 Found 11 hits in this display
Found 11 hits in this display Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Prostaglandin G/H synthase 1
(Homo sapiens (Human)) | BDBM50295287
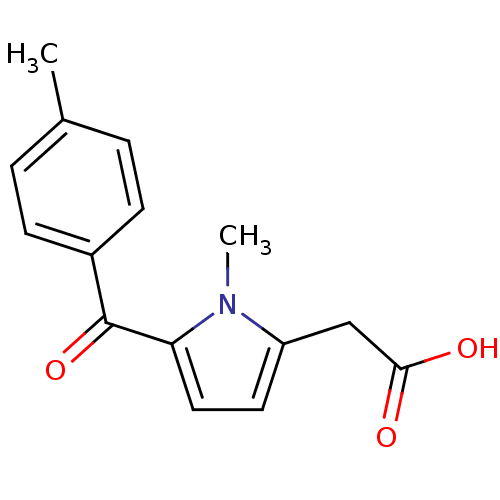
(2-(1-methyl-5-(4-methylbenzoyl)-1H-pyrrol-2-yl)ace...)Show InChI InChI=1S/C15H15NO3/c1-10-3-5-11(6-4-10)15(19)13-8-7-12(16(13)2)9-14(17)18/h3-8H,9H2,1-2H3,(H,17,18) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
| Article
PubMed
| 350 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
St. Bartholomew's and the Royal London School of Medicine and Dentistry
Curated by PDSP Ki Database
| |
Proc Natl Acad Sci U S A 96: 7563-8 (1999)
Article DOI: 10.1073/pnas.96.13.7563
BindingDB Entry DOI: 10.7270/Q21G0JT4 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50295287

(2-(1-methyl-5-(4-methylbenzoyl)-1H-pyrrol-2-yl)ace...)Show InChI InChI=1S/C15H15NO3/c1-10-3-5-11(6-4-10)15(19)13-8-7-12(16(13)2)9-14(17)18/h3-8H,9H2,1-2H3,(H,17,18) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
| DrugBank
Article
PubMed
| 820 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
St. Bartholomew's and the Royal London School of Medicine and Dentistry
Curated by PDSP Ki Database
| |
Proc Natl Acad Sci U S A 96: 7563-8 (1999)
Article DOI: 10.1073/pnas.96.13.7563
BindingDB Entry DOI: 10.7270/Q21G0JT4 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 1
(Homo sapiens (Human)) | BDBM50295287

(2-(1-methyl-5-(4-methylbenzoyl)-1H-pyrrol-2-yl)ace...)Show InChI InChI=1S/C15H15NO3/c1-10-3-5-11(6-4-10)15(19)13-8-7-12(16(13)2)9-14(17)18/h3-8H,9H2,1-2H3,(H,17,18) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
| Article
PubMed
| 1.08E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Dallas Department of Veterans Affairs Medical Center
Curated by PDSP Ki Database
| |
Am J Med 104: 413-21 (1998)
Article DOI: 10.1016/s0002-9343(98)00091-6
BindingDB Entry DOI: 10.7270/Q2F18X97 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50295287

(2-(1-methyl-5-(4-methylbenzoyl)-1H-pyrrol-2-yl)ace...)Show InChI InChI=1S/C15H15NO3/c1-10-3-5-11(6-4-10)15(19)13-8-7-12(16(13)2)9-14(17)18/h3-8H,9H2,1-2H3,(H,17,18) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
| DrugBank
Article
PubMed
| 1.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
St. Bartholomew's and the Royal London School of Medicine and Dentistry
Curated by PDSP Ki Database
| |
Proc Natl Acad Sci U S A 96: 7563-8 (1999)
Article DOI: 10.1073/pnas.96.13.7563
BindingDB Entry DOI: 10.7270/Q21G0JT4 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50295287

(2-(1-methyl-5-(4-methylbenzoyl)-1H-pyrrol-2-yl)ace...)Show InChI InChI=1S/C15H15NO3/c1-10-3-5-11(6-4-10)15(19)13-8-7-12(16(13)2)9-14(17)18/h3-8H,9H2,1-2H3,(H,17,18) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
| DrugBank
Article
PubMed
| 2.25E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Dallas Department of Veterans Affairs Medical Center
Curated by PDSP Ki Database
| |
Am J Med 104: 413-21 (1998)
Article DOI: 10.1016/s0002-9343(98)00091-6
BindingDB Entry DOI: 10.7270/Q2F18X97 |
More data for this
Ligand-Target Pair | |
Lactoylglutathione lyase
(Homo sapiens (Human)) | BDBM50295287

