 Found 5 hits in this display
Found 5 hits in this display Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
C-X-C chemokine receptor type 3
(Homo sapiens (Human)) | BDBM50337250
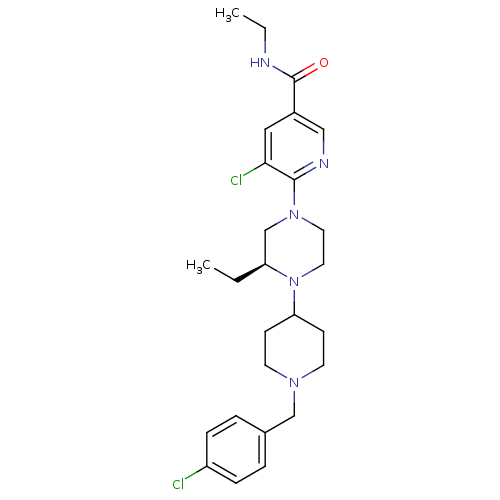
((S)-5-chloro-6-(4-(1-(4-chlorobenzyl)piperidin-4-y...)Show SMILES CCNC(=O)c1cnc(N2CCN([C@@H](CC)C2)C2CCN(Cc3ccc(Cl)cc3)CC2)c(Cl)c1 |r| Show InChI InChI=1S/C26H35Cl2N5O/c1-3-22-18-32(25-24(28)15-20(16-30-25)26(34)29-4-2)13-14-33(22)23-9-11-31(12-10-23)17-19-5-7-21(27)8-6-19/h5-8,15-16,22-23H,3-4,9-14,17-18H2,1-2H3,(H,29,34)/t22-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 3.16 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
VU University Amsterdam
Curated by ChEMBL
| Assay Description
Displacement of [125I]CXCL10 from human CXCR3 expressed in HEK293T cells |
Bioorg Med Chem Lett 19: 2252-7 (2009)
Article DOI: 10.1016/j.bmcl.2009.02.093
BindingDB Entry DOI: 10.7270/Q2DJ5GW6 |
More data for this
Ligand-Target Pair | |
C-X-C chemokine receptor type 3
(Homo sapiens (Human)) | BDBM50337250

((S)-5-chloro-6-(4-(1-(4-chlorobenzyl)piperidin-4-y...)Show SMILES CCNC(=O)c1cnc(N2CCN([C@@H](CC)C2)C2CCN(Cc3ccc(Cl)cc3)CC2)c(Cl)c1 |r| Show InChI InChI=1S/C26H35Cl2N5O/c1-3-22-18-32(25-24(28)15-20(16-30-25)26(34)29-4-2)13-14-33(22)23-9-11-31(12-10-23)17-19-5-7-21(27)8-6-19/h5-8,15-16,22-23H,3-4,9-14,17-18H2,1-2H3,(H,29,34)/t22-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Ligand Pharmaceuticals
Curated by ChEMBL
| Assay Description
Antagonist activity at human CXCR3 |
Bioorg Med Chem Lett 21: 1527-31 (2011)
Article DOI: 10.1016/j.bmcl.2010.12.114
BindingDB Entry DOI: 10.7270/Q2CZ37FJ |
More data for this
Ligand-Target Pair | |
C-X-C chemokine receptor type 3
(Homo sapiens (Human)) | BDBM50337250

((S)-5-chloro-6-(4-(1-(4-chlorobenzyl)piperidin-4-y...)Show SMILES CCNC(=O)c1cnc(N2CCN([C@@H](CC)C2)C2CCN(Cc3ccc(Cl)cc3)CC2)c(Cl)c1 |r| Show InChI InChI=1S/C26H35Cl2N5O/c1-3-22-18-32(25-24(28)15-20(16-30-25)26(34)29-4-2)13-14-33(22)23-9-11-31(12-10-23)17-19-5-7-21(27)8-6-19/h5-8,15-16,22-23H,3-4,9-14,17-18H2,1-2H3,(H,29,34)/t22-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [125I]CXCL10 from human CXCR3 expressed in mouse BA/F3 cells |
Bioorg Med Chem Lett 21: 6982-6 (2011)
Article DOI: 10.1016/j.bmcl.2011.09.120
BindingDB Entry DOI: 10.7270/Q2T72HVN |
More data for this
Ligand-Target Pair | |
C-X-C chemokine receptor type 3
(Mus musculus) | BDBM50337250

((S)-5-chloro-6-(4-(1-(4-chlorobenzyl)piperidin-4-y...)Show SMILES CCNC(=O)c1cnc(N2CCN([C@@H](CC)C2)C2CCN(Cc3ccc(Cl)cc3)CC2)c(Cl)c1 |r| Show InChI InChI=1S/C26H35Cl2N5O/c1-3-22-18-32(25-24(28)15-20(16-30-25)26(34)29-4-2)13-14-33(22)23-9-11-31(12-10-23)17-19-5-7-21(27)8-6-19/h5-8,15-16,22-23H,3-4,9-14,17-18H2,1-2H3,(H,29,34)/t22-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Ligand Pharmaceuticals
Curated by ChEMBL
| Assay Description
Antagonist activity at mouse CXCR3 |
Bioorg Med Chem Lett 21: 1527-31 (2011)
Article DOI: 10.1016/j.bmcl.2010.12.114
BindingDB Entry DOI: 10.7270/Q2CZ37FJ |
More data for this
Ligand-Target Pair | |
C-X-C chemokine receptor type 3
(Rattus norvegicus) | BDBM50337250

((S)-5-chloro-6-(4-(1-(4-chlorobenzyl)piperidin-4-y...)Show SMILES CCNC(=O)c1cnc(N2CCN([C@@H](CC)C2)C2CCN(Cc3ccc(Cl)cc3)CC2)c(Cl)c1 |r| Show InChI InChI=1S/C26H35Cl2N5O/c1-3-22-18-32(25-24(28)15-20(16-30-25)26(34)29-4-2)13-14-33(22)23-9-11-31(12-10-23)17-19-5-7-21(27)8-6-19/h5-8,15-16,22-23H,3-4,9-14,17-18H2,1-2H3,(H,29,34)/t22-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Ligand Pharmaceuticals
Curated by ChEMBL
| Assay Description
Antagonist activity at rat CXCR3 |
Bioorg Med Chem Lett 21: 1527-31 (2011)
Article DOI: 10.1016/j.bmcl.2010.12.114
BindingDB Entry DOI: 10.7270/Q2CZ37FJ |
More data for this
Ligand-Target Pair | |