 Found 12 hits in this display
Found 12 hits in this display Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Isocitrate dehydrogenase [NADP] cytoplasmic [R132H]
(Homo sapiens (Human)) | BDBM116236
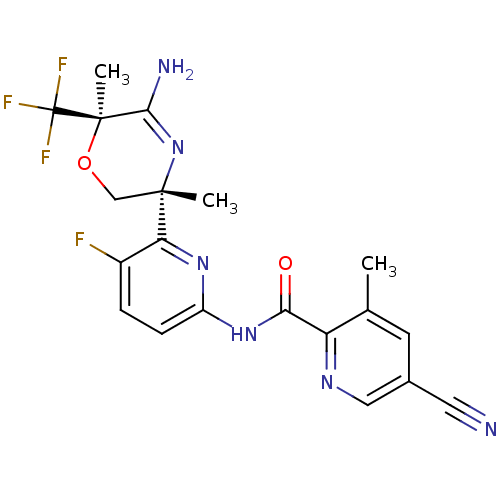
(US10035794, Example 11 | US10683287, Example 11 | ...)Show SMILES Cc1cc(cnc1C(=O)Nc1ccc(F)c(n1)[C@]1(C)CO[C@](C)(C(N)=N1)C(F)(F)F)C#N |r,c:26| Show InChI InChI=1S/C20H18F4N6O2/c1-10-6-11(7-25)8-27-14(10)16(31)29-13-5-4-12(21)15(28-13)18(2)9-32-19(3,17(26)30-18)20(22,23)24/h4-6,8H,9H2,1-3H3,(H2,26,30)(H,28,29,31)/t18-,19+/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis AG
US Patent
| Assay Description
Chinese hamster ovary cells are transfected with the human gene for amyloid precursor protein. The cells are plated at a density of 8000 cells/well i... |
US Patent US10035794 (2018)
BindingDB Entry DOI: 10.7270/Q2RB76M3 |
More data for this
Ligand-Target Pair | |
Amyloid-beta precursor protein
(Homo sapiens (Human)) | BDBM116236

(US10035794, Example 11 | US10683287, Example 11 | ...)Show SMILES Cc1cc(cnc1C(=O)Nc1ccc(F)c(n1)[C@]1(C)CO[C@](C)(C(N)=N1)C(F)(F)F)C#N |r,c:26| Show InChI InChI=1S/C20H18F4N6O2/c1-10-6-11(7-25)8-27-14(10)16(31)29-13-5-4-12(21)15(28-13)18(2)9-32-19(3,17(26)30-18)20(22,23)24/h4-6,8H,9H2,1-3H3,(H2,26,30)(H,28,29,31)/t18-,19+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of wild type APP751 (unknown origin) expressed in CHO cells |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01300
BindingDB Entry DOI: 10.7270/Q27D302Z |
More data for this
Ligand-Target Pair | |
Beta-secretase 1
(Homo sapiens (Human)) | BDBM116236

(US10035794, Example 11 | US10683287, Example 11 | ...)Show SMILES Cc1cc(cnc1C(=O)Nc1ccc(F)c(n1)[C@]1(C)CO[C@](C)(C(N)=N1)C(F)(F)F)C#N |r,c:26| Show InChI InChI=1S/C20H18F4N6O2/c1-10-6-11(7-25)8-27-14(10)16(31)29-13-5-4-12(21)15(28-13)18(2)9-32-19(3,17(26)30-18)20(22,23)24/h4-6,8H,9H2,1-3H3,(H2,26,30)(H,28,29,31)/t18-,19+/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | 4.5 | 25 |
Novartis AG
US Patent
| Assay Description
Recombinant BACE-1 (extracellular domain, expressed in baculovirus and purified using standard methods) at 0.1 to 10 nM concentrations is incubated w... |
US Patent US10035794 (2018)
BindingDB Entry DOI: 10.7270/Q2RB76M3 |
More data for this
Ligand-Target Pair | |
Beta-secretase 1
(Homo sapiens (Human)) | BDBM116236

