

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Group XIIB secretory phospholipase A2-like protein (Homo sapiens) | BDBM50534272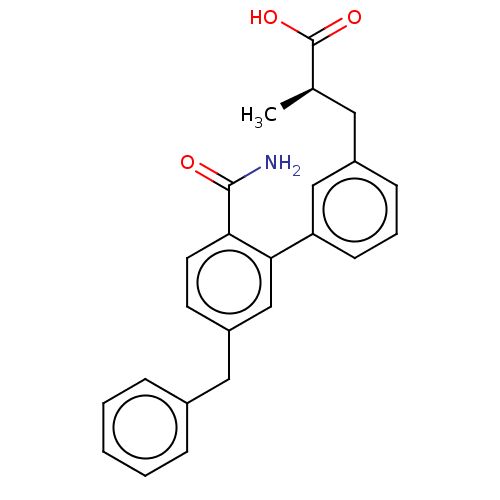 (CHEMBL4593409) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | n/a | n/a | <14 | n/a | n/a | n/a | n/a | n/a | n/a |
Astrazeneca Curated by ChEMBL | Assay Description Inhibition of sPLA2 in human HepG2 cells | ACS Med Chem Lett 7: 884-889 (2016) Article DOI: 10.1021/acsmedchemlett.6b00188 BindingDB Entry DOI: 10.7270/Q23N26W0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Group XIIB secretory phospholipase A2-like protein (Homo sapiens) | BDBM50534272 (CHEMBL4593409) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | n/a | n/a | 56 | n/a | n/a | n/a | n/a | n/a | n/a |
Astrazeneca Curated by ChEMBL | Assay Description Inhibition of sPLA2 in atherosclerotic plaque from coronary artery disease patient | ACS Med Chem Lett 7: 884-889 (2016) Article DOI: 10.1021/acsmedchemlett.6b00188 BindingDB Entry DOI: 10.7270/Q23N26W0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Group XIIB secretory phospholipase A2-like protein (Homo sapiens) | BDBM50055366 ((3-Aminooxalyl-1-benzyl-2-ethyl-1H-indol-4-yloxy)-...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 114 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Curated by ChEMBL | Assay Description Inhibition of human recombinant sPLA2 assessed as reduction in 16:0 LPC formation after 30 mins by HPLC-MS analysis | J Med Chem 62: 1999-2007 (2019) Article DOI: 10.1021/acs.jmedchem.8b01568 BindingDB Entry DOI: 10.7270/Q27S7S71 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Group XIIB secretory phospholipase A2-like protein (Homo sapiens) | BDBM50055366 ((3-Aminooxalyl-1-benzyl-2-ethyl-1H-indol-4-yloxy)-...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 114 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Curated by ChEMBL | Assay Description Inhibition of human recombinant sPLA2 assessed as reduction in 16:0 LPC formation after 30 mins by HPLC-MS analysis | J Med Chem 62: 1999-2007 (2019) Article DOI: 10.1021/acs.jmedchem.8b01568 BindingDB Entry DOI: 10.7270/Q27S7S71 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Group XIIB secretory phospholipase A2-like protein (Homo sapiens) | BDBM50055366 ((3-Aminooxalyl-1-benzyl-2-ethyl-1H-indol-4-yloxy)-...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 131 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Curated by ChEMBL | Assay Description Inhibition of human recombinant sPLA2 assessed as reduction in free FA formation after 30 mins by HPLC-MS analysis | J Med Chem 62: 1999-2007 (2019) Article DOI: 10.1021/acs.jmedchem.8b01568 BindingDB Entry DOI: 10.7270/Q27S7S71 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Group XIIB secretory phospholipase A2-like protein (Homo sapiens) | BDBM50055366 ((3-Aminooxalyl-1-benzyl-2-ethyl-1H-indol-4-yloxy)-...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 131 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Curated by ChEMBL | Assay Description Inhibition of human recombinant sPLA2 assessed as reduction in free FA formation after 30 mins by HPLC-MS analysis | J Med Chem 62: 1999-2007 (2019) Article DOI: 10.1021/acs.jmedchem.8b01568 BindingDB Entry DOI: 10.7270/Q27S7S71 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||