 Found 6 hits in this display
Found 6 hits in this display Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Type-2 angiotensin II receptor
(RAT) | BDBM82435
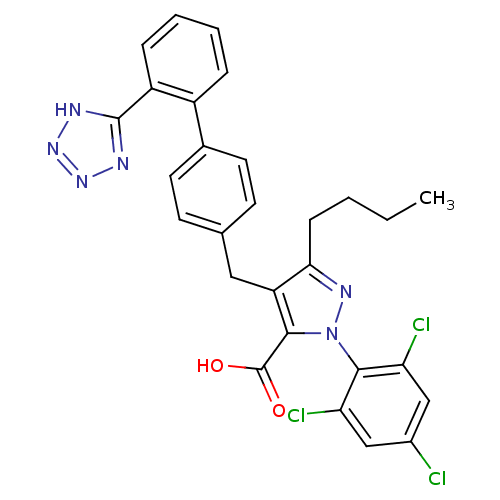
(1-[2,4,6-Trichlorophenyl]-3-butyl-4-[[2'-(1H-t...)Show SMILES CCCCc1nn(c(C(O)=O)c1Cc1ccc(cc1)-c1ccccc1-c1nnn[nH]1)-c1c(Cl)cc(Cl)cc1Cl |(13.29,.94,;11.96,.17,;10.62,.94,;9.29,.17,;7.95,.94,;7.79,2.47,;6.29,2.79,;5.52,1.46,;3.99,1.29,;3.36,-.11,;3.08,2.54,;6.55,.31,;6.23,-1.2,;7.37,-2.23,;7.05,-3.73,;8.2,-4.76,;9.66,-4.29,;9.98,-2.78,;8.84,-1.75,;10.8,-5.32,;12.27,-4.84,;13.41,-5.87,;13.09,-7.38,;11.63,-7.85,;10.48,-6.82,;9.02,-7.3,;7.77,-6.39,;6.53,-7.3,;7,-8.76,;8.54,-8.76,;5.91,4.28,;7.02,5.35,;8.5,4.93,;6.64,6.85,;5.16,7.27,;4.79,8.76,;4.06,6.2,;4.43,4.7,;3.32,3.63,)| Show InChI InChI=1S/C28H23Cl3N6O2/c1-2-3-8-24-21(25(28(38)39)37(34-24)26-22(30)14-18(29)15-23(26)31)13-16-9-11-17(12-10-16)19-6-4-5-7-20(19)27-32-35-36-33-27/h4-7,9-12,14-15H,2-3,8,13H2,1H3,(H,38,39)(H,32,33,35,36) | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| PubMed
| 0.25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 272: 612-8 (1995)
BindingDB Entry DOI: 10.7270/Q2FB51GP |
More data for this
Ligand-Target Pair | |
Type-2 angiotensin II receptor
(RAT) | BDBM82435

(1-[2,4,6-Trichlorophenyl]-3-butyl-4-[[2'-(1H-t...)Show SMILES CCCCc1nn(c(C(O)=O)c1Cc1ccc(cc1)-c1ccccc1-c1nnn[nH]1)-c1c(Cl)cc(Cl)cc1Cl |(13.29,.94,;11.96,.17,;10.62,.94,;9.29,.17,;7.95,.94,;7.79,2.47,;6.29,2.79,;5.52,1.46,;3.99,1.29,;3.36,-.11,;3.08,2.54,;6.55,.31,;6.23,-1.2,;7.37,-2.23,;7.05,-3.73,;8.2,-4.76,;9.66,-4.29,;9.98,-2.78,;8.84,-1.75,;10.8,-5.32,;12.27,-4.84,;13.41,-5.87,;13.09,-7.38,;11.63,-7.85,;10.48,-6.82,;9.02,-7.3,;7.77,-6.39,;6.53,-7.3,;7,-8.76,;8.54,-8.76,;5.91,4.28,;7.02,5.35,;8.5,4.93,;6.64,6.85,;5.16,7.27,;4.79,8.76,;4.06,6.2,;4.43,4.7,;3.32,3.63,)| Show InChI InChI=1S/C28H23Cl3N6O2/c1-2-3-8-24-21(25(28(38)39)37(34-24)26-22(30)14-18(29)15-23(26)31)13-16-9-11-17(12-10-16)19-6-4-5-7-20(19)27-32-35-36-33-27/h4-7,9-12,14-15H,2-3,8,13H2,1H3,(H,38,39)(H,32,33,35,36) | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| PubMed
| 570 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 272: 612-8 (1995)
BindingDB Entry DOI: 10.7270/Q2FB51GP |
More data for this
Ligand-Target Pair | |
Dipeptidase 1
(GUINEA PIG) | BDBM50042576
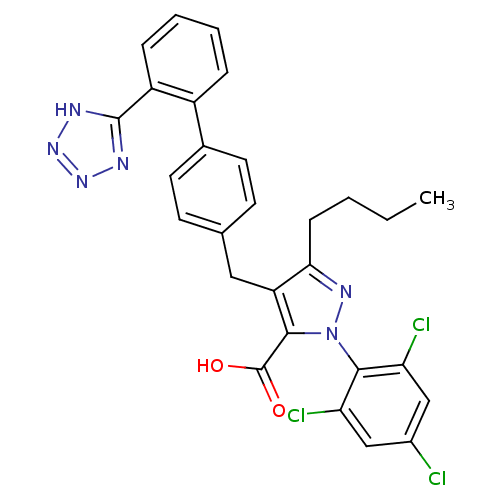