(US10035794, Example 11 | US10683287, Example 11 | ...)Show SMILES Cc1cc(cnc1C(=O)Nc1ccc(F)c(n1)[C@]1(C)CO[C@](C)(C(N)=N1)C(F)(F)F)C#N |r,c:26| Show InChI InChI=1S/C20H18F4N6O2/c1-10-6-11(7-25)8-27-14(10)16(31)29-13-5-4-12(21)15(28-13)18(2)9-32-19(3,17(26)30-18)20(22,23)24/h4-6,8H,9H2,1-3H3,(H2,26,30)(H,28,29,31)/t18-,19+/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | 4.5 | 25 |
Novartis AG
US Patent
| Assay Description
Recombinant BACE-1 (extracellular domain, expressed in baculovirus and purified using standard methods) at 0.1 to 10 nM concentrations is incubated w... |
US Patent US8637508 (2014)
BindingDB Entry DOI: 10.7270/Q2JD4VFD |
More data for this
Ligand-Target Pair | |
Beta-secretase 1
(Homo sapiens (Human)) | BDBM116236

(US10035794, Example 11 | US10683287, Example 11 | ...)Show SMILES Cc1cc(cnc1C(=O)Nc1ccc(F)c(n1)[C@]1(C)CO[C@](C)(C(N)=N1)C(F)(F)F)C#N |r,c:26| Show InChI InChI=1S/C20H18F4N6O2/c1-10-6-11(7-25)8-27-14(10)16(31)29-13-5-4-12(21)15(28-13)18(2)9-32-19(3,17(26)30-18)20(22,23)24/h4-6,8H,9H2,1-3H3,(H2,26,30)(H,28,29,31)/t18-,19+/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis AG
US Patent
| Assay Description
Recombinant BACE-1 (extracellular domain, expressed in baculovirus and purified using standard methods) at 0.1 to 10 nM concentrations is incubated w... |
US Patent US10683287 (2020)
BindingDB Entry DOI: 10.7270/Q2DZ0CCG |
More data for this
Ligand-Target Pair | |
Beta-secretase 1
(Homo sapiens (Human)) | BDBM116236

(US10035794, Example 11 | US10683287, Example 11 | ...)Show SMILES Cc1cc(cnc1C(=O)Nc1ccc(F)c(n1)[C@]1(C)CO[C@](C)(C(N)=N1)C(F)(F)F)C#N |r,c:26| Show InChI InChI=1S/C20H18F4N6O2/c1-10-6-11(7-25)8-27-14(10)16(31)29-13-5-4-12(21)15(28-13)18(2)9-32-19(3,17(26)30-18)20(22,23)24/h4-6,8H,9H2,1-3H3,(H2,26,30)(H,28,29,31)/t18-,19+/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human recombinant BACE1 catalytic domain using FRET substrate with BACE-cleavable sequence |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01300
BindingDB Entry DOI: 10.7270/Q27D302Z |
More data for this
Ligand-Target Pair | |
Beta-secretase 2
(Homo sapiens (Human)) | BDBM116236

(US10035794, Example 11 | US10683287, Example 11 | ...)Show SMILES Cc1cc(cnc1C(=O)Nc1ccc(F)c(n1)[C@]1(C)CO[C@](C)(C(N)=N1)C(F)(F)F)C#N |r,c:26| Show InChI InChI=1S/C20H18F4N6O2/c1-10-6-11(7-25)8-27-14(10)16(31)29-13-5-4-12(21)15(28-13)18(2)9-32-19(3,17(26)30-18)20(22,23)24/h4-6,8H,9H2,1-3H3,(H2,26,30)(H,28,29,31)/t18-,19+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human recombinant BACE2 catalytic domain using FRET substrate with BACE-cleavable sequence |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01300
BindingDB Entry DOI: 10.7270/Q27D302Z |
More data for this
Ligand-Target Pair | |
Beta-secretase 2
(Homo sapiens (Human)) | BDBM116236

(US10035794, Example 11 | US10683287, Example 11 | ...)Show SMILES Cc1cc(cnc1C(=O)Nc1ccc(F)c(n1)[C@]1(C)CO[C@](C)(C(N)=N1)C(F)(F)F)C#N |r,c:26| Show InChI InChI=1S/C20H18F4N6O2/c1-10-6-11(7-25)8-27-14(10)16(31)29-13-5-4-12(21)15(28-13)18(2)9-32-19(3,17(26)30-18)20(22,23)24/h4-6,8H,9H2,1-3H3,(H2,26,30)(H,28,29,31)/t18-,19+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | 4.5 | 25 |
Novartis AG
US Patent
| Assay Description
Recombinant BACE-2 (extracellular domain, expressed in baculovirus and purified using standard methods) at 0.1 to 10 nM concentrations is incubated w... |
US Patent US10035794 (2018)
BindingDB Entry DOI: 10.7270/Q2RB76M3 |
More data for this
Ligand-Target Pair | |
Beta-secretase 2
(Homo sapiens (Human)) | BDBM116236

