

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Serine protease HTRA1 [158-480] (Homo sapiens (Human)) | BDBM521765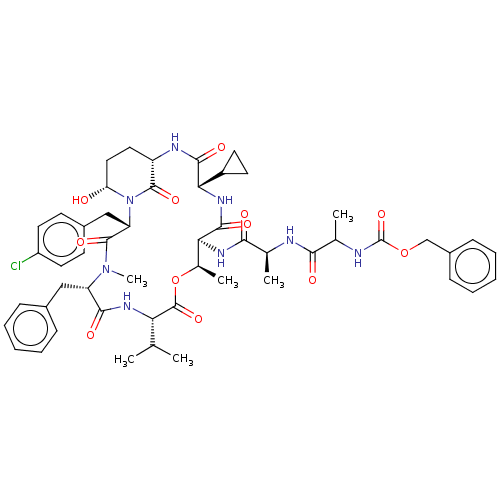 (US11149067, Compound Ahp26) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 65 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description In general, proteolytic activity was tested by monitoring the cleavage of the specific chromogenic substrate at 405 nm wavelength for 60 or 120 minut... | Citation and Details BindingDB Entry DOI: 10.7270/Q2RR22D0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease HTRA1 [158-480] (Homo sapiens (Human)) | BDBM521770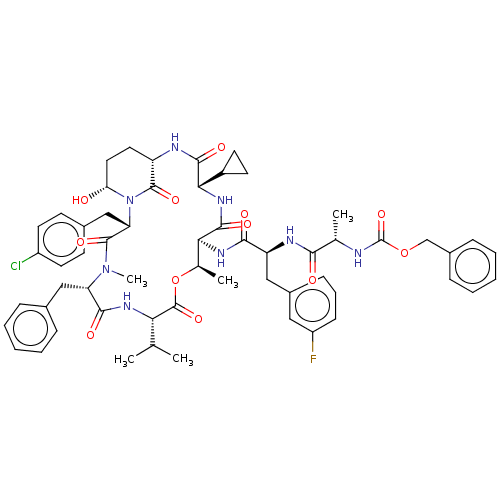 (US11149067, Compound Ahp33) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 610 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description In general, proteolytic activity was tested by monitoring the cleavage of the specific chromogenic substrate at 405 nm wavelength for 60 or 120 minut... | Citation and Details BindingDB Entry DOI: 10.7270/Q2RR22D0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease HTRA1 [158-480] (Homo sapiens (Human)) | BDBM521764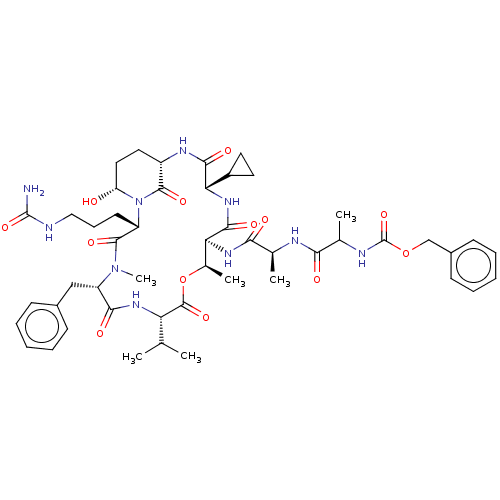 (US11149067, Compound Ahp25) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description In general, proteolytic activity was tested by monitoring the cleavage of the specific chromogenic substrate at 405 nm wavelength for 60 or 120 minut... | Citation and Details BindingDB Entry DOI: 10.7270/Q2RR22D0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease HTRA1 [158-480] (Homo sapiens (Human)) | BDBM521766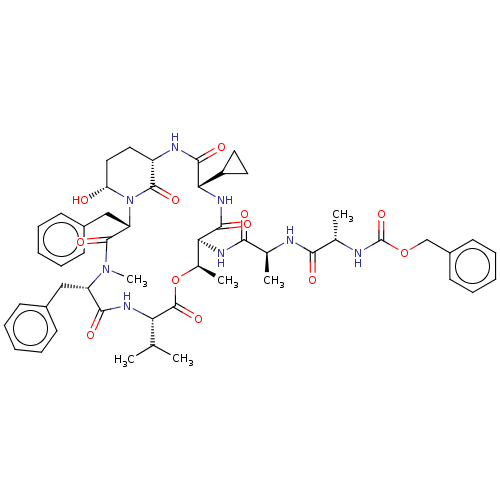 (US11149067, Compound Ahp27) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 950 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description In general, proteolytic activity was tested by monitoring the cleavage of the specific chromogenic substrate at 405 nm wavelength for 60 or 120 minut... | Citation and Details BindingDB Entry DOI: 10.7270/Q2RR22D0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease HTRA1 [158-480] (Homo sapiens (Human)) | BDBM521759 (US11149067, Compound Ahp19) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 1.24E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description In general, proteolytic activity was tested by monitoring the cleavage of the specific chromogenic substrate at 405 nm wavelength for 60 or 120 minut... | Citation and Details BindingDB Entry DOI: 10.7270/Q2RR22D0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease HTRA1 [158-480] (Homo sapiens (Human)) | BDBM521773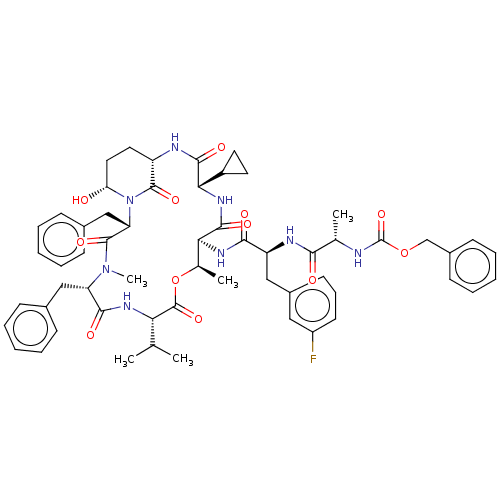 (US11149067, Compound Ahp36) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 1.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description In general, proteolytic activity was tested by monitoring the cleavage of the specific chromogenic substrate at 405 nm wavelength for 60 or 120 minut... | Citation and Details BindingDB Entry DOI: 10.7270/Q2RR22D0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease HTRA1 [158-480] (Homo sapiens (Human)) | BDBM521774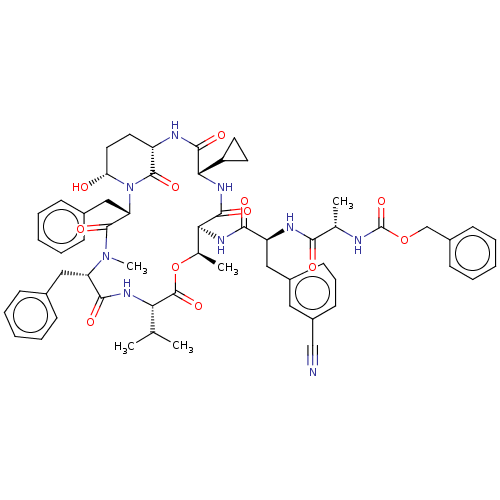 (US11149067, Compound Ahp37) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 1.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description In general, proteolytic activity was tested by monitoring the cleavage of the specific chromogenic substrate at 405 nm wavelength for 60 or 120 minut... | Citation and Details BindingDB Entry DOI: 10.7270/Q2RR22D0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease HTRA1 [158-480] (Homo sapiens (Human)) | BDBM521763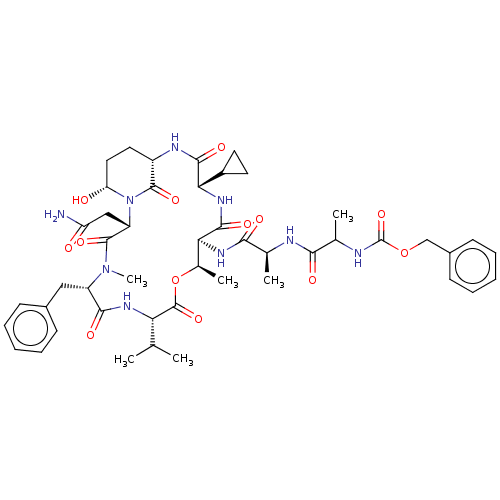 (US11149067, Compound Ahp24) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 1.66E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description In general, proteolytic activity was tested by monitoring the cleavage of the specific chromogenic substrate at 405 nm wavelength for 60 or 120 minut... | Citation and Details BindingDB Entry DOI: 10.7270/Q2RR22D0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease HTRA1 [158-480] (Homo sapiens (Human)) | BDBM521768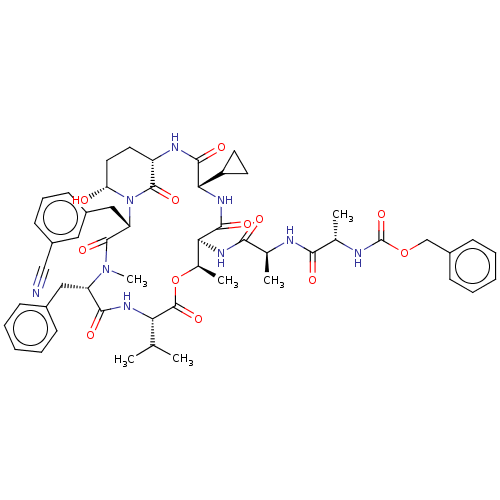 (US11149067, Compound Ahp29) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 1.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description In general, proteolytic activity was tested by monitoring the cleavage of the specific chromogenic substrate at 405 nm wavelength for 60 or 120 minut... | Citation and Details BindingDB Entry DOI: 10.7270/Q2RR22D0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease HTRA1 [158-480] (Homo sapiens (Human)) | BDBM521751 (US11149067, Compound Ahp10) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 2.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description In general, proteolytic activity was tested by monitoring the cleavage of the specific chromogenic substrate at 405 nm wavelength for 60 or 120 minut... | Citation and Details BindingDB Entry DOI: 10.7270/Q2RR22D0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease HTRA1 [158-480] (Homo sapiens (Human)) | BDBM521767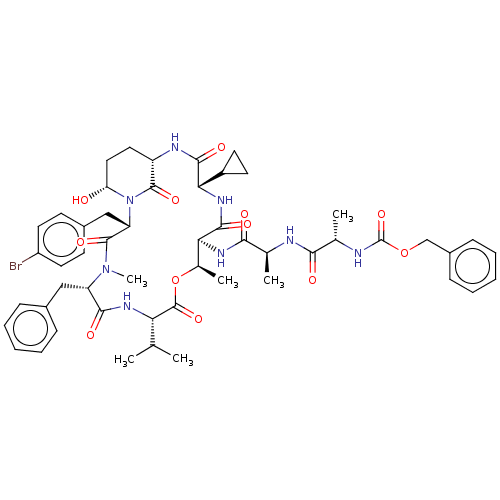 (US11149067, Compound Ahp28) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 2.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description In general, proteolytic activity was tested by monitoring the cleavage of the specific chromogenic substrate at 405 nm wavelength for 60 or 120 minut... | Citation and Details BindingDB Entry DOI: 10.7270/Q2RR22D0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease HTRA1 [158-480] (Homo sapiens (Human)) | BDBM521754 (US11149067, Compound Ahp14) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 2.97E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description In general, proteolytic activity was tested by monitoring the cleavage of the specific chromogenic substrate at 405 nm wavelength for 60 or 120 minut... | Citation and Details BindingDB Entry DOI: 10.7270/Q2RR22D0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease HTRA1 [158-480] (Homo sapiens (Human)) | BDBM521769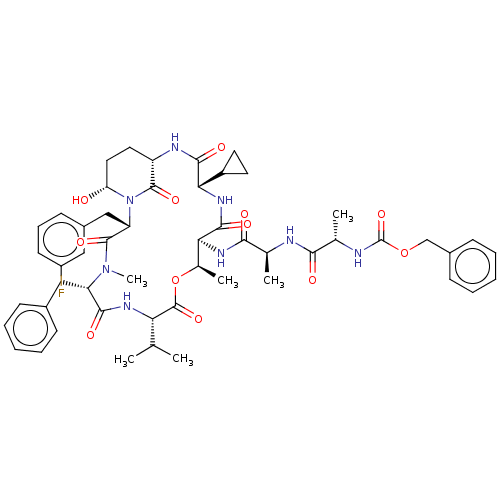 (US11149067, Compound Ahp30) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description In general, proteolytic activity was tested by monitoring the cleavage of the specific chromogenic substrate at 405 nm wavelength for 60 or 120 minut... | Citation and Details BindingDB Entry DOI: 10.7270/Q2RR22D0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease HTRA1 [158-480] (Homo sapiens (Human)) | BDBM521753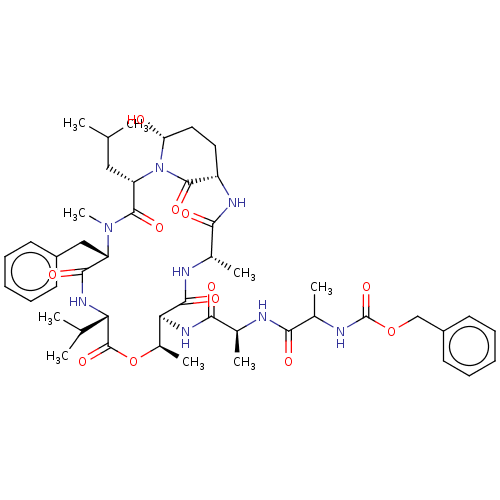 (US11149067, Compound Ahp13) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 4.26E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description In general, proteolytic activity was tested by monitoring the cleavage of the specific chromogenic substrate at 405 nm wavelength for 60 or 120 minut... | Citation and Details BindingDB Entry DOI: 10.7270/Q2RR22D0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease HTRA1 [158-480] (Homo sapiens (Human)) | BDBM521762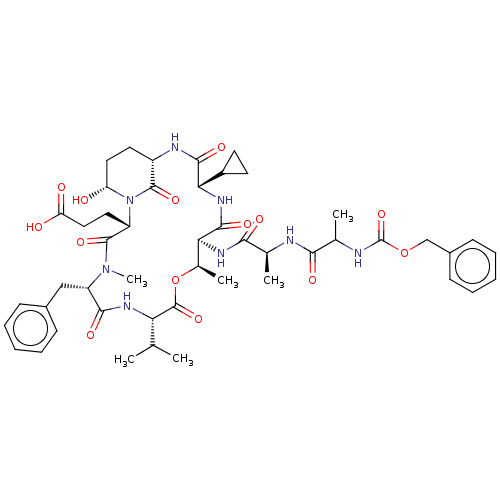 (US11149067, Compound Ahp22) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 5.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description In general, proteolytic activity was tested by monitoring the cleavage of the specific chromogenic substrate at 405 nm wavelength for 60 or 120 minut... | Citation and Details BindingDB Entry DOI: 10.7270/Q2RR22D0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease HTRA1 [158-480] (Homo sapiens (Human)) | BDBM521772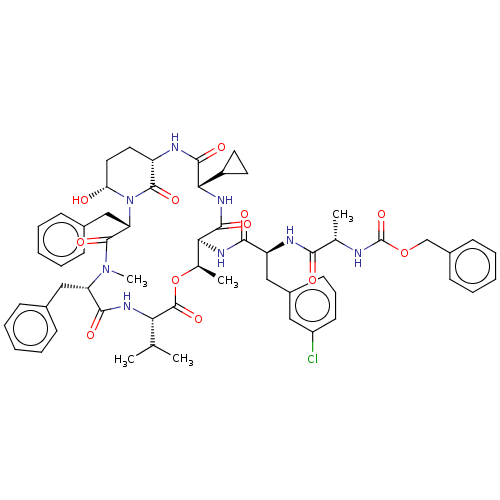 (US11149067, Compound Ahp35) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 6.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description In general, proteolytic activity was tested by monitoring the cleavage of the specific chromogenic substrate at 405 nm wavelength for 60 or 120 minut... | Citation and Details BindingDB Entry DOI: 10.7270/Q2RR22D0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease HTRA1 [158-480] (Homo sapiens (Human)) | BDBM521755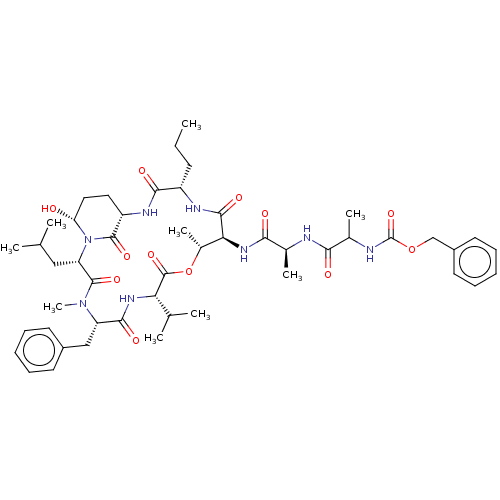 (US11149067, Compound Ahp15) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 7.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description In general, proteolytic activity was tested by monitoring the cleavage of the specific chromogenic substrate at 405 nm wavelength for 60 or 120 minut... | Citation and Details BindingDB Entry DOI: 10.7270/Q2RR22D0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease HTRA1 [158-480] (Homo sapiens (Human)) | BDBM521742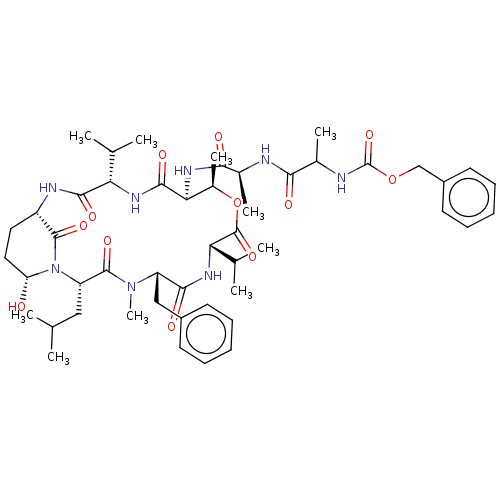 (US11149067, Compound Ahp1) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 8.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description In general, proteolytic activity was tested by monitoring the cleavage of the specific chromogenic substrate at 405 nm wavelength for 60 or 120 minut... | Citation and Details BindingDB Entry DOI: 10.7270/Q2RR22D0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease HTRA1 [158-480] (Homo sapiens (Human)) | BDBM521747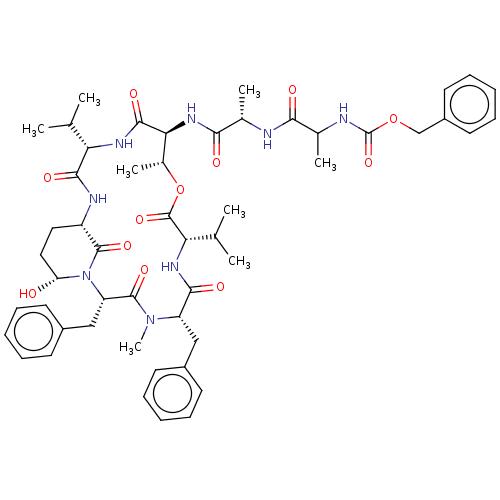 (US11149067, Compound Ahp6) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 1.03E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description In general, proteolytic activity was tested by monitoring the cleavage of the specific chromogenic substrate at 405 nm wavelength for 60 or 120 minut... | Citation and Details BindingDB Entry DOI: 10.7270/Q2RR22D0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease HTRA1 [158-480] (Homo sapiens (Human)) | BDBM521771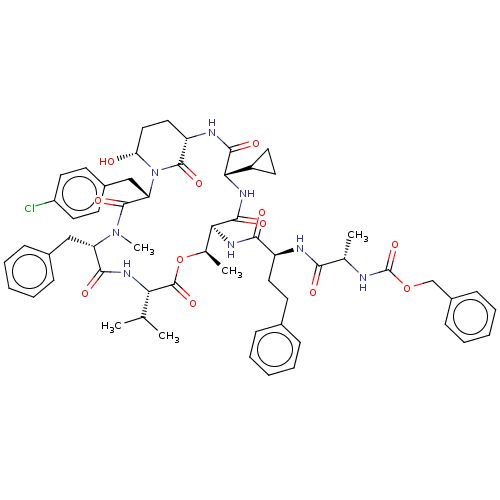 (US11149067, Compound Ahp34) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 4.34E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description In general, proteolytic activity was tested by monitoring the cleavage of the specific chromogenic substrate at 405 nm wavelength for 60 or 120 minut... | Citation and Details BindingDB Entry DOI: 10.7270/Q2RR22D0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease HTRA1 [158-480] (Homo sapiens (Human)) | BDBM521758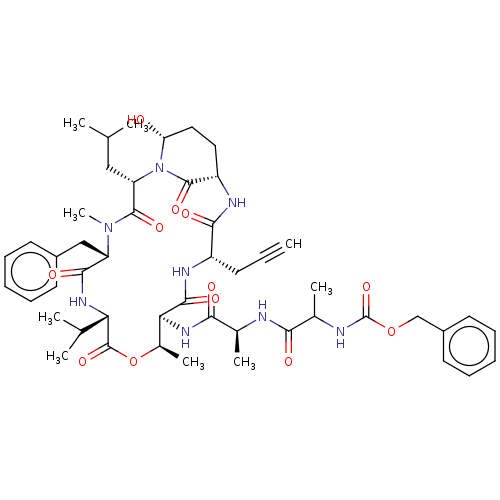 (US11149067, Compound Ahp18) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description In general, proteolytic activity was tested by monitoring the cleavage of the specific chromogenic substrate at 405 nm wavelength for 60 or 120 minut... | Citation and Details BindingDB Entry DOI: 10.7270/Q2RR22D0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease HTRA1 [158-480] (Homo sapiens (Human)) | BDBM521757 (US11149067, Compound Ahp17) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description In general, proteolytic activity was tested by monitoring the cleavage of the specific chromogenic substrate at 405 nm wavelength for 60 or 120 minut... | Citation and Details BindingDB Entry DOI: 10.7270/Q2RR22D0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease HTRA1 [158-480] (Homo sapiens (Human)) | BDBM521756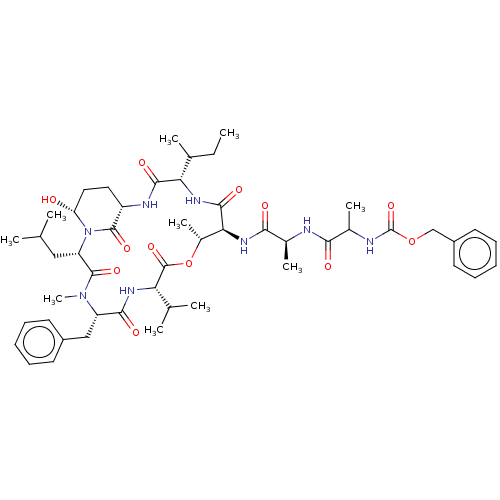 (US11149067, Compound Ahp16) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description In general, proteolytic activity was tested by monitoring the cleavage of the specific chromogenic substrate at 405 nm wavelength for 60 or 120 minut... | Citation and Details BindingDB Entry DOI: 10.7270/Q2RR22D0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease HTRA1 [158-480] (Homo sapiens (Human)) | BDBM521752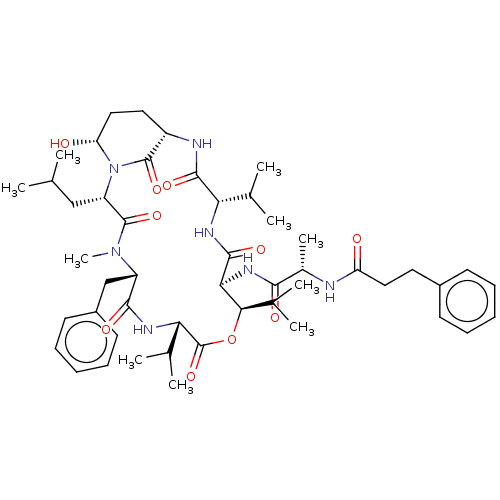 (US11149067, Compound Ahp11) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description In general, proteolytic activity was tested by monitoring the cleavage of the specific chromogenic substrate at 405 nm wavelength for 60 or 120 minut... | Citation and Details BindingDB Entry DOI: 10.7270/Q2RR22D0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease HTRA1 [158-480] (Homo sapiens (Human)) | BDBM521750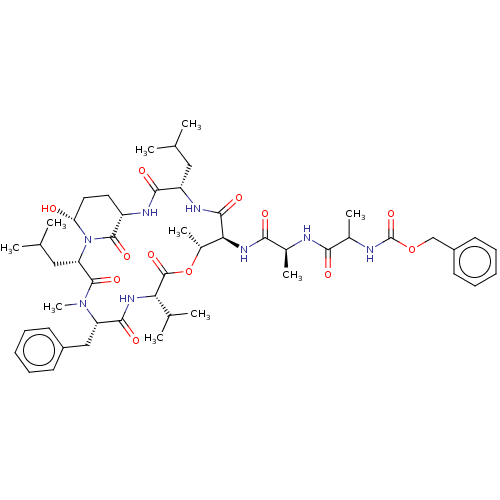 (US11149067, Compound Ahp9) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description In general, proteolytic activity was tested by monitoring the cleavage of the specific chromogenic substrate at 405 nm wavelength for 60 or 120 minut... | Citation and Details BindingDB Entry DOI: 10.7270/Q2RR22D0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease HTRA1 [158-480] (Homo sapiens (Human)) | BDBM521761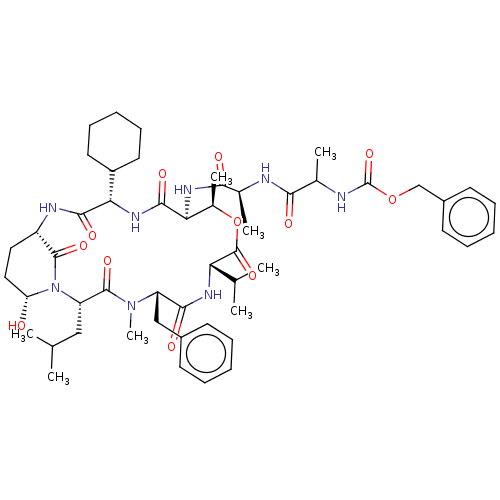 (US11149067, Compound Ahp21) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description In general, proteolytic activity was tested by monitoring the cleavage of the specific chromogenic substrate at 405 nm wavelength for 60 or 120 minut... | Citation and Details BindingDB Entry DOI: 10.7270/Q2RR22D0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease HTRA1 [158-480] (Homo sapiens (Human)) | BDBM521760 (US11149067, Compound Ahp20) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description In general, proteolytic activity was tested by monitoring the cleavage of the specific chromogenic substrate at 405 nm wavelength for 60 or 120 minut... | Citation and Details BindingDB Entry DOI: 10.7270/Q2RR22D0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease HTRA1 [158-480] (Homo sapiens (Human)) | BDBM521748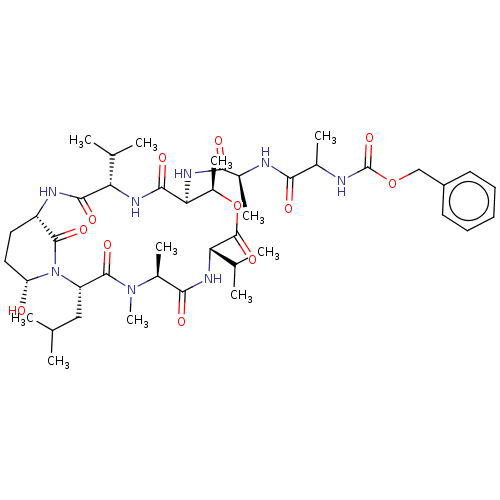 (US11149067, Compound Ahp7) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description In general, proteolytic activity was tested by monitoring the cleavage of the specific chromogenic substrate at 405 nm wavelength for 60 or 120 minut... | Citation and Details BindingDB Entry DOI: 10.7270/Q2RR22D0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease HTRA1 [158-480] (Homo sapiens (Human)) | BDBM521745 (US11149067, Compound Ahp4) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description In general, proteolytic activity was tested by monitoring the cleavage of the specific chromogenic substrate at 405 nm wavelength for 60 or 120 minut... | Citation and Details BindingDB Entry DOI: 10.7270/Q2RR22D0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease HTRA1 [158-480] (Homo sapiens (Human)) | BDBM521746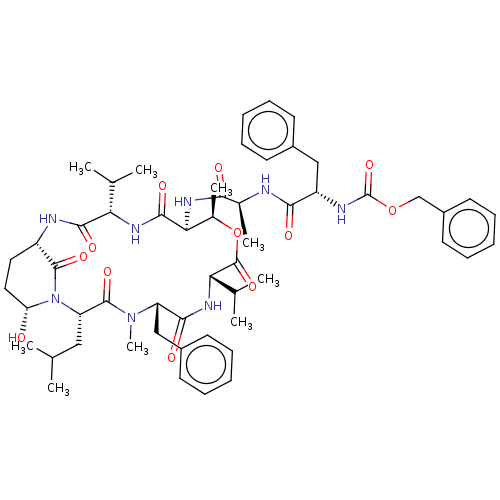 (US11149067, Compound Ahp5) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description In general, proteolytic activity was tested by monitoring the cleavage of the specific chromogenic substrate at 405 nm wavelength for 60 or 120 minut... | Citation and Details BindingDB Entry DOI: 10.7270/Q2RR22D0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease HTRA1 [158-480] (Homo sapiens (Human)) | BDBM521743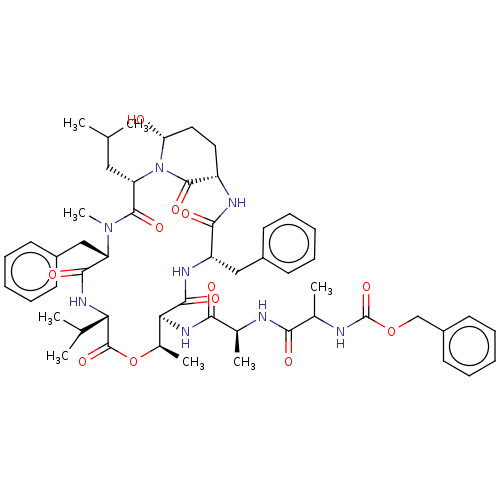 (US11149067, Compound Ahp2) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description In general, proteolytic activity was tested by monitoring the cleavage of the specific chromogenic substrate at 405 nm wavelength for 60 or 120 minut... | Citation and Details BindingDB Entry DOI: 10.7270/Q2RR22D0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease HTRA1 [158-480] (Homo sapiens (Human)) | BDBM521744 (US11149067, Compound Ahp3) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description In general, proteolytic activity was tested by monitoring the cleavage of the specific chromogenic substrate at 405 nm wavelength for 60 or 120 minut... | Citation and Details BindingDB Entry DOI: 10.7270/Q2RR22D0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease HTRA1 [158-480] (Homo sapiens (Human)) | BDBM521749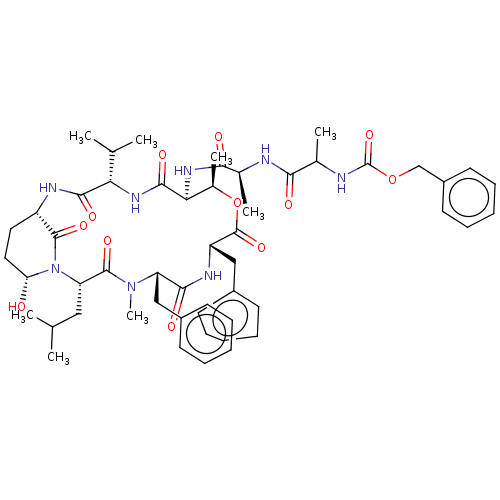 (US11149067, Compound Ahp8) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description In general, proteolytic activity was tested by monitoring the cleavage of the specific chromogenic substrate at 405 nm wavelength for 60 or 120 minut... | Citation and Details BindingDB Entry DOI: 10.7270/Q2RR22D0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease HTRA1 (Homo sapiens (Human)) | BDBM496984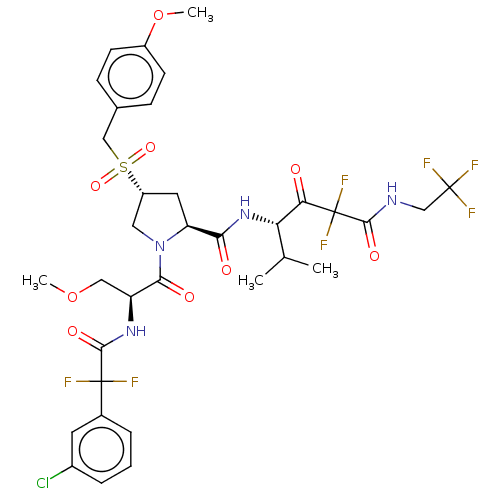 (US11001555, Example 7) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.262 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffman-La Roche Inc. US Patent | Assay Description Enzyme activity is measured by observing the increase in fluorescence intensity caused by cleavage of a peptide substrate containing a fluorophore, w... | US Patent US11001555 (2021) BindingDB Entry DOI: 10.7270/Q2G44TD5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease HTRA1 (Homo sapiens (Human)) | BDBM475923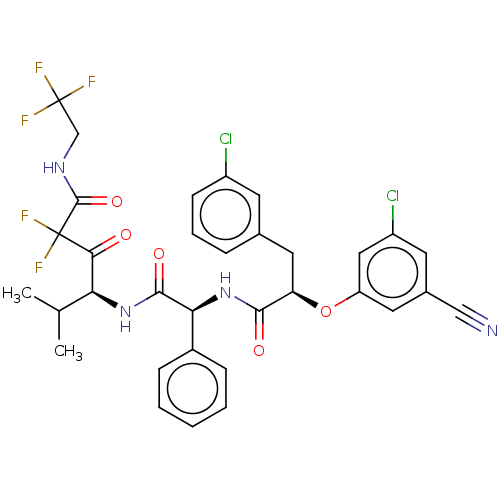 ((4S)-4-[[(2S)-2-[[(2R)-2- (3-chloro-5- cyanophenox...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.293 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc. US Patent | Assay Description Enzyme activity is measured by observing the increase in fluorescence intensity caused by cleavage of a peptide substrate containing a fluorophore, w... | US Patent US10865182 (2020) BindingDB Entry DOI: 10.7270/Q2MC9330 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease HTRA1 (Homo sapiens (Human)) | BDBM514085 (US11059858, Example 42) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Enzyme activity is measured by observing the increase in fluorescence intensity caused by cleavage of a peptide substrate containing a fluorophore, w... | Citation and Details BindingDB Entry DOI: 10.7270/Q25H7KDD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease HTRA1 (Homo sapiens (Human)) | BDBM514076 (US11059858, Example 33) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Enzyme activity is measured by observing the increase in fluorescence intensity caused by cleavage of a peptide substrate containing a fluorophore, w... | Citation and Details BindingDB Entry DOI: 10.7270/Q25H7KDD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease HTRA1 (Homo sapiens (Human)) | BDBM514094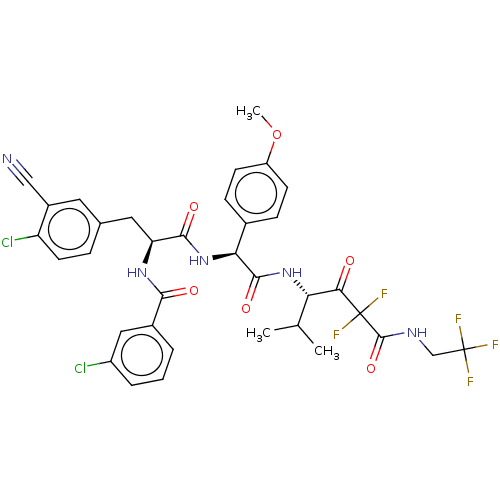 (US11059858, Example 48) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Enzyme activity is measured by observing the increase in fluorescence intensity caused by cleavage of a peptide substrate containing a fluorophore, w... | Citation and Details BindingDB Entry DOI: 10.7270/Q25H7KDD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease HTRA1 (Homo sapiens (Human)) | BDBM496983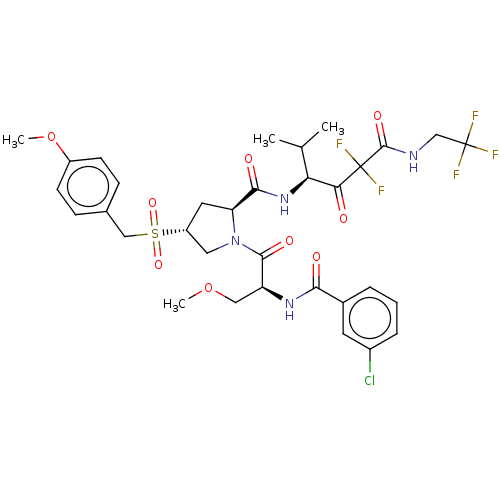 (US11001555, Example 6) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.351 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffman-La Roche Inc. US Patent | Assay Description Enzyme activity is measured by observing the increase in fluorescence intensity caused by cleavage of a peptide substrate containing a fluorophore, w... | US Patent US11001555 (2021) BindingDB Entry DOI: 10.7270/Q2G44TD5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease HTRA1 (Homo sapiens (Human)) | BDBM514091 (US11059858, Example 45) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Enzyme activity is measured by observing the increase in fluorescence intensity caused by cleavage of a peptide substrate containing a fluorophore, w... | Citation and Details BindingDB Entry DOI: 10.7270/Q25H7KDD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease HTRA1 (Homo sapiens (Human)) | BDBM514098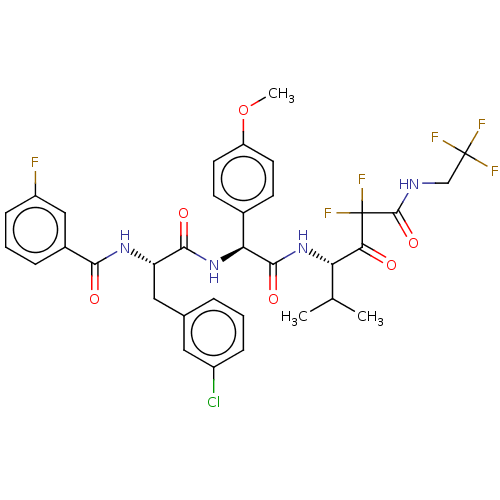 (N-[(2S)-3-(3-Chlorophenyl)-1-[[(1S)-2-[[(3S)-5,5-d...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Enzyme activity is measured by observing the increase in fluorescence intensity caused by cleavage of a peptide substrate containing a fluorophore, w... | Citation and Details BindingDB Entry DOI: 10.7270/Q25H7KDD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease HTRA1 (Homo sapiens (Human)) | BDBM514104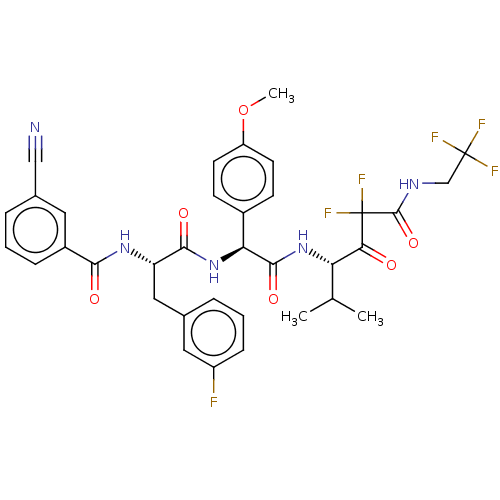 (US11059858, Example 58) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Enzyme activity is measured by observing the increase in fluorescence intensity caused by cleavage of a peptide substrate containing a fluorophore, w... | Citation and Details BindingDB Entry DOI: 10.7270/Q25H7KDD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease HTRA1 (Homo sapiens (Human)) | BDBM499311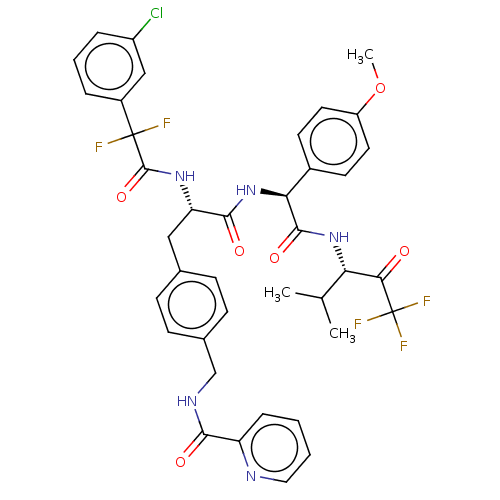 (US11014963, Example 216) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc. US Patent | Assay Description Enzyme activity is measured by observing the increase in fluorescence intensity caused by cleavage of a peptide substrate containing a fluorophore, w... | US Patent US11014963 (2021) BindingDB Entry DOI: 10.7270/Q22R3VSM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease HTRA1 (Homo sapiens (Human)) | BDBM514056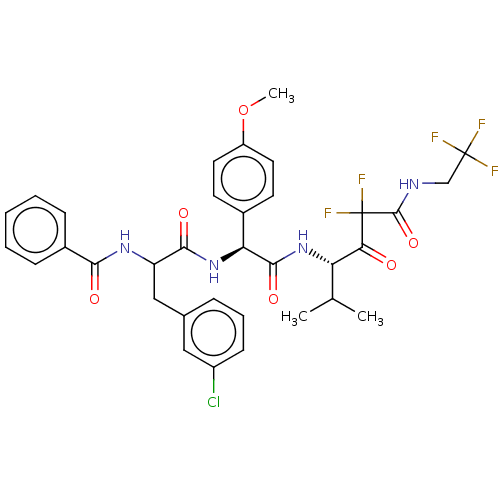 (US11059858, Example 15) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Enzyme activity is measured by observing the increase in fluorescence intensity caused by cleavage of a peptide substrate containing a fluorophore, w... | Citation and Details BindingDB Entry DOI: 10.7270/Q25H7KDD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease HTRA1 (Homo sapiens (Human)) | BDBM514090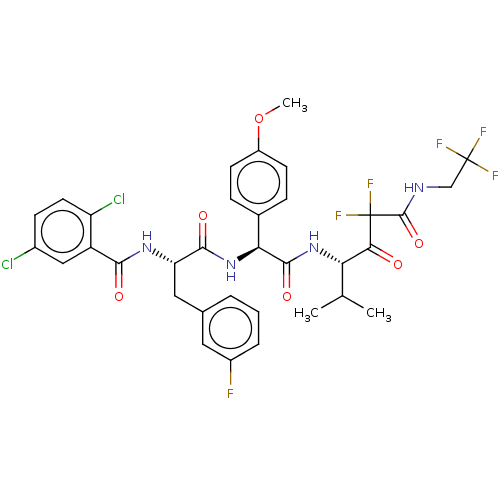 (US11059858, Example 44) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Enzyme activity is measured by observing the increase in fluorescence intensity caused by cleavage of a peptide substrate containing a fluorophore, w... | Citation and Details BindingDB Entry DOI: 10.7270/Q25H7KDD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease HTRA1 (Homo sapiens (Human)) | BDBM475927 ((4S)-4-[[(2S)-2-[[(2R)-2- (3-chlorophenoxy)-3-(3- ...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.461 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc. US Patent | Assay Description Enzyme activity is measured by observing the increase in fluorescence intensity caused by cleavage of a peptide substrate containing a fluorophore, w... | US Patent US10865182 (2020) BindingDB Entry DOI: 10.7270/Q2MC9330 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease HTRA1 (Homo sapiens (Human)) | BDBM496981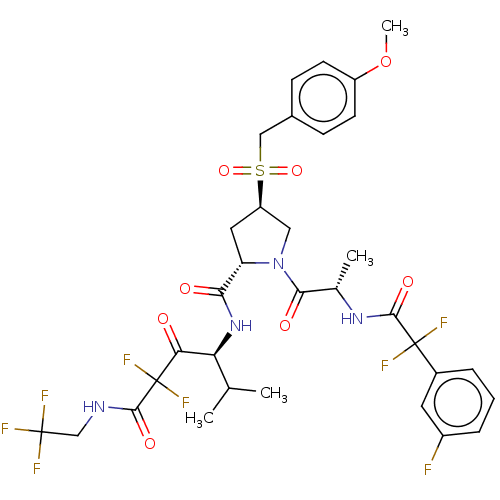 (US11001555, Example 4) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.471 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffman-La Roche Inc. US Patent | Assay Description Enzyme activity is measured by observing the increase in fluorescence intensity caused by cleavage of a peptide substrate containing a fluorophore, w... | US Patent US11001555 (2021) BindingDB Entry DOI: 10.7270/Q2G44TD5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease HTRA1 (Homo sapiens (Human)) | BDBM499107 (US11014963, Example 17) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.490 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc. US Patent | Assay Description Enzyme activity is measured by observing the increase in fluorescence intensity caused by cleavage of a peptide substrate containing a fluorophore, w... | US Patent US11014963 (2021) BindingDB Entry DOI: 10.7270/Q22R3VSM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease HTRA1 (Homo sapiens (Human)) | BDBM499266 (US11014963, Example 166) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc. US Patent | Assay Description Enzyme activity is measured by observing the increase in fluorescence intensity caused by cleavage of a peptide substrate containing a fluorophore, w... | US Patent US11014963 (2021) BindingDB Entry DOI: 10.7270/Q22R3VSM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease HTRA1 (Homo sapiens (Human)) | BDBM514102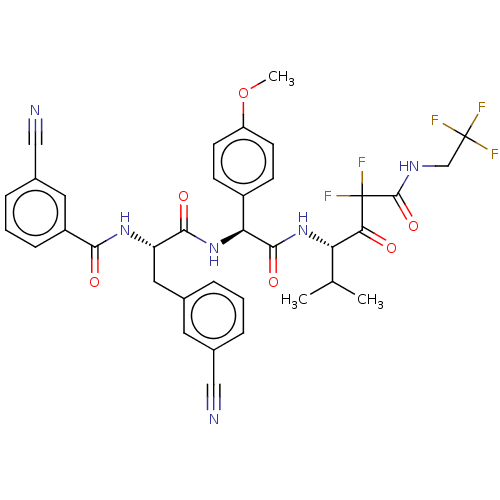 (US11059858, Example 56) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Enzyme activity is measured by observing the increase in fluorescence intensity caused by cleavage of a peptide substrate containing a fluorophore, w... | Citation and Details BindingDB Entry DOI: 10.7270/Q25H7KDD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 885 total ) | Next | Last >> |