 Found 6 hits in this display
Found 6 hits in this display Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
C-X-C chemokine receptor type 3
(Homo sapiens (Human)) | BDBM50229382
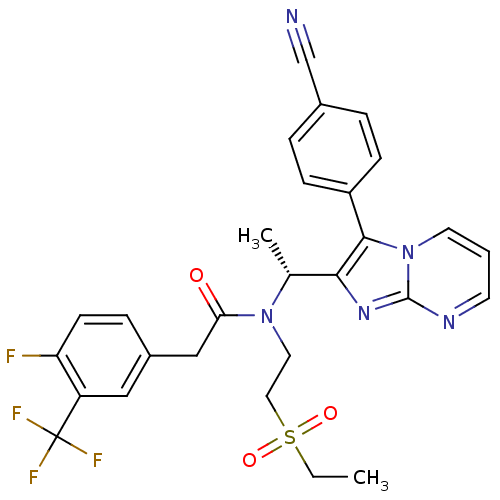
((R)-N-(1-(3-(4-cyanophenyl)imidazo[1,2-a]pyrimidin...)Show SMILES CCS(=O)(=O)CCN([C@H](C)c1nc2ncccn2c1-c1ccc(cc1)C#N)C(=O)Cc1ccc(F)c(c1)C(F)(F)F Show InChI InChI=1S/C28H25F4N5O3S/c1-3-41(39,40)14-13-36(24(38)16-20-7-10-23(29)22(15-20)28(30,31)32)18(2)25-26(21-8-5-19(17-33)6-9-21)37-12-4-11-34-27(37)35-25/h4-12,15,18H,3,13-14,16H2,1-2H3/t18-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Displacement of [125I]-IP-10 from CXCR3 receptor expressed in human PBMC in RPMI-1640 buffer supplemented with 0.5% BSA |
Bioorg Med Chem Lett 18: 688-93 (2008)
Article DOI: 10.1016/j.bmcl.2007.11.060
BindingDB Entry DOI: 10.7270/Q2WQ03HW |
More data for this
Ligand-Target Pair | |
C-X-C chemokine receptor type 3
(Homo sapiens (Human)) | BDBM50229382

((R)-N-(1-(3-(4-cyanophenyl)imidazo[1,2-a]pyrimidin...)Show SMILES CCS(=O)(=O)CCN([C@H](C)c1nc2ncccn2c1-c1ccc(cc1)C#N)C(=O)Cc1ccc(F)c(c1)C(F)(F)F Show InChI InChI=1S/C28H25F4N5O3S/c1-3-41(39,40)14-13-36(24(38)16-20-7-10-23(29)22(15-20)28(30,31)32)18(2)25-26(21-8-5-19(17-33)6-9-21)37-12-4-11-34-27(37)35-25/h4-12,15,18H,3,13-14,16H2,1-2H3/t18-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Displacement of [125I]IP10 from CXCR3 receptor expressed in PBMC |
Bioorg Med Chem Lett 19: 5200-4 (2009)
Article DOI: 10.1016/j.bmcl.2009.07.021
BindingDB Entry DOI: 10.7270/Q2W95980 |
More data for this
Ligand-Target Pair | |
C-X-C chemokine receptor type 3
(Homo sapiens (Human)) | BDBM50229382

((R)-N-(1-(3-(4-cyanophenyl)imidazo[1,2-a]pyrimidin...)Show SMILES CCS(=O)(=O)CCN([C@H](C)c1nc2ncccn2c1-c1ccc(cc1)C#N)C(=O)Cc1ccc(F)c(c1)C(F)(F)F Show InChI InChI=1S/C28H25F4N5O3S/c1-3-41(39,40)14-13-36(24(38)16-20-7-10-23(29)22(15-20)28(30,31)32)18(2)25-26(21-8-5-19(17-33)6-9-21)37-12-4-11-34-27(37)35-25/h4-12,15,18H,3,13-14,16H2,1-2H3/t18-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 79 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Displacement of [125I]IP10 from CXCR3 receptor expressed in PBMC in presence of human plasma |
Bioorg Med Chem Lett 19: 5200-4 (2009)
Article DOI: 10.1016/j.bmcl.2009.07.021
BindingDB Entry DOI: 10.7270/Q2W95980 |
More data for this
Ligand-Target Pair | |
C-X-C chemokine receptor type 3
(Homo sapiens (Human)) | BDBM50229382