(US10035794, Example 11 | US10683287, Example 11 | ...)Show SMILES Cc1cc(cnc1C(=O)Nc1ccc(F)c(n1)[C@]1(C)CO[C@](C)(C(N)=N1)C(F)(F)F)C#N |r,c:26| Show InChI InChI=1S/C20H18F4N6O2/c1-10-6-11(7-25)8-27-14(10)16(31)29-13-5-4-12(21)15(28-13)18(2)9-32-19(3,17(26)30-18)20(22,23)24/h4-6,8H,9H2,1-3H3,(H2,26,30)(H,28,29,31)/t18-,19+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis AG
US Patent
| Assay Description
Recombinant BACE-2 (extracellular domain, expressed in baculovirus and purified using standard methods) at 0.1 to 10 nM concentrations is incubated w... |
US Patent US10683287 (2020)
BindingDB Entry DOI: 10.7270/Q2DZ0CCG |
More data for this
Ligand-Target Pair | |
Beta-secretase 2
(Homo sapiens (Human)) | BDBM116236

(US10035794, Example 11 | US10683287, Example 11 | ...)Show SMILES Cc1cc(cnc1C(=O)Nc1ccc(F)c(n1)[C@]1(C)CO[C@](C)(C(N)=N1)C(F)(F)F)C#N |r,c:26| Show InChI InChI=1S/C20H18F4N6O2/c1-10-6-11(7-25)8-27-14(10)16(31)29-13-5-4-12(21)15(28-13)18(2)9-32-19(3,17(26)30-18)20(22,23)24/h4-6,8H,9H2,1-3H3,(H2,26,30)(H,28,29,31)/t18-,19+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | 4.5 | 25 |
Novartis AG
US Patent
| Assay Description
Recombinant BACE-2 (extracellular domain, expressed in baculovirus and purified using standard methods) at 0.1 to 10 nM concentrations is incubated w... |
US Patent US8637508 (2014)
BindingDB Entry DOI: 10.7270/Q2JD4VFD |
More data for this
Ligand-Target Pair | |
Beta-secretase 1
(Homo sapiens (Human)) | BDBM116236

(US10035794, Example 11 | US10683287, Example 11 | ...)Show SMILES Cc1cc(cnc1C(=O)Nc1ccc(F)c(n1)[C@]1(C)CO[C@](C)(C(N)=N1)C(F)(F)F)C#N |r,c:26| Show InChI InChI=1S/C20H18F4N6O2/c1-10-6-11(7-25)8-27-14(10)16(31)29-13-5-4-12(21)15(28-13)18(2)9-32-19(3,17(26)30-18)20(22,23)24/h4-6,8H,9H2,1-3H3,(H2,26,30)(H,28,29,31)/t18-,19+/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 77 | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human BACE1 extracellular domain expressed in baculovirus using fluorescence-quenched peptide substrate derived from APP se... |
Bioorg Med Chem Lett 24: 2033-45 (2014)
Article DOI: 10.1016/j.bmcl.2014.03.025
BindingDB Entry DOI: 10.7270/Q2H41T0Z |
More data for this
Ligand-Target Pair | |
Cathepsin D
(Homo sapiens (Human)) | BDBM116236

(US10035794, Example 11 | US10683287, Example 11 | ...)Show SMILES Cc1cc(cnc1C(=O)Nc1ccc(F)c(n1)[C@]1(C)CO[C@](C)(C(N)=N1)C(F)(F)F)C#N |r,c:26| Show InChI InChI=1S/C20H18F4N6O2/c1-10-6-11(7-25)8-27-14(10)16(31)29-13-5-4-12(21)15(28-13)18(2)9-32-19(3,17(26)30-18)20(22,23)24/h4-6,8H,9H2,1-3H3,(H2,26,30)(H,28,29,31)/t18-,19+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.78E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human Cathepsin D using Mca-GKPILFFRLK(DNP)D-R-NH2 as a substrate |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01300
BindingDB Entry DOI: 10.7270/Q27D302Z |
More data for this
Ligand-Target Pair | |