 Found 4 hits in this display
Found 4 hits in this display Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Dimer of Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM1143
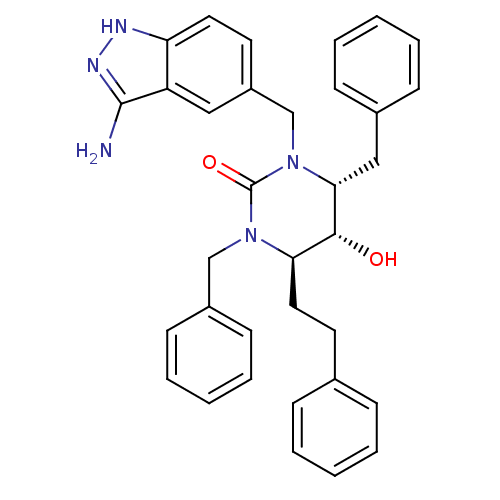
((4R,5R,6R)-1-[(3-amino-1H-indazol-5-yl)methyl]-3,6...)Show SMILES Nc1n[nH]c2ccc(CN3[C@H](Cc4ccccc4)[C@H](O)[C@@H](CCc4ccccc4)N(Cc4ccccc4)C3=O)cc12 |r| Show InChI InChI=1S/C34H35N5O2/c35-33-28-20-27(16-18-29(28)36-37-33)23-39-31(21-25-12-6-2-7-13-25)32(40)30(19-17-24-10-4-1-5-11-24)38(34(39)41)22-26-14-8-3-9-15-26/h1-16,18,20,30-32,40H,17,19,21-23H2,(H3,35,36,37)/t30-,31-,32-/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.280 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
DuPont Pharmaceuticals Company
| Assay Description
Inhibition of HIV protease was measured by assay of the cleavage of a fluorescent peptide substrate. The fluorescent product (2-aminobenzoyl-Ala-Thr-... |
J Med Chem 42: 135-52 (1999)
Article DOI: 10.1021/jm9803626
BindingDB Entry DOI: 10.7270/Q28050S9 |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM1143

((4R,5R,6R)-1-[(3-amino-1H-indazol-5-yl)methyl]-3,6...)Show SMILES Nc1n[nH]c2ccc(CN3[C@H](Cc4ccccc4)[C@H](O)[C@@H](CCc4ccccc4)N(Cc4ccccc4)C3=O)cc12 |r| Show InChI InChI=1S/C34H35N5O2/c35-33-28-20-27(16-18-29(28)36-37-33)23-39-31(21-25-12-6-2-7-13-25)32(40)30(19-17-24-10-4-1-5-11-24)38(34(39)41)22-26-14-8-3-9-15-26/h1-16,18,20,30-32,40H,17,19,21-23H2,(H3,35,36,37)/t30-,31-,32-/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.280 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
DuPont Pharmaceuticals Company
| Assay Description
Inhibition constant of HIV protease inhibitors |
J Med Chem 42: 135-52 (1999)
Article DOI: 10.1021/jm9803626
BindingDB Entry DOI: 10.7270/Q28050S9 |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM1143

((4R,5R,6R)-1-[(3-amino-1H-indazol-5-yl)methyl]-3,6...)Show SMILES Nc1n[nH]c2ccc(CN3[C@H](Cc4ccccc4)[C@H](O)[C@@H](CCc4ccccc4)N(Cc4ccccc4)C3=O)cc12 |r| Show InChI InChI=1S/C34H35N5O2/c35-33-28-20-27(16-18-29(28)36-37-33)23-39-31(21-25-12-6-2-7-13-25)32(40)30(19-17-24-10-4-1-5-11-24)38(34(39)41)22-26-14-8-3-9-15-26/h1-16,18,20,30-32,40H,17,19,21-23H2,(H3,35,36,37)/t30-,31-,32-/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| PubMed
| 0.281 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Florida
Curated by ChEMBL
| Assay Description
Inhibitory activity of compound against HIV-1 aspartyl protease. |
Bioorg Med Chem Lett 12: 3453-7 (2002)
BindingDB Entry DOI: 10.7270/Q2B27WG3 |
More data for this
Ligand-Target Pair | |
Protease
(Human immunodeficiency virus 1 (HIV-1)) | BDBM50488747

(CHEMBL2296973)Show SMILES Nc1n[nH]c2ccc(CN3C(Cc4ccccc4)C(O)C(CCc4ccccc4)N(Cc4ccccc4)C3=O)cc12 Show InChI InChI=1S/C34H35N5O2/c35-33-28-20-27(16-18-29(28)36-37-33)23-39-31(21-25-12-6-2-7-13-25)32(40)30(19-17-24-10-4-1-5-11-24)38(34(39)41)22-26-14-8-3-9-15-26/h1-16,18,20,30-32,40H,17,19,21-23H2,(H3,35,36,37) | PDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
| 0.282 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of Human immunodeficiency virus 1 protease |
Citation and Details
Article DOI: 10.1007/s00044-012-0275-8
BindingDB Entry DOI: 10.7270/Q2R49TP4 |
More data for this
Ligand-Target Pair | |