(5-Butyl-4-[2'-(1H-tetrazol-5-yl)-biphenyl-4-ylmeth...)Show SMILES CCCCc1nn(c(C(O)=O)c1Cc1ccc(cc1)-c1ccccc1-c1nnn[nH]1)-c1c(Cl)cc(Cl)cc1Cl |(-2.96,-14.32,;-1.41,-14.32,;-.64,-13.02,;.92,-13.03,;1.82,-11.79,;1.36,-10.32,;2.6,-9.41,;3.84,-10.32,;5.31,-9.83,;5.63,-8.33,;6.79,-10.57,;3.37,-11.79,;4.27,-13.03,;5.81,-13.02,;6.57,-14.35,;8.11,-14.34,;8.86,-13.02,;8.09,-11.69,;6.56,-11.7,;10.41,-13,;11.18,-14.34,;12.71,-14.32,;13.48,-13,;12.69,-11.64,;11.16,-11.67,;10.63,-10.21,;11.87,-9.31,;11.4,-7.85,;9.86,-7.83,;9.38,-9.3,;2.2,-7.88,;3.31,-6.78,;4.76,-6.54,;2.91,-5.28,;1.4,-4.86,;.98,-3.34,;.29,-5.99,;.69,-7.47,;-.47,-8.57,)| Show InChI InChI=1S/C28H23Cl3N6O2/c1-2-3-8-24-21(25(28(38)39)37(34-24)26-22(30)14-18(29)15-23(26)31)13-16-9-11-17(12-10-16)19-6-4-5-7-20(19)27-32-35-36-33-27/h4-7,9-12,14-15H,2-3,8,13H2,1H3,(H,38,39)(H,32,33,35,36) | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 8.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
In vitro apparent inhibition constant of porcine renal Dehydropeptidase-1. |
Bioorg Med Chem Lett 9: 2741-6 (1999)
BindingDB Entry DOI: 10.7270/Q24Q7T6C |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor
(RABBIT) | BDBM50042576

(5-Butyl-4-[2'-(1H-tetrazol-5-yl)-biphenyl-4-ylmeth...)Show SMILES CCCCc1nn(c(C(O)=O)c1Cc1ccc(cc1)-c1ccccc1-c1nnn[nH]1)-c1c(Cl)cc(Cl)cc1Cl |(-2.96,-14.32,;-1.41,-14.32,;-.64,-13.02,;.92,-13.03,;1.82,-11.79,;1.36,-10.32,;2.6,-9.41,;3.84,-10.32,;5.31,-9.83,;5.63,-8.33,;6.79,-10.57,;3.37,-11.79,;4.27,-13.03,;5.81,-13.02,;6.57,-14.35,;8.11,-14.34,;8.86,-13.02,;8.09,-11.69,;6.56,-11.7,;10.41,-13,;11.18,-14.34,;12.71,-14.32,;13.48,-13,;12.69,-11.64,;11.16,-11.67,;10.63,-10.21,;11.87,-9.31,;11.4,-7.85,;9.86,-7.83,;9.38,-9.3,;2.2,-7.88,;3.31,-6.78,;4.76,-6.54,;2.91,-5.28,;1.4,-4.86,;.98,-3.34,;.29,-5.99,;.69,-7.47,;-.47,-8.57,)| Show InChI InChI=1S/C28H23Cl3N6O2/c1-2-3-8-24-21(25(28(38)39)37(34-24)26-22(30)14-18(29)15-23(26)31)13-16-9-11-17(12-10-16)19-6-4-5-7-20(19)27-32-35-36-33-27/h4-7,9-12,14-15H,2-3,8,13H2,1H3,(H,38,39)(H,32,33,35,36) | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Evaluation of Angiotensin II antagonistic activity by displacement of [125I]-Sar Ile-AII at the rabbit aorta Angiotensin II receptor, type 1 |
J Med Chem 36: 3595-605 (1994)
BindingDB Entry DOI: 10.7270/Q25Q4V5H |
More data for this
Ligand-Target Pair | |
Metallo-beta-lactamase type 2
(Bacteroides fragilis) | BDBM50042576