((R)-N-(1-(3-(4-cyanophenyl)imidazo[1,2-a]pyrimidin...)Show SMILES CCS(=O)(=O)CCN([C@H](C)c1nc2ncccn2c1-c1ccc(cc1)C#N)C(=O)Cc1ccc(F)c(c1)C(F)(F)F Show InChI InChI=1S/C28H25F4N5O3S/c1-3-41(39,40)14-13-36(24(38)16-20-7-10-23(29)22(15-20)28(30,31)32)18(2)25-26(21-8-5-19(17-33)6-9-21)37-12-4-11-34-27(37)35-25/h4-12,15,18H,3,13-14,16H2,1-2H3/t18-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 79 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Displacement of [125I]-IP-10 from CXCR3 receptor expressed in human PBMC in EDTA-anti-coagulated human plasma |
Bioorg Med Chem Lett 18: 688-93 (2008)
Article DOI: 10.1016/j.bmcl.2007.11.060
BindingDB Entry DOI: 10.7270/Q2WQ03HW |
More data for this
Ligand-Target Pair | |
C-X-C chemokine receptor type 3
(Homo sapiens (Human)) | BDBM50229382

((R)-N-(1-(3-(4-cyanophenyl)imidazo[1,2-a]pyrimidin...)Show SMILES CCS(=O)(=O)CCN([C@H](C)c1nc2ncccn2c1-c1ccc(cc1)C#N)C(=O)Cc1ccc(F)c(c1)C(F)(F)F Show InChI InChI=1S/C28H25F4N5O3S/c1-3-41(39,40)14-13-36(24(38)16-20-7-10-23(29)22(15-20)28(30,31)32)18(2)25-26(21-8-5-19(17-33)6-9-21)37-12-4-11-34-27(37)35-25/h4-12,15,18H,3,13-14,16H2,1-2H3/t18-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 643 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Antagonistic activity to CXCR3 receptor expressed in PBMC assessed as inhibition of ITAC-mediated cell migration in presence of 100% human plasma |
Bioorg Med Chem Lett 19: 5200-4 (2009)
Article DOI: 10.1016/j.bmcl.2009.07.021
BindingDB Entry DOI: 10.7270/Q2W95980 |
More data for this
Ligand-Target Pair | |
C-X-C chemokine receptor type 3
(Homo sapiens (Human)) | BDBM50229382

((R)-N-(1-(3-(4-cyanophenyl)imidazo[1,2-a]pyrimidin...)Show SMILES CCS(=O)(=O)CCN([C@H](C)c1nc2ncccn2c1-c1ccc(cc1)C#N)C(=O)Cc1ccc(F)c(c1)C(F)(F)F Show InChI InChI=1S/C28H25F4N5O3S/c1-3-41(39,40)14-13-36(24(38)16-20-7-10-23(29)22(15-20)28(30,31)32)18(2)25-26(21-8-5-19(17-33)6-9-21)37-12-4-11-34-27(37)35-25/h4-12,15,18H,3,13-14,16H2,1-2H3/t18-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 643 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Antagonist activity at CXCR3 assessed as ITAC-mediated migration of human PBMC in presence of 100% human plasma |
Bioorg Med Chem Lett 18: 688-93 (2008)
Article DOI: 10.1016/j.bmcl.2007.11.060
BindingDB Entry DOI: 10.7270/Q2WQ03HW |
More data for this
Ligand-Target Pair | |