acetic a...)Show SMILES CCCCC\C=C/C\C=C/C\C=C/C\C=C/CCCC(=O)NCC(O)=O Show InChI InChI=1S/C22H35NO3/c1-2-3-4-5-6-7-8-9-10-11-12-13-14-15-16-17-18-19-21(24)23-20-22(25)26/h6-7,9-10,12-13,15-16H,2-5,8,11,14,17-20H2,1H3,(H,23,24)(H,25,26)/b7-6-,10-9-,13-12-,16-15- | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Antagonist activity at Prolink1-tagged human GPR18 receptor expressed in CHO cells assessed as inhibition of THC-induced beta arrestin recruitment af... |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.0c00208
BindingDB Entry DOI: 10.7270/Q2PZ5DJH |
More data for this
Ligand-Target Pair | |
N-arachidonyl glycine receptor
(Homo sapiens (Human)) | BDBM50056486

(((5Z,8Z)-Icosa-5,8,11,14-tetraenoylamino)-acetic a...)Show SMILES CCCCC\C=C/C\C=C/C\C=C/C\C=C/CCCC(=O)NCC(O)=O Show InChI InChI=1S/C22H35NO3/c1-2-3-4-5-6-7-8-9-10-11-12-13-14-15-16-17-18-19-21(24)23-20-22(25)26/h6-7,9-10,12-13,15-16H,2-5,8,11,14,17-20H2,1H3,(H,23,24)(H,25,26)/b7-6-,10-9-,13-12-,16-15- | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Agonist activity at Prolink1-tagged human GPR18 receptor expressed in CHO cells assessed as induction of beta-arrestin recruitment after 90 mins by b... |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.0c00208
BindingDB Entry DOI: 10.7270/Q2PZ5DJH |
More data for this
Ligand-Target Pair | |
N-arachidonyl glycine receptor
(Homo sapiens (Human)) | BDBM50056486

(((5Z,8Z)-Icosa-5,8,11,14-tetraenoylamino)-acetic a...)Show SMILES CCCCC\C=C/C\C=C/C\C=C/C\C=C/CCCC(=O)NCC(O)=O Show InChI InChI=1S/C22H35NO3/c1-2-3-4-5-6-7-8-9-10-11-12-13-14-15-16-17-18-19-21(24)23-20-22(25)26/h6-7,9-10,12-13,15-16H,2-5,8,11,14,17-20H2,1H3,(H,23,24)(H,25,26)/b7-6-,10-9-,13-12-,16-15- | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 20 | n/a | n/a | n/a | n/a |
University of Bonn
Curated by ChEMBL
| Assay Description
Agonist activity at recombinant full length human GPR18 expressed in CHO cells assessed as forskolin-stimulated cAMP accumulation after 10 mins by en... |
Eur J Med Chem 155: 381-397 (2018)
Article DOI: 10.1016/j.ejmech.2018.05.050
BindingDB Entry DOI: 10.7270/Q22F7R1X |
More data for this
Ligand-Target Pair | |
N-arachidonyl glycine receptor
(Homo sapiens (Human)) | BDBM50056486

(((5Z,8Z)-Icosa-5,8,11,14-tetraenoylamino)-acetic a...)Show SMILES CCCCC\C=C/C\C=C/C\C=C/C\C=C/CCCC(=O)NCC(O)=O Show InChI InChI=1S/C22H35NO3/c1-2-3-4-5-6-7-8-9-10-11-12-13-14-15-16-17-18-19-21(24)23-20-22(25)26/h6-7,9-10,12-13,15-16H,2-5,8,11,14,17-20H2,1H3,(H,23,24)(H,25,26)/b7-6-,10-9-,13-12-,16-15- | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 44 | n/a | n/a | n/a | n/a |
University of Bonn
Curated by ChEMBL
| Assay Description
Agonist activity at recombinant human GPR18 expressed in HEK293 cells assessed as induction of p44/42 MAPK phosphorylation after 5 mins by Western bl... |
Eur J Med Chem 155: 381-397 (2018)
Article DOI: 10.1016/j.ejmech.2018.05.050
BindingDB Entry DOI: 10.7270/Q22F7R1X |
More data for this
Ligand-Target Pair | |