(5-Butyl-4-[2'-(1H-tetrazol-5-yl)-biphenyl-4-ylmeth...)Show SMILES CCCCc1nn(c(C(O)=O)c1Cc1ccc(cc1)-c1ccccc1-c1nnn[nH]1)-c1c(Cl)cc(Cl)cc1Cl |(-2.96,-14.32,;-1.41,-14.32,;-.64,-13.02,;.92,-13.03,;1.82,-11.79,;1.36,-10.32,;2.6,-9.41,;3.84,-10.32,;5.31,-9.83,;5.63,-8.33,;6.79,-10.57,;3.37,-11.79,;4.27,-13.03,;5.81,-13.02,;6.57,-14.35,;8.11,-14.34,;8.86,-13.02,;8.09,-11.69,;6.56,-11.7,;10.41,-13,;11.18,-14.34,;12.71,-14.32,;13.48,-13,;12.69,-11.64,;11.16,-11.67,;10.63,-10.21,;11.87,-9.31,;11.4,-7.85,;9.86,-7.83,;9.38,-9.3,;2.2,-7.88,;3.31,-6.78,;4.76,-6.54,;2.91,-5.28,;1.4,-4.86,;.98,-3.34,;.29,-5.99,;.69,-7.47,;-.47,-8.57,)| Show InChI InChI=1S/C28H23Cl3N6O2/c1-2-3-8-24-21(25(28(38)39)37(34-24)26-22(30)14-18(29)15-23(26)31)13-16-9-11-17(12-10-16)19-6-4-5-7-20(19)27-32-35-36-33-27/h4-7,9-12,14-15H,2-3,8,13H2,1H3,(H,38,39)(H,32,33,35,36) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 3.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of metallo-beta-lactamase (MBL) from Bacteroides fragilis. |
Bioorg Med Chem Lett 9: 2741-6 (1999)
BindingDB Entry DOI: 10.7270/Q24Q7T6C |
More data for this
Ligand-Target Pair | |
Beta-lactamase
(Pseudomonas aeruginosa) | BDBM50042576

(5-Butyl-4-[2'-(1H-tetrazol-5-yl)-biphenyl-4-ylmeth...)Show SMILES CCCCc1nn(c(C(O)=O)c1Cc1ccc(cc1)-c1ccccc1-c1nnn[nH]1)-c1c(Cl)cc(Cl)cc1Cl |(-2.96,-14.32,;-1.41,-14.32,;-.64,-13.02,;.92,-13.03,;1.82,-11.79,;1.36,-10.32,;2.6,-9.41,;3.84,-10.32,;5.31,-9.83,;5.63,-8.33,;6.79,-10.57,;3.37,-11.79,;4.27,-13.03,;5.81,-13.02,;6.57,-14.35,;8.11,-14.34,;8.86,-13.02,;8.09,-11.69,;6.56,-11.7,;10.41,-13,;11.18,-14.34,;12.71,-14.32,;13.48,-13,;12.69,-11.64,;11.16,-11.67,;10.63,-10.21,;11.87,-9.31,;11.4,-7.85,;9.86,-7.83,;9.38,-9.3,;2.2,-7.88,;3.31,-6.78,;4.76,-6.54,;2.91,-5.28,;1.4,-4.86,;.98,-3.34,;.29,-5.99,;.69,-7.47,;-.47,-8.57,)| Show InChI InChI=1S/C28H23Cl3N6O2/c1-2-3-8-24-21(25(28(38)39)37(34-24)26-22(30)14-18(29)15-23(26)31)13-16-9-11-17(12-10-16)19-6-4-5-7-20(19)27-32-35-36-33-27/h4-7,9-12,14-15H,2-3,8,13H2,1H3,(H,38,39)(H,32,33,35,36) | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | >2.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of metallo-beta-lactamase (MBL) from Pseudomonas aeruginosa mediated by the plasmid-borne IMP-1 enzyme. |
Bioorg Med Chem Lett 9: 2741-6 (1999)
BindingDB Entry DOI: 10.7270/Q24Q7T6C |
More data for this
Ligand-Target Pair | |