

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Ataxin-2 (Homo sapiens (Human)) | BDBM258501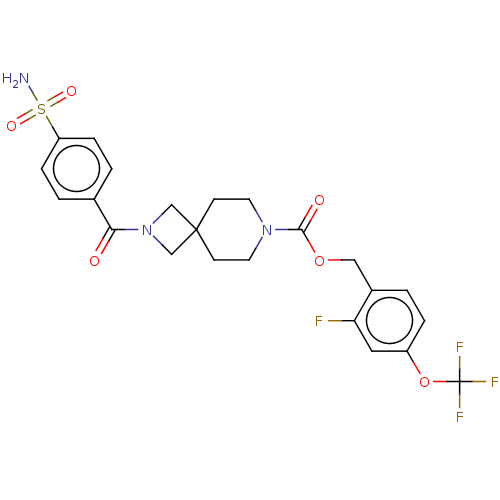 (US10633384, Example 1.23 | US9493486, 1.23) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc. US Patent | Assay Description ATX inhibition was measured by a fluorescence quenching assay using a specifically labeled substrate analogue (MR121 substrate). To obtain this MR121... | US Patent US10633384 (2020) BindingDB Entry DOI: 10.7270/Q2K077BX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ataxin-2 (Homo sapiens (Human)) | BDBM258574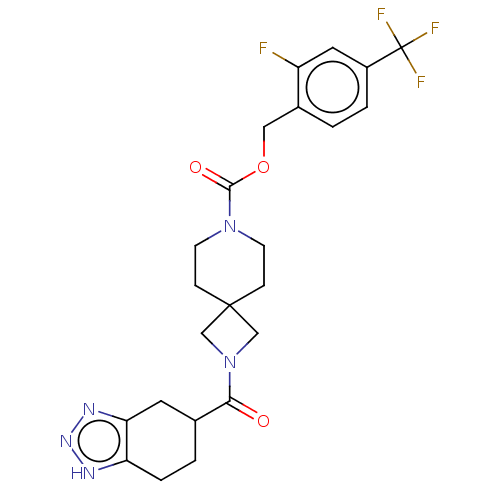 (US10633384, Example 12.36 | US9493486, 12.36) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc. US Patent | Assay Description ATX inhibition was measured by a fluorescence quenching assay using a specifically labeled substrate analogue (MR121 substrate). To obtain this MR121... | US Patent US10633384 (2020) BindingDB Entry DOI: 10.7270/Q2K077BX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ataxin-2 (Homo sapiens (Human)) | BDBM258575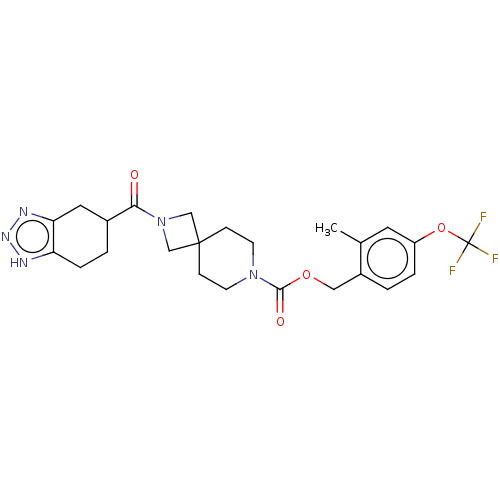 (US10633384, Example 12.37 | US9493486, 12.37) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc. US Patent | Assay Description ATX inhibition was measured by a fluorescence quenching assay using a specifically labeled substrate analogue (MR121 substrate). To obtain this MR121... | US Patent US10633384 (2020) BindingDB Entry DOI: 10.7270/Q2K077BX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ataxin-2 (Homo sapiens (Human)) | BDBM258580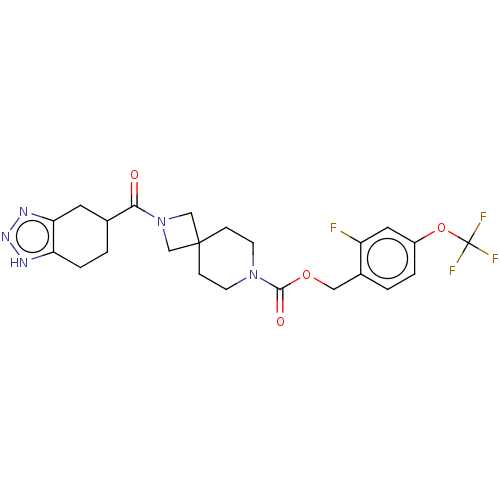 (US10633384, Example 12.42 | US9493486, 12.42) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc. US Patent | Assay Description ATX inhibition was measured by a fluorescence quenching assay using a specifically labeled substrate analogue (MR121 substrate). To obtain this MR121... | US Patent US10633384 (2020) BindingDB Entry DOI: 10.7270/Q2K077BX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ataxin-2 (Homo sapiens (Human)) | BDBM258581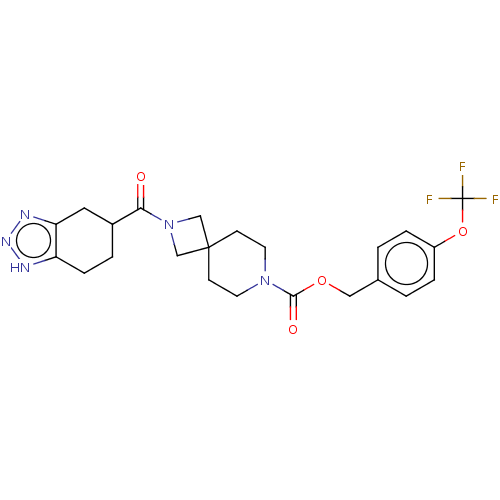 (US10633384, Example 12.43 | US9493486, 12.43) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc. US Patent | Assay Description ATX inhibition was measured by a fluorescence quenching assay using a specifically labeled substrate analogue (MR121 substrate). To obtain this MR121... | US Patent US10633384 (2020) BindingDB Entry DOI: 10.7270/Q2K077BX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ataxin-2 (Homo sapiens (Human)) | BDBM258585 (US10633384, 13.01 | US9493486, 13.01) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc. US Patent | Assay Description ATX inhibition was measured by a fluorescence quenching assay using a specifically labeled substrate analogue (MR121 substrate). To obtain this MR121... | US Patent US10633384 (2020) BindingDB Entry DOI: 10.7270/Q2K077BX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ataxin-2 (Homo sapiens (Human)) | BDBM440743 ((?)-2-Fluoro-4-(trifluoromethoxy)benzyl 2-(4,5,6,7...) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc. US Patent | Assay Description ATX inhibition was measured by a fluorescence quenching assay using a specifically labeled substrate analogue (MR121 substrate). To obtain this MR121... | US Patent US10633384 (2020) BindingDB Entry DOI: 10.7270/Q2K077BX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ataxin-2 (Homo sapiens (Human)) | BDBM442737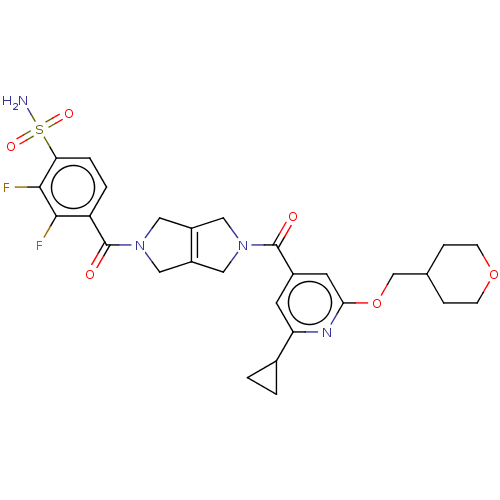 (4-[5-[2-cyclopyropyl-6-[(1- | US10647719, Example ...) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc. US Patent | Assay Description Human carbonic anhydrase II (hCA-II) inhibition was measured by an absorbance method using 4-nitrophenyl acetate (4-NPA) as its substrate. 4-NPA can ... | US Patent US10647719 (2020) BindingDB Entry DOI: 10.7270/Q2V98C4V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ataxin-2 (Homo sapiens (Human)) | BDBM442707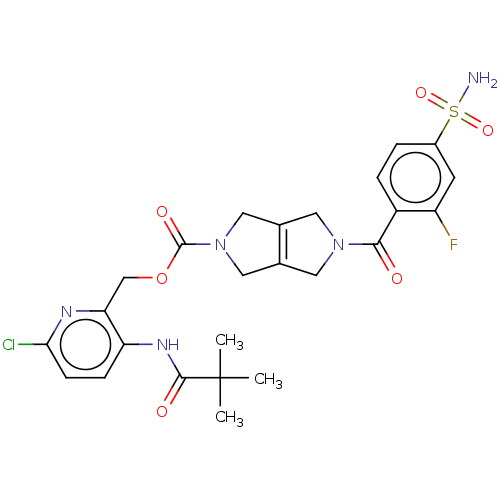 (US10647719, Example 1.18 | [6-chloro-3-(2,2-) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc. US Patent | Assay Description Human carbonic anhydrase II (hCA-II) inhibition was measured by an absorbance method using 4-nitrophenyl acetate (4-NPA) as its substrate. 4-NPA can ... | US Patent US10647719 (2020) BindingDB Entry DOI: 10.7270/Q2V98C4V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ataxin-2 (Homo sapiens (Human)) | BDBM442712 (US10647719, Example 1.23 | [6-chloro-3-(2,2-) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc. US Patent | Assay Description Human carbonic anhydrase II (hCA-II) inhibition was measured by an absorbance method using 4-nitrophenyl acetate (4-NPA) as its substrate. 4-NPA can ... | US Patent US10647719 (2020) BindingDB Entry DOI: 10.7270/Q2V98C4V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ataxin-2 (Homo sapiens (Human)) | BDBM442717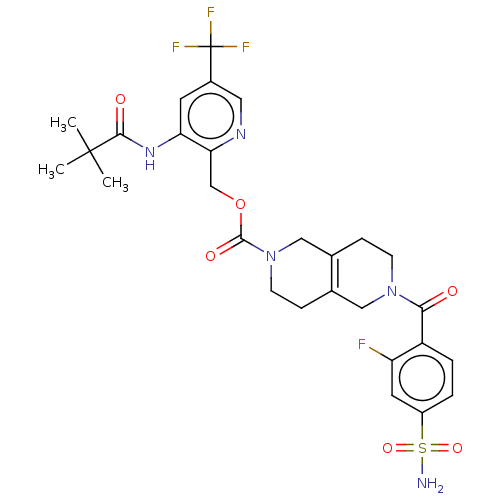 (BDBM442732 | US10647719, Example 1.28 | [3-(2,2-di...) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc. US Patent | Assay Description Human carbonic anhydrase II (hCA-II) inhibition was measured by an absorbance method using 4-nitrophenyl acetate (4-NPA) as its substrate. 4-NPA can ... | US Patent US10647719 (2020) BindingDB Entry DOI: 10.7270/Q2V98C4V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ataxin-2 (Homo sapiens (Human)) | BDBM442718 (US10647719, Example 2.00) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc. US Patent | Assay Description Human carbonic anhydrase II (hCA-II) inhibition was measured by an absorbance method using 4-nitrophenyl acetate (4-NPA) as its substrate. 4-NPA can ... | US Patent US10647719 (2020) BindingDB Entry DOI: 10.7270/Q2V98C4V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ataxin-2 (Homo sapiens (Human)) | BDBM241106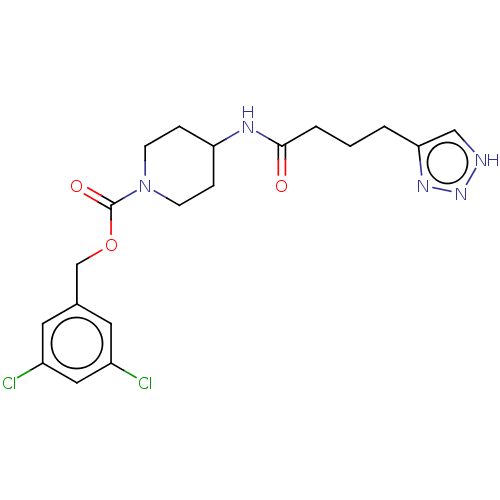 (US9409895, 17 | US9630945, 17) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | US Patent | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis AG US Patent | Assay Description ATX activity was determined by measurement of released choline in reactions containing ATX (10 nM), choline oxidase (0.1 U/ml), HRP (100 U/ml), ample... | US Patent US9630945 (2017) BindingDB Entry DOI: 10.7270/Q25Q4Z5R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ataxin-2 (Homo sapiens (Human)) | BDBM258579 (US10633384, Example 12.41 | US9493486, 12.41) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc. US Patent | Assay Description ATX inhibition was measured by a fluorescence quenching assay using a specifically labeled substrate analogue (MR121 substrate). To obtain this MR121... | US Patent US10633384 (2020) BindingDB Entry DOI: 10.7270/Q2K077BX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ataxin-2 (Homo sapiens (Human)) | BDBM442709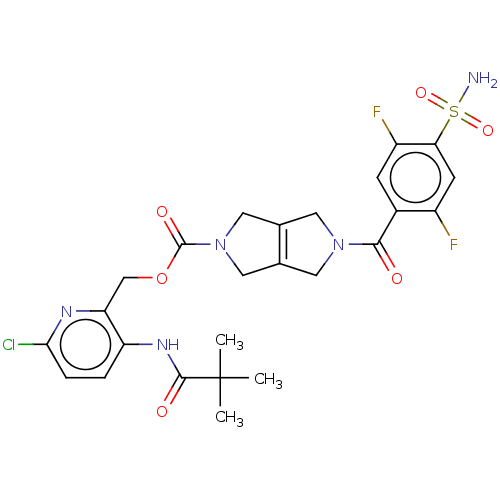 (US10647719, Example 1.20 | [6-chloro-3-(2,2-) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc. US Patent | Assay Description Human carbonic anhydrase II (hCA-II) inhibition was measured by an absorbance method using 4-nitrophenyl acetate (4-NPA) as its substrate. 4-NPA can ... | US Patent US10647719 (2020) BindingDB Entry DOI: 10.7270/Q2V98C4V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ataxin-2 (Homo sapiens (Human)) | BDBM442711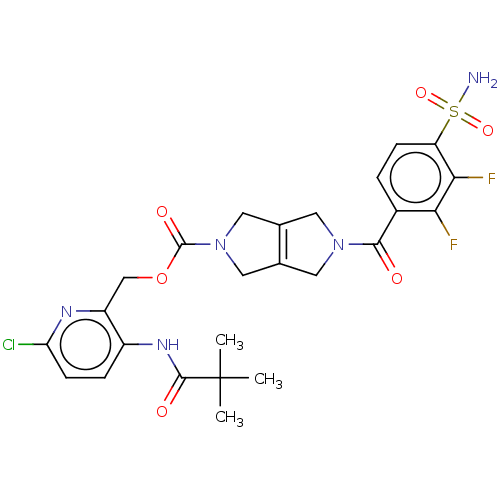 (US10647719, Example 1.22 | [6-chloro-3-(2,2-) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc. US Patent | Assay Description Human carbonic anhydrase II (hCA-II) inhibition was measured by an absorbance method using 4-nitrophenyl acetate (4-NPA) as its substrate. 4-NPA can ... | US Patent US10647719 (2020) BindingDB Entry DOI: 10.7270/Q2V98C4V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ataxin-2 (Homo sapiens (Human)) | BDBM323247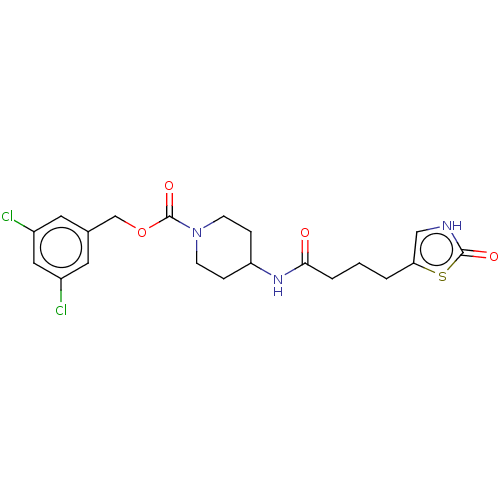 (3,5-Dichlorobenzyl 4-(4-(2-oxo-2,3-dihydrothiazol-...) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis AG US Patent | Assay Description ATX activity was determined by measurement of released choline in reactions containing ATX (10 nM), choline oxidase (0.1 U/ml), HRP (100 U/ml), ample... | US Patent US9630945 (2017) BindingDB Entry DOI: 10.7270/Q25Q4Z5R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ataxin-2 (Homo sapiens (Human)) | BDBM442736 (4-[5-[2-cyclopropyl-6-(oxan-4- | US10647719, Examp...) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc. US Patent | Assay Description Human carbonic anhydrase II (hCA-II) inhibition was measured by an absorbance method using 4-nitrophenyl acetate (4-NPA) as its substrate. 4-NPA can ... | US Patent US10647719 (2020) BindingDB Entry DOI: 10.7270/Q2V98C4V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ataxin-2 (Homo sapiens (Human)) | BDBM442735 (US10647719, Example 1.32 | [3-(2,2-dimethylpropano...) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc. US Patent | Assay Description Human carbonic anhydrase II (hCA-II) inhibition was measured by an absorbance method using 4-nitrophenyl acetate (4-NPA) as its substrate. 4-NPA can ... | US Patent US10647719 (2020) BindingDB Entry DOI: 10.7270/Q2V98C4V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ataxin-2 (Homo sapiens (Human)) | BDBM258576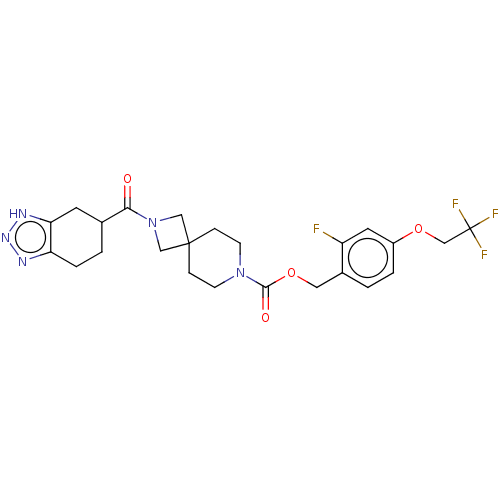 (US10633384, Example 12.38 | US9493486, 12.38) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc. US Patent | Assay Description ATX inhibition was measured by a fluorescence quenching assay using a specifically labeled substrate analogue (MR121 substrate). To obtain this MR121... | US Patent US10633384 (2020) BindingDB Entry DOI: 10.7270/Q2K077BX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ataxin-2 (Homo sapiens (Human)) | BDBM258578 (US10633384, Example 12.40 | US9493486, 12.40) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc. US Patent | Assay Description ATX inhibition was measured by a fluorescence quenching assay using a specifically labeled substrate analogue (MR121 substrate). To obtain this MR121... | US Patent US10633384 (2020) BindingDB Entry DOI: 10.7270/Q2K077BX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ataxin-2 (Homo sapiens (Human)) | BDBM241108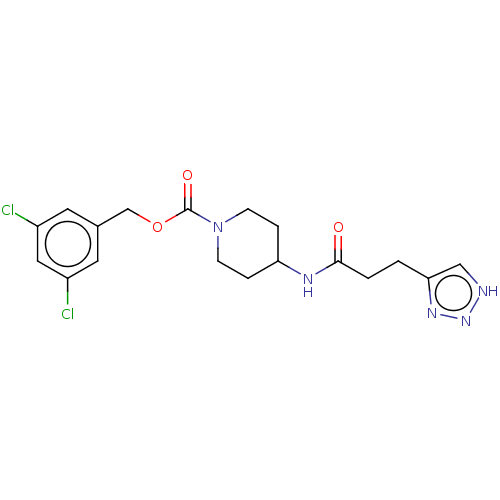 (US9409895, 19 | US9630945, 19) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | US Patent | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis AG US Patent | Assay Description ATX activity was determined by measurement of released choline in reactions containing ATX (10 nM), choline oxidase (0.1 U/ml), HRP (100 U/ml), ample... | US Patent US9630945 (2017) BindingDB Entry DOI: 10.7270/Q25Q4Z5R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ataxin-2 (Homo sapiens (Human)) | BDBM241153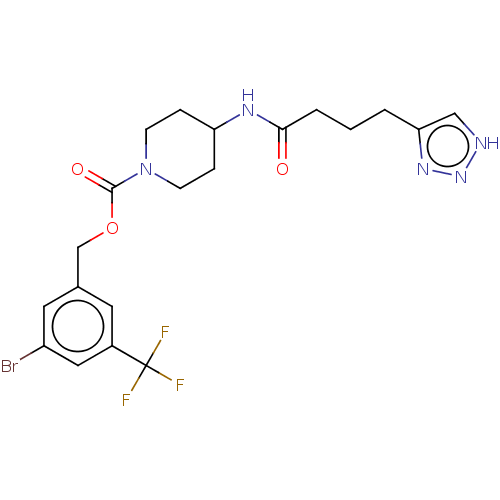 (US9409895, 63 | US9630945, 63) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis AG US Patent | Assay Description ATX activity was determined by measurement of released choline in reactions containing ATX (10 nM), choline oxidase (0.1 U/ml), HRP (100 U/ml), ample... | US Patent US9630945 (2017) BindingDB Entry DOI: 10.7270/Q25Q4Z5R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ataxin-2 (Homo sapiens (Human)) | BDBM352285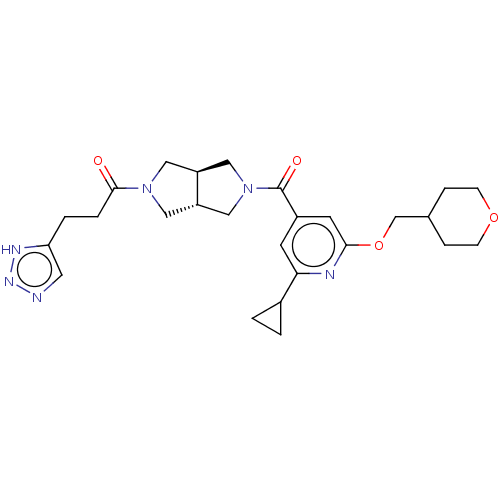 (CHEM-US-00050 | US9802944, Example 1.13) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc. US Patent | Assay Description ATX inhibition was measured by a fluorescence quenching assay using a specifically labeled substrate analogue (MR121 substrate). To obtain this MR121... | US Patent US9802944 (2017) BindingDB Entry DOI: 10.7270/Q2SF2Z9M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ataxin-2 (Homo sapiens (Human)) | BDBM352340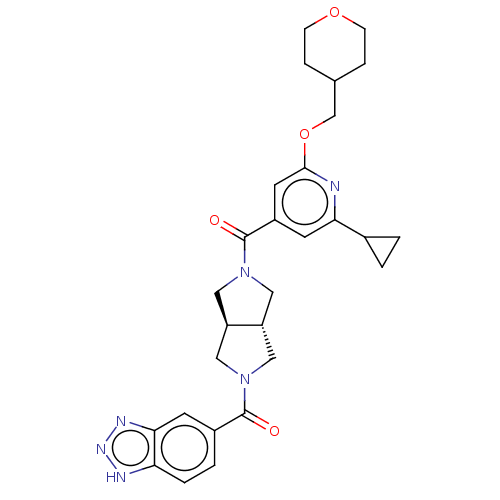 (US11098048, Example 2.01 | US9802944, Example 2.01) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc. US Patent | Assay Description ATX inhibition was measured by a fluorescence quenching assay using a specifically labeled substrate analogue (MR121 substrate). To obtain this MR121... | US Patent US9802944 (2017) BindingDB Entry DOI: 10.7270/Q2SF2Z9M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ataxin-2 (Homo sapiens (Human)) | BDBM352271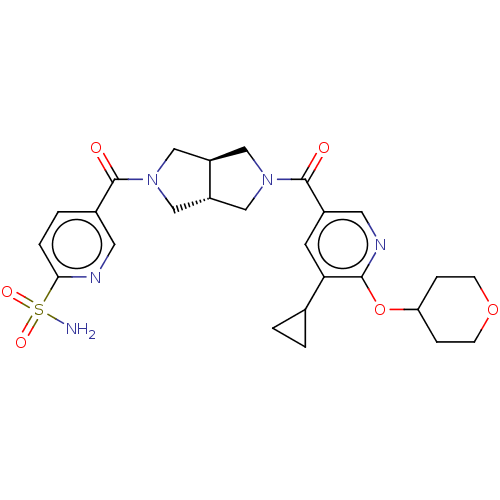 (US9802944, Example 1) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffman-La Roche Inc. US Patent | Assay Description Assay working solutions were made as follows:Assay buffer (50 mM Tris-HCl, 140 mM NaCl, 5 mM KCl, 1 mM CaCl2, 1 mM MgCl2, 0.01% Triton-X-100, pH 8.0;... | US Patent US10654857 (2020) BindingDB Entry DOI: 10.7270/Q2NK3J1K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ataxin-2 (Homo sapiens (Human)) | BDBM352339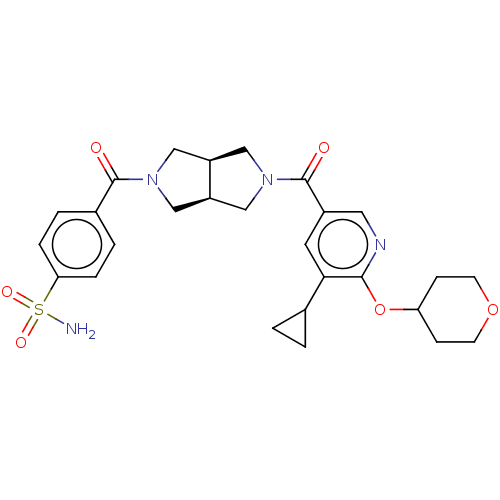 (4-((3aR,6aS)-5-(5-Cyclopropyl-6-(tetrahydro-2H-pyr...) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffman-La Roche Inc. US Patent | Assay Description Assay working solutions were made as follows:Assay buffer (50 mM Tris-HCl, 140 mM NaCl, 5 mM KCl, 1 mM CaCl2, 1 mM MgCl2, 0.01% Triton-X-100, pH 8.0;... | US Patent US10654857 (2020) BindingDB Entry DOI: 10.7270/Q2NK3J1K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ataxin-2 (Homo sapiens (Human)) | BDBM352339 (4-((3aR,6aS)-5-(5-Cyclopropyl-6-(tetrahydro-2H-pyr...) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffman-La Roche Inc. US Patent | Assay Description Assay working solutions were made as follows:Assay buffer (50 mM Tris-HCl, 140 mM NaCl, 5 mM KCl, 1 mM CaCl2, 1 mM MgCl2, 0.01% Triton-X-100, pH 8.0;... | US Patent US10654857 (2020) BindingDB Entry DOI: 10.7270/Q2NK3J1K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ataxin-2 (Homo sapiens (Human)) | BDBM258584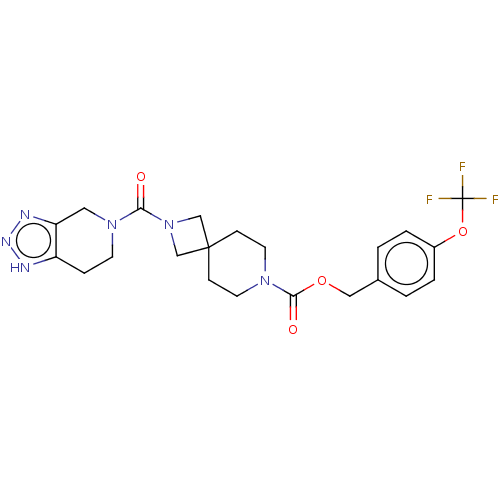 (US10633384, Example 13 | US9493486, 13) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc. US Patent | Assay Description ATX inhibition was measured by a fluorescence quenching assay using a specifically labeled substrate analogue (MR121 substrate). To obtain this MR121... | US Patent US10633384 (2020) BindingDB Entry DOI: 10.7270/Q2K077BX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ataxin-2 (Homo sapiens (Human)) | BDBM442710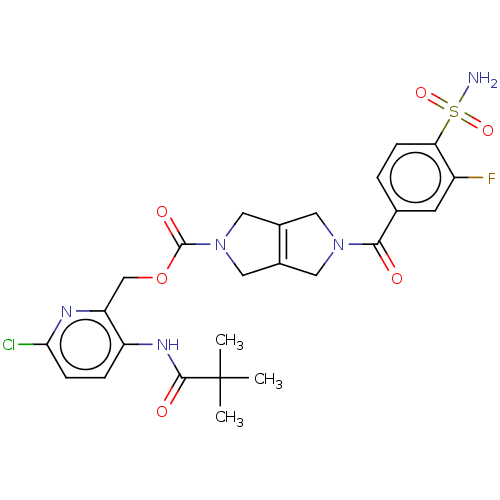 (US10647719, Example 1.21 | [6-chloro-3-(2,2-) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc. US Patent | Assay Description Human carbonic anhydrase II (hCA-II) inhibition was measured by an absorbance method using 4-nitrophenyl acetate (4-NPA) as its substrate. 4-NPA can ... | US Patent US10647719 (2020) BindingDB Entry DOI: 10.7270/Q2V98C4V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ataxin-2 (Homo sapiens (Human)) | BDBM442715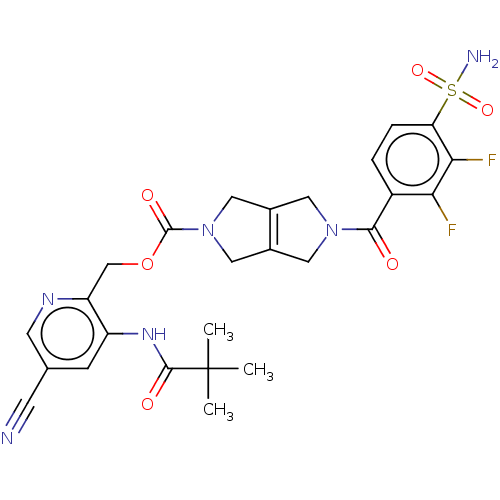 (US10647719, Example 1.26 | [5-cyano-3-(2,2-) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc. US Patent | Assay Description Human carbonic anhydrase II (hCA-II) inhibition was measured by an absorbance method using 4-nitrophenyl acetate (4-NPA) as its substrate. 4-NPA can ... | US Patent US10647719 (2020) BindingDB Entry DOI: 10.7270/Q2V98C4V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ataxin-2 (Homo sapiens (Human)) | BDBM352353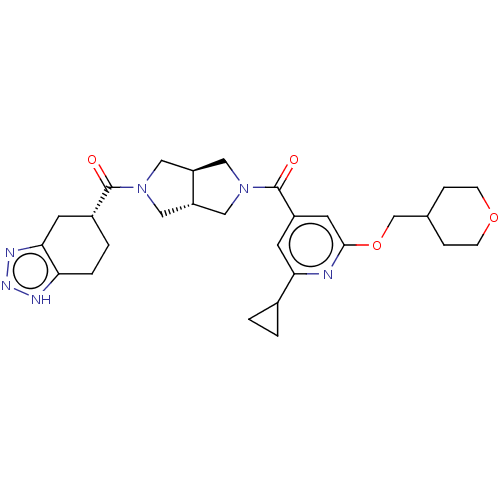 (((3aR,6aR)-hexahydropyrrolo[3,4- | US9802944, Exam...) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc. US Patent | Assay Description ATX inhibition was measured by a fluorescence quenching assay using a specifically labeled substrate analogue (MR121 substrate). To obtain this MR121... | US Patent US9802944 (2017) BindingDB Entry DOI: 10.7270/Q2SF2Z9M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ataxin-2 (Homo sapiens (Human)) | BDBM442741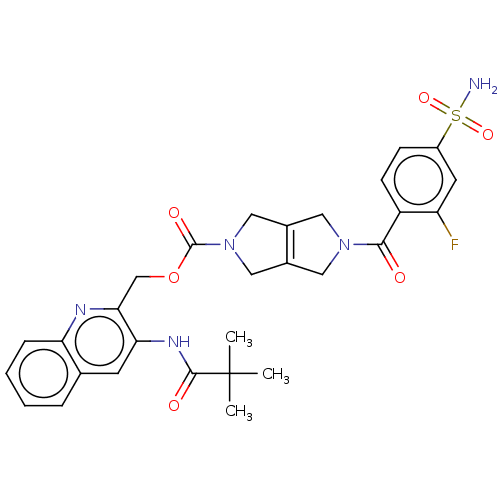 (US10647719, Example 4.01 | [3-(2,2-) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 3.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc. US Patent | Assay Description Human carbonic anhydrase II (hCA-II) inhibition was measured by an absorbance method using 4-nitrophenyl acetate (4-NPA) as its substrate. 4-NPA can ... | US Patent US10647719 (2020) BindingDB Entry DOI: 10.7270/Q2V98C4V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ataxin-2 (Homo sapiens (Human)) | BDBM442740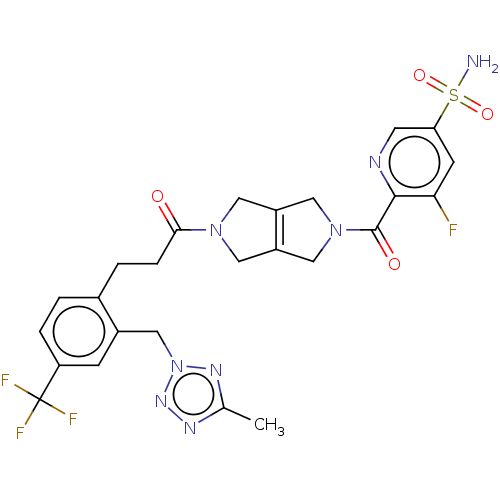 (US10647719, Example 4.00) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 3.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc. US Patent | Assay Description Human carbonic anhydrase II (hCA-II) inhibition was measured by an absorbance method using 4-nitrophenyl acetate (4-NPA) as its substrate. 4-NPA can ... | US Patent US10647719 (2020) BindingDB Entry DOI: 10.7270/Q2V98C4V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ataxin-2 (Homo sapiens (Human)) | BDBM241109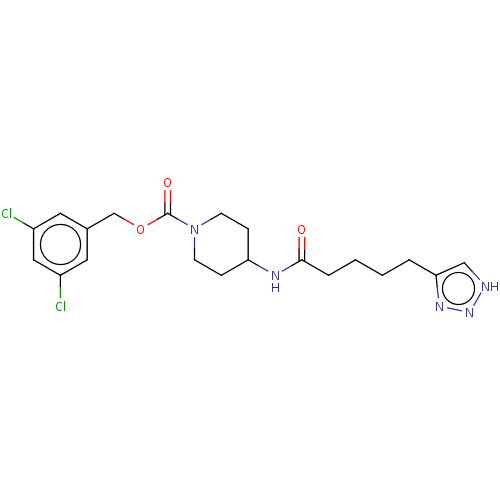 (US9409895, 20 | US9630945, 20) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis AG US Patent | Assay Description ATX activity was determined by measurement of released choline in reactions containing ATX (10 nM), choline oxidase (0.1 U/ml), HRP (100 U/ml), ample... | US Patent US9630945 (2017) BindingDB Entry DOI: 10.7270/Q25Q4Z5R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ataxin-2 (Homo sapiens (Human)) | BDBM241156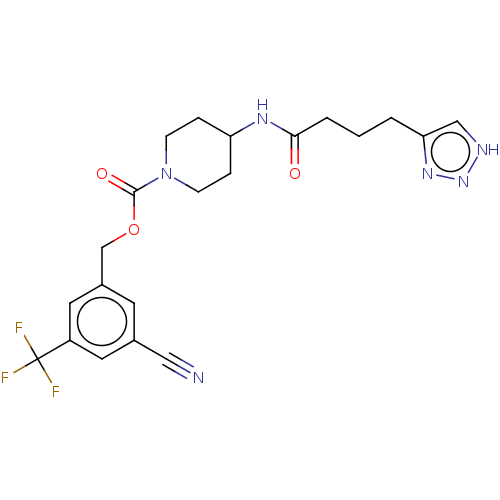 (US9409895, 66 | US9630945, 66) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis AG US Patent | Assay Description ATX activity was determined by measurement of released choline in reactions containing ATX (10 nM), choline oxidase (0.1 U/ml), HRP (100 U/ml), ample... | US Patent US9630945 (2017) BindingDB Entry DOI: 10.7270/Q25Q4Z5R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ataxin-2 (Homo sapiens (Human)) | BDBM352316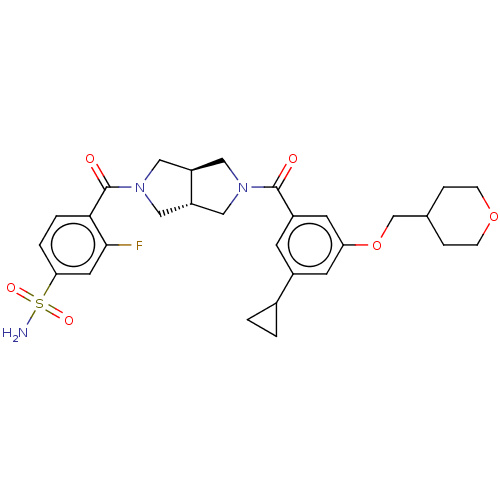 (CHEM-US-00055 | US9802944, Example 1.18) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc. US Patent | Assay Description ATX inhibition was measured by a fluorescence quenching assay using a specifically labeled substrate analogue (MR121 substrate). To obtain this MR121... | US Patent US9802944 (2017) BindingDB Entry DOI: 10.7270/Q2SF2Z9M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ataxin-2 (Homo sapiens (Human)) | BDBM352271 (US9802944, Example 1) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffman-La Roche Inc. US Patent | Assay Description Assay working solutions were made as follows:Assay buffer (50 mM Tris-HCl, 140 mM NaCl, 5 mM KCl, 1 mM CaCl2, 1 mM MgCl2, 0.01% Triton-X-100, pH 8.0;... | US Patent US10654857 (2020) BindingDB Entry DOI: 10.7270/Q2NK3J1K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ataxin-2 (Homo sapiens (Human)) | BDBM442706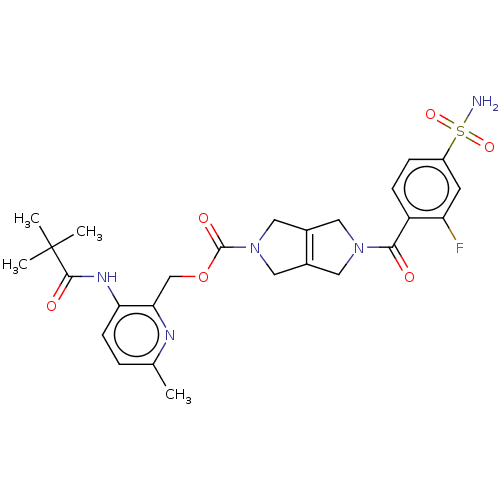 (US10647719, Example 1.17 | [3-(2,2-dimethylpropano...) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc. US Patent | Assay Description Human carbonic anhydrase II (hCA-II) inhibition was measured by an absorbance method using 4-nitrophenyl acetate (4-NPA) as its substrate. 4-NPA can ... | US Patent US10647719 (2020) BindingDB Entry DOI: 10.7270/Q2V98C4V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ataxin-2 (Homo sapiens (Human)) | BDBM442708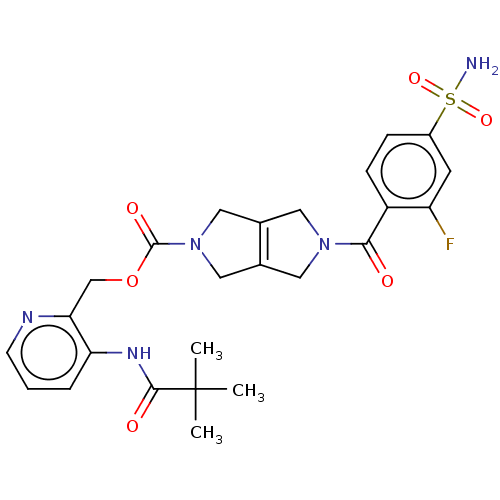 (US10647719, Example 1.19 | [3-(2,2-) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc. US Patent | Assay Description Human carbonic anhydrase II (hCA-II) inhibition was measured by an absorbance method using 4-nitrophenyl acetate (4-NPA) as its substrate. 4-NPA can ... | US Patent US10647719 (2020) BindingDB Entry DOI: 10.7270/Q2V98C4V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ataxin-2 (Homo sapiens (Human)) | BDBM241157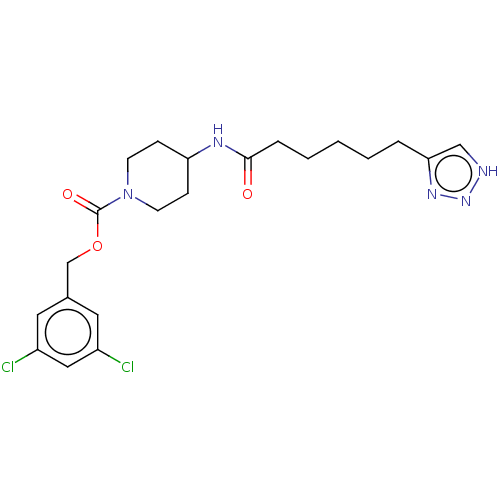 (US9409895, 67 | US9630945, 67) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis AG US Patent | Assay Description ATX activity was determined by measurement of released choline in reactions containing ATX (10 nM), choline oxidase (0.1 U/ml), HRP (100 U/ml), ample... | US Patent US9630945 (2017) BindingDB Entry DOI: 10.7270/Q25Q4Z5R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ataxin-2 (Homo sapiens (Human)) | BDBM442733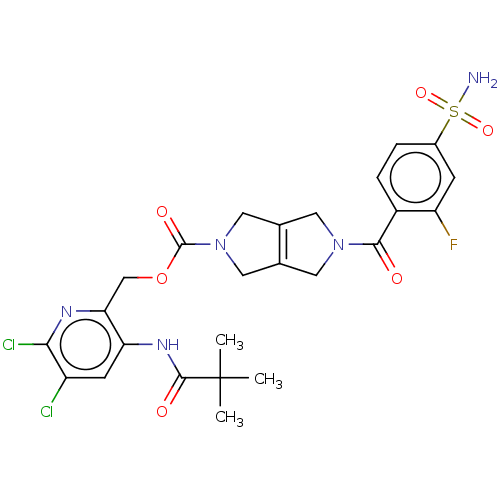 (US10647719, Example 1.30 | [5,6-dichloro-3-(2,2-) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 4.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc. US Patent | Assay Description Human carbonic anhydrase II (hCA-II) inhibition was measured by an absorbance method using 4-nitrophenyl acetate (4-NPA) as its substrate. 4-NPA can ... | US Patent US10647719 (2020) BindingDB Entry DOI: 10.7270/Q2V98C4V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ataxin-2 (Homo sapiens (Human)) | BDBM258573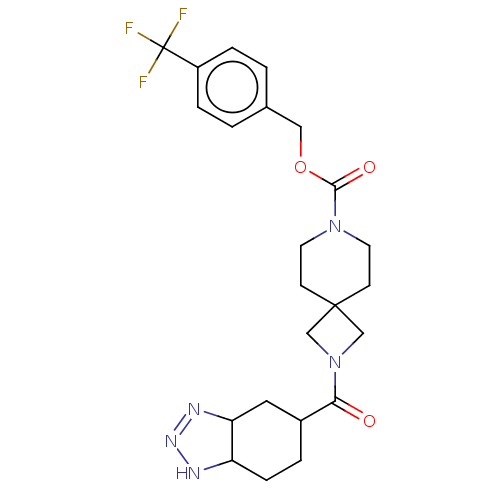 (US10633384, Example 12.35 | US9493486, 12.35) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 4.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc. US Patent | Assay Description ATX inhibition was measured by a fluorescence quenching assay using a specifically labeled substrate analogue (MR121 substrate). To obtain this MR121... | US Patent US10633384 (2020) BindingDB Entry DOI: 10.7270/Q2K077BX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ataxin-2 (Homo sapiens (Human)) | BDBM352349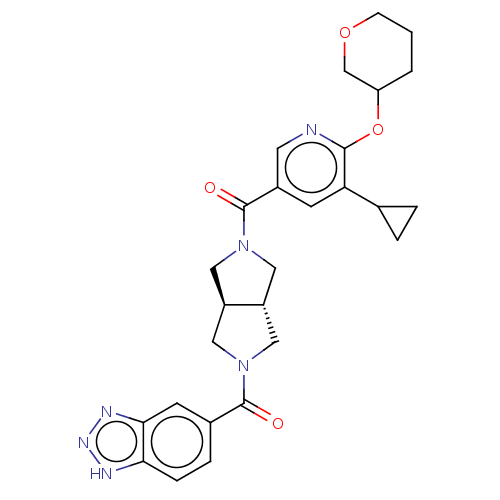 (US11098048, Example 2.06 | US9802944, Example 2.06) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 4.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc. US Patent | Assay Description ATX inhibition was measured by a fluorescence quenching assay using a specifically labeled substrate analogue (MR121 substrate). To obtain this MR121... | US Patent US9802944 (2017) BindingDB Entry DOI: 10.7270/Q2SF2Z9M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ataxin-2 (Homo sapiens (Human)) | BDBM352339 (4-((3aR,6aS)-5-(5-Cyclopropyl-6-(tetrahydro-2H-pyr...) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 4.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffman-La Roche Inc. US Patent | Assay Description Assay working solutions were made as follows:Assay buffer (50 mM Tris-HCl, 140 mM NaCl, 5 mM KCl, 1 mM CaCl2, 1 mM MgCl2, 0.01% Triton-X-100, pH 8.0;... | US Patent US10654857 (2020) BindingDB Entry DOI: 10.7270/Q2NK3J1K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ataxin-2 (Homo sapiens (Human)) | BDBM241152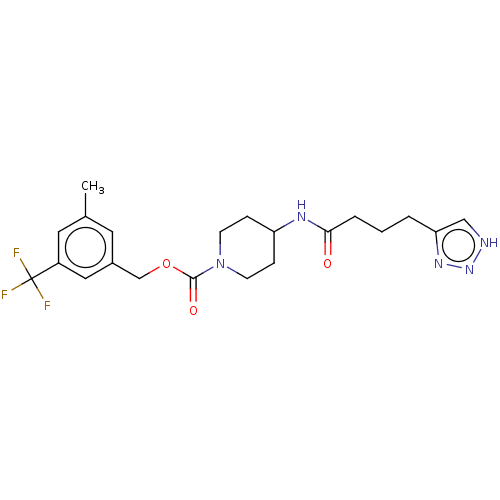 (US9409895, 62 | US9630945, 62) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis AG US Patent | Assay Description ATX activity was determined by measurement of released choline in reactions containing ATX (10 nM), choline oxidase (0.1 U/ml), HRP (100 U/ml), ample... | US Patent US9630945 (2017) BindingDB Entry DOI: 10.7270/Q25Q4Z5R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ataxin-2 (Homo sapiens (Human)) | BDBM323244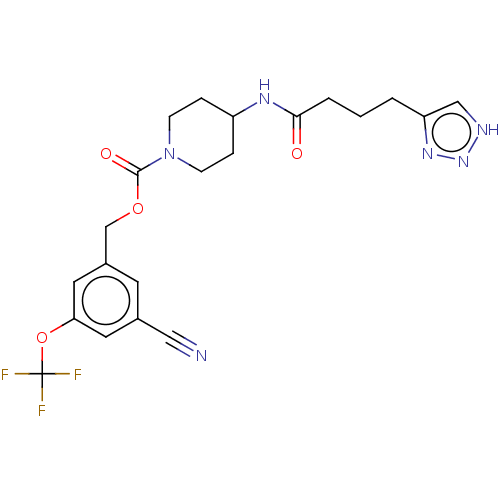 (3-Cyano-5-(trifluoromethoxy)benzyl 4-(4-(1H-1,2,3-...) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis AG US Patent | Assay Description ATX activity was determined by measurement of released choline in reactions containing ATX (10 nM), choline oxidase (0.1 U/ml), HRP (100 U/ml), ample... | US Patent US9630945 (2017) BindingDB Entry DOI: 10.7270/Q25Q4Z5R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ataxin-2 (Homo sapiens (Human)) | BDBM352280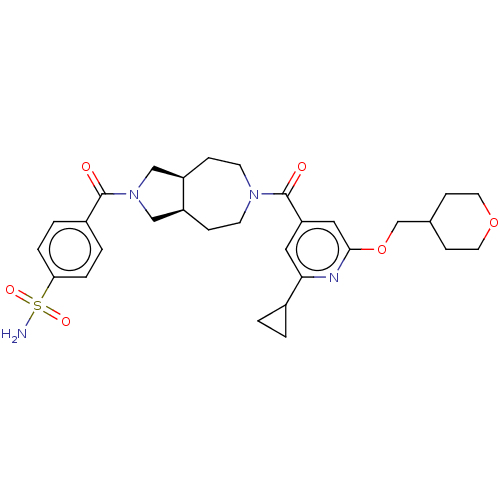 (CHEM-US-00046 | US9802944, Example 1.09) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc. US Patent | Assay Description ATX inhibition was measured by a fluorescence quenching assay using a specifically labeled substrate analogue (MR121 substrate). To obtain this MR121... | US Patent US9802944 (2017) BindingDB Entry DOI: 10.7270/Q2SF2Z9M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ataxin-2 (Homo sapiens (Human)) | BDBM352332 (BDBM352333 | CHEM-US-00058 | US9802944, Example 1....) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc. US Patent | Assay Description ATX inhibition was measured by a fluorescence quenching assay using a specifically labeled substrate analogue (MR121 substrate). To obtain this MR121... | US Patent US9802944 (2017) BindingDB Entry DOI: 10.7270/Q2SF2Z9M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ataxin-2 (Homo sapiens (Human)) | BDBM352366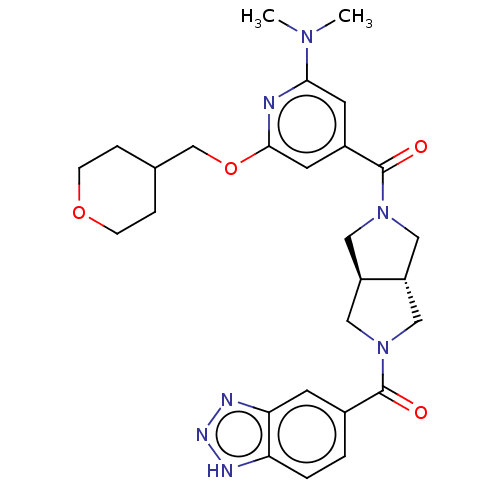 (US11098048, Example 2.23 | US9802944, Example 2.23) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc. US Patent | Assay Description ATX inhibition was measured by a fluorescence quenching assay using a specifically labeled substrate analogue (MR121 substrate). To obtain this MR121... | US Patent US9802944 (2017) BindingDB Entry DOI: 10.7270/Q2SF2Z9M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 417 total ) | Next | Last >> |