 Found 8 hits in this display
Found 8 hits in this display Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Bifunctional ligase/repressor BirA
(Staphylococcus aureus) | BDBM161472
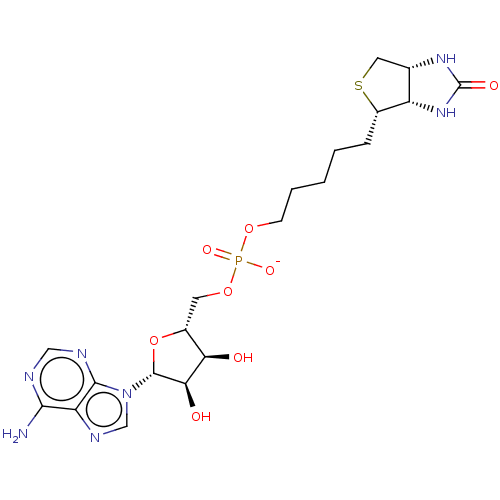
(US9108978, 2.02)Show SMILES Nc1ncnc2n(cnc12)[C@@H]1O[C@H](COP([O-])(=O)OCCCCC[C@@H]2SC[C@@H]3NC(=O)N[C@H]23)[C@@H](O)[C@H]1O |r| Show InChI InChI=1S/C20H30N7O8PS/c21-17-14-18(23-8-22-17)27(9-24-14)19-16(29)15(28)11(35-19)6-34-36(31,32)33-5-3-1-2-4-12-13-10(7-37-12)25-20(30)26-13/h8-13,15-16,19,28-29H,1-7H2,(H,31,32)(H2,21,22,23)(H2,25,26,30)/p-1/t10-,11+,12-,13-,15+,16+,19+/m0/s1 | PDB
KEGG
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| 30 | -44.7 | 203 | n/a | n/a | n/a | n/a | 8.0 | 37 |
Monash University; Adelaide Research & Innovation Pty Ltd
US Patent
| Assay Description
Quantitation of BPL catalysed 3H-biotin incorporation into the biotin domain substrate was performed as previously described by Polyak et al, J. Biol... |
US Patent US9108978 (2015)
BindingDB Entry DOI: 10.7270/Q2T43RVW |
More data for this
Ligand-Target Pair | |
Bifunctional ligase/repressor BirA
(Escherichia coli) | BDBM50039492
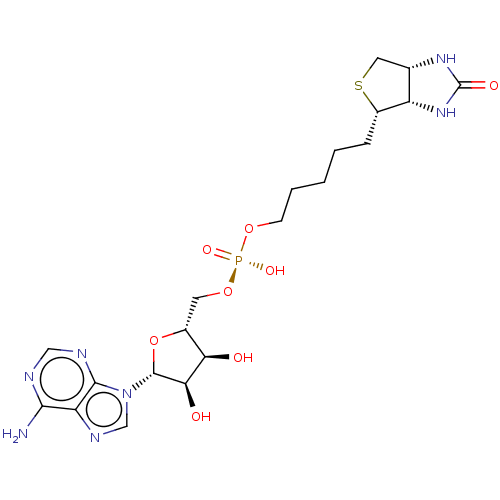
(CHEMBL1231498)Show SMILES Nc1ncnc2n(cnc12)[C@@H]1O[C@H](CO[P@@](O)(=O)OCCCCC[C@@H]2SC[C@@H]3NC(=O)N[C@H]23)[C@@H](O)[C@H]1O |r| Show InChI InChI=1S/C20H30N7O8PS/c21-17-14-18(23-8-22-17)27(9-24-14)19-16(29)15(28)11(35-19)6-34-36(31,32)33-5-3-1-2-4-12-13-10(7-37-12)25-20(30)26-13/h8-13,15-16,19,28-29H,1-7H2,(H,31,32)(H2,21,22,23)(H2,25,26,30)/t10-,11+,12-,13-,15+,16+,19+/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
DrugBank
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| 87 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Adelaide
Curated by ChEMBL
| Assay Description
Inhibition of Escherichia coli recombinant BPL using 3H-biotin as substrate after 10 mins by liquid scintillation counting analysis |
ACS Med Chem Lett 6: 216-20 (2015)
Article DOI: 10.1021/ml500475n
BindingDB Entry DOI: 10.7270/Q2VT1TTQ |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Biotin--protein ligase
(Homo sapiens (Human)) | BDBM50039492

(CHEMBL1231498)Show SMILES Nc1ncnc2n(cnc12)[C@@H]1O[C@H](CO[P@@](O)(=O)OCCCCC[C@@H]2SC[C@@H]3NC(=O)N[C@H]23)[C@@H](O)[C@H]1O |r| Show InChI InChI=1S/C20H30N7O8PS/c21-17-14-18(23-8-22-17)27(9-24-14)19-16(29)15(28)11(35-19)6-34-36(31,32)33-5-3-1-2-4-12-13-10(7-37-12)25-20(30)26-13/h8-13,15-16,19,28-29H,1-7H2,(H,31,32)(H2,21,22,23)(H2,25,26,30)/t10-,11+,12-,13-,15+,16+,19+/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
DrugBank
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 182 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Adelaide
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant BPL using 3H-biotin as substrate after 10 mins by liquid scintillation counting analysis |
ACS Med Chem Lett 6: 216-20 (2015)
Article DOI: 10.1021/ml500475n
BindingDB Entry DOI: 10.7270/Q2VT1TTQ |
More data for this
Ligand-Target Pair | |
Bifunctional ligase/repressor BirA
(Escherichia coli) | BDBM161472

(US9108978, 2.02)Show SMILES Nc1ncnc2n(cnc12)[C@@H]1O[C@H](COP([O-])(=O)OCCCCC[C@@H]2SC[C@@H]3NC(=O)N[C@H]23)[C@@H](O)[C@H]1O |r| Show InChI InChI=1S/C20H30N7O8PS/c21-17-14-18(23-8-22-17)27(9-24-14)19-16(29)15(28)11(35-19)6-34-36(31,32)33-5-3-1-2-4-12-13-10(7-37-12)25-20(30)26-13/h8-13,15-16,19,28-29H,1-7H2,(H,31,32)(H2,21,22,23)(H2,25,26,30)/p-1/t10-,11+,12-,13-,15+,16+,19+/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| 225 | -39.5 | n/a | n/a | n/a | n/a | n/a | 8.0 | 37 |
Monash University; Adelaide Research & Innovation Pty Ltd
US Patent
| Assay Description
Quantitation of BPL catalysed 3H-biotin incorporation into the biotin domain substrate was performed as previously described by Polyak et al, J. Biol... |
US Patent US9108978 (2015)
BindingDB Entry DOI: 10.7270/Q2T43RVW |
More data for this
Ligand-Target Pair | |
Bifunctional ligase/repressor BirA
(Escherichia coli) | BDBM161472

(US9108978, 2.02)Show SMILES Nc1ncnc2n(cnc12)[C@@H]1O[C@H](COP([O-])(=O)OCCCCC[C@@H]2SC[C@@H]3NC(=O)N[C@H]23)[C@@H](O)[C@H]1O |r| Show InChI InChI=1S/C20H30N7O8PS/c21-17-14-18(23-8-22-17)27(9-24-14)19-16(29)15(28)11(35-19)6-34-36(31,32)33-5-3-1-2-4-12-13-10(7-37-12)25-20(30)26-13/h8-13,15-16,19,28-29H,1-7H2,(H,31,32)(H2,21,22,23)(H2,25,26,30)/p-1/t10-,11+,12-,13-,15+,16+,19+/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| 420 | -37.9 | n/a | n/a | n/a | n/a | n/a | 8.0 | 37 |
Monash University; Adelaide Research & Innovation Pty Ltd
US Patent
| Assay Description
Quantitation of BPL catalysed 3H-biotin incorporation into the biotin domain substrate was performed as previously described by Polyak et al, J. Biol... |
US Patent US9108978 (2015)
BindingDB Entry DOI: 10.7270/Q2T43RVW |
More data for this
Ligand-Target Pair | |
Biotin--protein ligase
(Homo sapiens (Human)) | BDBM50039492