(2-(1-methyl-5-(4-methylbenzoyl)-1H-pyrrol-2-yl)ace...)Show InChI InChI=1S/C15H15NO3/c1-10-3-5-11(6-4-10)15(19)13-8-7-12(16(13)2)9-14(17)18/h3-8H,9H2,1-2H3,(H,17,18) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
| Article
PubMed
| 8.78E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sun Yat-sen University
Curated by ChEMBL
| Assay Description
Inhibition of glyoxalase 1 |
Bioorg Med Chem 19: 1189-96 (2011)
Article DOI: 10.1016/j.bmc.2010.12.039
BindingDB Entry DOI: 10.7270/Q2222V2X |
More data for this
Ligand-Target Pair | |
Lactoylglutathione lyase
(Homo sapiens (Human)) | BDBM50295287

(2-(1-methyl-5-(4-methylbenzoyl)-1H-pyrrol-2-yl)ace...)Show InChI InChI=1S/C15H15NO3/c1-10-3-5-11(6-4-10)15(19)13-8-7-12(16(13)2)9-14(17)18/h3-8H,9H2,1-2H3,(H,17,18) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
| Article
PubMed
| 8.78E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sun Yat-Sen University
Curated by ChEMBL
| Assay Description
Inhibition of human glyoxalase 1 |
Bioorg Med Chem Lett 21: 4243-7 (2011)
Article DOI: 10.1016/j.bmcl.2011.05.095
BindingDB Entry DOI: 10.7270/Q23R0T76 |
More data for this
Ligand-Target Pair | |
Albumin
(Homo sapiens (Human)) | BDBM50295287

(2-(1-methyl-5-(4-methylbenzoyl)-1H-pyrrol-2-yl)ace...)Show InChI InChI=1S/C15H15NO3/c1-10-3-5-11(6-4-10)15(19)13-8-7-12(16(13)2)9-14(17)18/h3-8H,9H2,1-2H3,(H,17,18) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
| Article
PubMed
| 4.16E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Molecular Discovery Ltd.
Curated by ChEMBL
| Assay Description
Binding affinity towards human serum albumin |
J Med Chem 48: 2469-79 (2005)
Article DOI: 10.1021/jm049227l
BindingDB Entry DOI: 10.7270/Q2125WDN |
More data for this
Ligand-Target Pair | |
Lactoylglutathione lyase
(Homo sapiens (Human)) | BDBM50295287

(2-(1-methyl-5-(4-methylbenzoyl)-1H-pyrrol-2-yl)ace...)Show InChI InChI=1S/C15H15NO3/c1-10-3-5-11(6-4-10)15(19)13-8-7-12(16(13)2)9-14(17)18/h3-8H,9H2,1-2H3,(H,17,18) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
| Article
PubMed
| 1.01E+6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sun Yat-sen University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant His-tagged glyoxalase 1 expressed in Escherichia coli BL21 (DE3) preincubated for 20 mins by Dixon plot analysis |
Bioorg Med Chem 19: 1189-96 (2011)
Article DOI: 10.1016/j.bmc.2010.12.039
BindingDB Entry DOI: 10.7270/Q2222V2X |
More data for this
Ligand-Target Pair | |
Interleukin-8
(Homo sapiens (Human)) | BDBM50295287

(2-(1-methyl-5-(4-methylbenzoyl)-1H-pyrrol-2-yl)ace...)Show InChI InChI=1S/C15H15NO3/c1-10-3-5-11(6-4-10)15(19)13-8-7-12(16(13)2)9-14(17)18/h3-8H,9H2,1-2H3,(H,17,18) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
| Article
PubMed
| n/a | n/a | 90 | n/a | n/a | n/a | n/a | n/a | n/a |
Grupo Uriach
Curated by ChEMBL
| Assay Description
Inhibition of CXCL8-induced chemotaxis in human polymorphonuclear leukocyte pretreated for 15 mins measured after 4 hrs by cell migration assay |
Bioorg Med Chem Lett 19: 4026-30 (2009)
Article DOI: 10.1016/j.bmcl.2009.06.027
BindingDB Entry DOI: 10.7270/Q2V124TW |
More data for this
Ligand-Target Pair | |
Aldo-keto reductase family 1 member B1
(Homo sapiens (Human)) | BDBM50295287

(2-(1-methyl-5-(4-methylbenzoyl)-1H-pyrrol-2-yl)ace...)Show InChI InChI=1S/C15H15NO3/c1-10-3-5-11(6-4-10)15(19)13-8-7-12(16(13)2)9-14(17)18/h3-8H,9H2,1-2H3,(H,17,18) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
| PDB
Article
| n/a | n/a | 2.39E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of Homo sapiens (human) aldose reductase |
Citation and Details
Article DOI: 10.1007/s00044-012-0367-5
BindingDB Entry DOI: 10.7270/Q2XP77VN |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |