 Found 5 hits in this display
Found 5 hits in this display Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
RNA-directed RNA polymerase
(Hepatitis C virus) | BDBM50181935
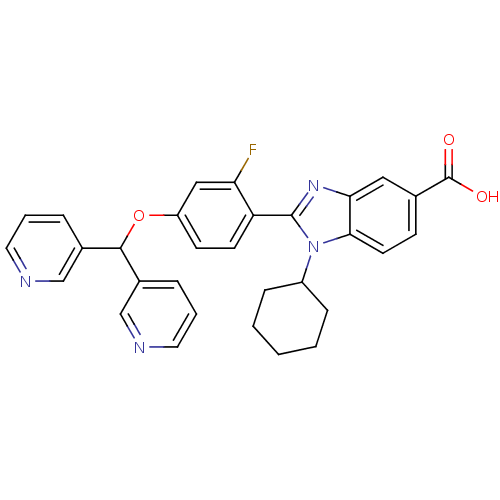
(1-Cyclohexyl-2-[4-(di-pyridin-3-yl-methoxy)-2-fluo...)Show SMILES OC(=O)c1ccc2n(C3CCCCC3)c(nc2c1)-c1ccc(OC(c2cccnc2)c2cccnc2)cc1F Show InChI InChI=1S/C31H27FN4O3/c32-26-17-24(39-29(21-6-4-14-33-18-21)22-7-5-15-34-19-22)11-12-25(26)30-35-27-16-20(31(37)38)10-13-28(27)36(30)23-8-2-1-3-9-23/h4-7,10-19,23,29H,1-3,8-9H2,(H,37,38) | PDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Central Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibitory activity against HCV 1b NS5B RNA dependent RNA polymerase |
Bioorg Med Chem Lett 16: 1859-63 (2006)
Article DOI: 10.1016/j.bmcl.2006.01.032
BindingDB Entry DOI: 10.7270/Q2BK1BX6 |
More data for this
Ligand-Target Pair | |
RNA-directed RNA polymerase
(Hepatitis C virus) | BDBM50181935

(1-Cyclohexyl-2-[4-(di-pyridin-3-yl-methoxy)-2-fluo...)Show SMILES OC(=O)c1ccc2n(C3CCCCC3)c(nc2c1)-c1ccc(OC(c2cccnc2)c2cccnc2)cc1F Show InChI InChI=1S/C31H27FN4O3/c32-26-17-24(39-29(21-6-4-14-33-18-21)22-7-5-15-34-19-22)11-12-25(26)30-35-27-16-20(31(37)38)10-13-28(27)36(30)23-8-2-1-3-9-23/h4-7,10-19,23,29H,1-3,8-9H2,(H,37,38) | PDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Central Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibitory activity against HCV 1a NS5B RNA dependent RNA polymerase |
Bioorg Med Chem Lett 16: 1859-63 (2006)
Article DOI: 10.1016/j.bmcl.2006.01.032
BindingDB Entry DOI: 10.7270/Q2BK1BX6 |
More data for this
Ligand-Target Pair | |
DNA polymerase beta
(Homo sapiens (Human)) | BDBM50181935

(1-Cyclohexyl-2-[4-(di-pyridin-3-yl-methoxy)-2-fluo...)Show SMILES OC(=O)c1ccc2n(C3CCCCC3)c(nc2c1)-c1ccc(OC(c2cccnc2)c2cccnc2)cc1F Show InChI InChI=1S/C31H27FN4O3/c32-26-17-24(39-29(21-6-4-14-33-18-21)22-7-5-15-34-19-22)11-12-25(26)30-35-27-16-20(31(37)38)10-13-28(27)36(30)23-8-2-1-3-9-23/h4-7,10-19,23,29H,1-3,8-9H2,(H,37,38) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Central Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibitory activity against DNA polymerase beta |
Bioorg Med Chem Lett 16: 1859-63 (2006)
Article DOI: 10.1016/j.bmcl.2006.01.032
BindingDB Entry DOI: 10.7270/Q2BK1BX6 |
More data for this
Ligand-Target Pair | |
DNA polymerase alpha catalytic subunit
(Homo sapiens (Human)) | BDBM50181935

(1-Cyclohexyl-2-[4-(di-pyridin-3-yl-methoxy)-2-fluo...)Show SMILES OC(=O)c1ccc2n(C3CCCCC3)c(nc2c1)-c1ccc(OC(c2cccnc2)c2cccnc2)cc1F Show InChI InChI=1S/C31H27FN4O3/c32-26-17-24(39-29(21-6-4-14-33-18-21)22-7-5-15-34-19-22)11-12-25(26)30-35-27-16-20(31(37)38)10-13-28(27)36(30)23-8-2-1-3-9-23/h4-7,10-19,23,29H,1-3,8-9H2,(H,37,38) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Central Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibitory activity against DNA polymerase alpha |
Bioorg Med Chem Lett 16: 1859-63 (2006)
Article DOI: 10.1016/j.bmcl.2006.01.032
BindingDB Entry DOI: 10.7270/Q2BK1BX6 |
More data for this
Ligand-Target Pair | |
RNA-directed RNA polymerase
(Hepatitis C virus) | BDBM50181935

(1-Cyclohexyl-2-[4-(di-pyridin-3-yl-methoxy)-2-fluo...)Show SMILES OC(=O)c1ccc2n(C3CCCCC3)c(nc2c1)-c1ccc(OC(c2cccnc2)c2cccnc2)cc1F Show InChI InChI=1S/C31H27FN4O3/c32-26-17-24(39-29(21-6-4-14-33-18-21)22-7-5-15-34-19-22)11-12-25(26)30-35-27-16-20(31(37)38)10-13-28(27)36(30)23-8-2-1-3-9-23/h4-7,10-19,23,29H,1-3,8-9H2,(H,37,38) | PDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 2.60E+3 | n/a | n/a | n/a | n/a |
Central Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of HCV RNA replication in Huh5-2 cells after 48 hrs |
Bioorg Med Chem Lett 16: 1859-63 (2006)
Article DOI: 10.1016/j.bmcl.2006.01.032
BindingDB Entry DOI: 10.7270/Q2BK1BX6 |
More data for this
Ligand-Target Pair | |