(CHEMBL1231498)Show SMILES Nc1ncnc2n(cnc12)[C@@H]1O[C@H](CO[P@@](O)(=O)OCCCCC[C@@H]2SC[C@@H]3NC(=O)N[C@H]23)[C@@H](O)[C@H]1O |r| Show InChI InChI=1S/C20H30N7O8PS/c21-17-14-18(23-8-22-17)27(9-24-14)19-16(29)15(28)11(35-19)6-34-36(31,32)33-5-3-1-2-4-12-13-10(7-37-12)25-20(30)26-13/h8-13,15-16,19,28-29H,1-7H2,(H,31,32)(H2,21,22,23)(H2,25,26,30)/t10-,11+,12-,13-,15+,16+,19+/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
DrugBank
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 7.54E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Nebraska-Lincoln
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant HLCS using P67 as substrate after 2 hrs relative to vehicle-treated control |
Bioorg Med Chem Lett 24: 5568-71 (2014)
Article DOI: 10.1016/j.bmcl.2014.11.010
BindingDB Entry DOI: 10.7270/Q2W097KM |
More data for this
Ligand-Target Pair | |
Biotin--protein ligase
(Homo sapiens (Human)) | BDBM50039492

(CHEMBL1231498)Show SMILES Nc1ncnc2n(cnc12)[C@@H]1O[C@H](CO[P@@](O)(=O)OCCCCC[C@@H]2SC[C@@H]3NC(=O)N[C@H]23)[C@@H](O)[C@H]1O |r| Show InChI InChI=1S/C20H30N7O8PS/c21-17-14-18(23-8-22-17)27(9-24-14)19-16(29)15(28)11(35-19)6-34-36(31,32)33-5-3-1-2-4-12-13-10(7-37-12)25-20(30)26-13/h8-13,15-16,19,28-29H,1-7H2,(H,31,32)(H2,21,22,23)(H2,25,26,30)/t10-,11+,12-,13-,15+,16+,19+/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
DrugBank
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 8.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Nebraska-Lincoln
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant HLCS using P67 as substrate after 2 hrs relative to vehicle-treated control |
Bioorg Med Chem Lett 24: 5568-71 (2014)
Article DOI: 10.1016/j.bmcl.2014.11.010
BindingDB Entry DOI: 10.7270/Q2W097KM |
More data for this
Ligand-Target Pair | |
Bifunctional ligase/repressor BirA
(Escherichia coli) | BDBM50039492

(CHEMBL1231498)Show SMILES Nc1ncnc2n(cnc12)[C@@H]1O[C@H](CO[P@@](O)(=O)OCCCCC[C@@H]2SC[C@@H]3NC(=O)N[C@H]23)[C@@H](O)[C@H]1O |r| Show InChI InChI=1S/C20H30N7O8PS/c21-17-14-18(23-8-22-17)27(9-24-14)19-16(29)15(28)11(35-19)6-34-36(31,32)33-5-3-1-2-4-12-13-10(7-37-12)25-20(30)26-13/h8-13,15-16,19,28-29H,1-7H2,(H,31,32)(H2,21,22,23)(H2,25,26,30)/t10-,11+,12-,13-,15+,16+,19+/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
DrugBank
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| n/a | n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a |
University of Nebraska-Lincoln
Curated by ChEMBL
| Assay Description
Binding affinity to Escherichia coli biotin protein ligase |
Bioorg Med Chem Lett 24: 5568-71 (2014)
Article DOI: 10.1016/j.bmcl.2014.11.010
BindingDB Entry DOI: 10.7270/Q2W097KM |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |