

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Thrombospondin-1 (Homo sapiens) | BDBM50541838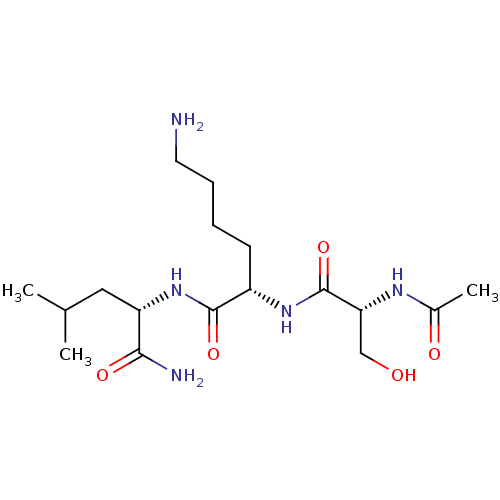 (CHEMBL4649483) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.380 | n/a | n/a | n/a | n/a | n/a | n/a |
Southern Research Institute Curated by ChEMBL | Assay Description Inhibition of TSP1 in human myeloma cells assessed reduction in TGFbeta activity incubated for 1 hr by ELISA | ACS Med Chem Lett 11: 1130-1136 (2020) Article DOI: 10.1021/acsmedchemlett.9b00540 BindingDB Entry DOI: 10.7270/Q2M330BV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thrombospondin-1 (Homo sapiens) | BDBM50541831 (CHEMBL4644535) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Southern Research Institute Curated by ChEMBL | Assay Description Inhibition of TSP1 in human myeloma cells assessed reduction in TGFbeta activity incubated for 1 hr by ELISA | ACS Med Chem Lett 11: 1130-1136 (2020) Article DOI: 10.1021/acsmedchemlett.9b00540 BindingDB Entry DOI: 10.7270/Q2M330BV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thrombospondin-1 (Homo sapiens) | BDBM50541837 (CHEMBL4642848) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Southern Research Institute Curated by ChEMBL | Assay Description Inhibition of TSP1 in human myeloma cells assessed reduction in TGFbeta activity incubated for 1 hr by ELISA | ACS Med Chem Lett 11: 1130-1136 (2020) Article DOI: 10.1021/acsmedchemlett.9b00540 BindingDB Entry DOI: 10.7270/Q2M330BV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thrombospondin-1 (Homo sapiens) | BDBM50541836 (CHEMBL4642780) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Southern Research Institute Curated by ChEMBL | Assay Description Inhibition of TSP1 in human myeloma cells assessed reduction in TGFbeta activity incubated for 1 hr by ELISA | ACS Med Chem Lett 11: 1130-1136 (2020) Article DOI: 10.1021/acsmedchemlett.9b00540 BindingDB Entry DOI: 10.7270/Q2M330BV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thrombospondin-1 (Homo sapiens) | BDBM50541828 (CHEMBL4633877) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Southern Research Institute Curated by ChEMBL | Assay Description Inhibition of TSP1 in human myeloma cells assessed reduction in TGFbeta activity incubated for 1 hr by ELISA | ACS Med Chem Lett 11: 1130-1136 (2020) Article DOI: 10.1021/acsmedchemlett.9b00540 BindingDB Entry DOI: 10.7270/Q2M330BV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thrombospondin-1 (Homo sapiens) | BDBM50541833 (CHEMBL4636492) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Southern Research Institute Curated by ChEMBL | Assay Description Inhibition of TSP1 in human myeloma cells assessed reduction in TGFbeta activity incubated for 1 hr by ELISA | ACS Med Chem Lett 11: 1130-1136 (2020) Article DOI: 10.1021/acsmedchemlett.9b00540 BindingDB Entry DOI: 10.7270/Q2M330BV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thrombospondin-1 (Homo sapiens) | BDBM50541832 (CHEMBL4648099) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Southern Research Institute Curated by ChEMBL | Assay Description Inhibition of TSP1 in human myeloma cells assessed reduction in TGFbeta activity incubated for 1 hr by ELISA | ACS Med Chem Lett 11: 1130-1136 (2020) Article DOI: 10.1021/acsmedchemlett.9b00540 BindingDB Entry DOI: 10.7270/Q2M330BV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thrombospondin-1 (Homo sapiens) | BDBM50541830 (CHEMBL4647669) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Southern Research Institute Curated by ChEMBL | Assay Description Inhibition of TSP1 in human myeloma cells assessed reduction in TGFbeta activity incubated for 1 hr by ELISA | ACS Med Chem Lett 11: 1130-1136 (2020) Article DOI: 10.1021/acsmedchemlett.9b00540 BindingDB Entry DOI: 10.7270/Q2M330BV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thrombospondin-1 (Homo sapiens) | BDBM50541826 (CHEMBL4644244) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 5.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Southern Research Institute Curated by ChEMBL | Assay Description Inhibition of TSP1 in human myeloma cells assessed reduction in TGFbeta activity incubated for 1 hr by ELISA | ACS Med Chem Lett 11: 1130-1136 (2020) Article DOI: 10.1021/acsmedchemlett.9b00540 BindingDB Entry DOI: 10.7270/Q2M330BV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thrombospondin-1 (Homo sapiens) | BDBM50541839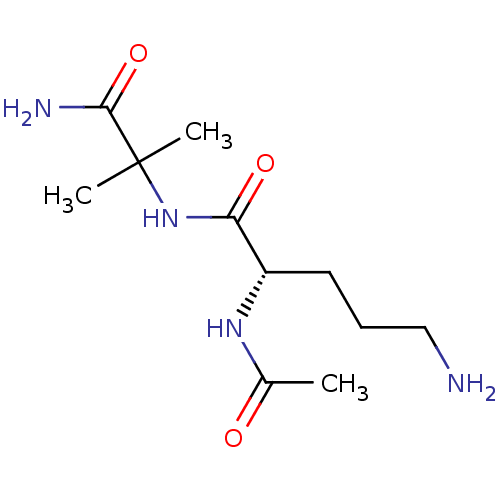 (CHEMBL4649293) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 7.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Southern Research Institute Curated by ChEMBL | Assay Description Inhibition of TSP1 in human myeloma cells assessed reduction in TGFbeta activity incubated for 1 hr by ELISA | ACS Med Chem Lett 11: 1130-1136 (2020) Article DOI: 10.1021/acsmedchemlett.9b00540 BindingDB Entry DOI: 10.7270/Q2M330BV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thrombospondin-1 (Homo sapiens) | BDBM50541827 (CHEMBL4644046) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 9.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Southern Research Institute Curated by ChEMBL | Assay Description Inhibition of TSP1 in human myeloma cells assessed reduction in TGFbeta activity incubated for 1 hr by ELISA | ACS Med Chem Lett 11: 1130-1136 (2020) Article DOI: 10.1021/acsmedchemlett.9b00540 BindingDB Entry DOI: 10.7270/Q2M330BV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thrombospondin-1 (Homo sapiens) | BDBM50541834 (CHEMBL4644197) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Southern Research Institute Curated by ChEMBL | Assay Description Inhibition of TSP1 in human myeloma cells assessed reduction in TGFbeta activity incubated for 1 hr by ELISA | ACS Med Chem Lett 11: 1130-1136 (2020) Article DOI: 10.1021/acsmedchemlett.9b00540 BindingDB Entry DOI: 10.7270/Q2M330BV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thrombospondin-1 (Homo sapiens) | BDBM50541835 (CHEMBL4645518) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
Southern Research Institute Curated by ChEMBL | Assay Description Inhibition of TSP1 in human myeloma cells assessed reduction in TGFbeta activity incubated for 1 hr by ELISA | ACS Med Chem Lett 11: 1130-1136 (2020) Article DOI: 10.1021/acsmedchemlett.9b00540 BindingDB Entry DOI: 10.7270/Q2M330BV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thrombospondin-1 (Homo sapiens) | BDBM50541829 (CHEMBL4638959) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | Article PubMed | n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Southern Research Institute Curated by ChEMBL | Assay Description Inhibition of TSP1 in human myeloma cells assessed reduction in TGFbeta activity incubated for 1 hr by ELISA | ACS Med Chem Lett 11: 1130-1136 (2020) Article DOI: 10.1021/acsmedchemlett.9b00540 BindingDB Entry DOI: 10.7270/Q2M330BV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thrombospondin-1 (Homo sapiens) | BDBM521734 (US11149066, Compound 9) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | n/a | 0.230 | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description As referred to herein, the TSP1 inhibitory activity refers to the activity to inhibit one or more effects of TSP1, including angiostatic effect. The ... | Citation and Details BindingDB Entry DOI: 10.7270/Q2WH2T4B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thrombospondin-1 (Homo sapiens) | BDBM521735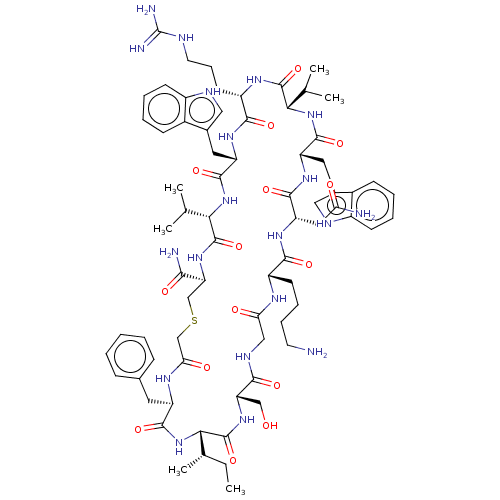 (US11149066, Compound 10) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | n/a | 0.0910 | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description As referred to herein, the TSP1 inhibitory activity refers to the activity to inhibit one or more effects of TSP1, including angiostatic effect. The ... | Citation and Details BindingDB Entry DOI: 10.7270/Q2WH2T4B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thrombospondin-1 (Homo sapiens) | BDBM521736 (US11149066, Compound 11) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | n/a | 0.140 | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description As referred to herein, the TSP1 inhibitory activity refers to the activity to inhibit one or more effects of TSP1, including angiostatic effect. The ... | Citation and Details BindingDB Entry DOI: 10.7270/Q2WH2T4B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thrombospondin-1 (Homo sapiens) | BDBM521737 (US11149066, Compound 12) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | n/a | 0.380 | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description As referred to herein, the TSP1 inhibitory activity refers to the activity to inhibit one or more effects of TSP1, including angiostatic effect. The ... | Citation and Details BindingDB Entry DOI: 10.7270/Q2WH2T4B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thrombospondin-1 (Homo sapiens) | BDBM521738 (US11149066, Compound 13) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | n/a | 0.0960 | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description As referred to herein, the TSP1 inhibitory activity refers to the activity to inhibit one or more effects of TSP1, including angiostatic effect. The ... | Citation and Details BindingDB Entry DOI: 10.7270/Q2WH2T4B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thrombospondin-1 (Homo sapiens) | BDBM521739 (US11149066, Compound 24) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | n/a | 0.120 | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description As referred to herein, the TSP1 inhibitory activity refers to the activity to inhibit one or more effects of TSP1, including angiostatic effect. The ... | Citation and Details BindingDB Entry DOI: 10.7270/Q2WH2T4B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thrombospondin-1 (Homo sapiens) | BDBM521740 (US11149066, Compound 58) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | n/a | 0.0910 | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description As referred to herein, the TSP1 inhibitory activity refers to the activity to inhibit one or more effects of TSP1, including angiostatic effect. The ... | Citation and Details BindingDB Entry DOI: 10.7270/Q2WH2T4B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thrombospondin-1 (Homo sapiens) | BDBM521733 (US11149066, Compound 7) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | n/a | 0.120 | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description As referred to herein, the TSP1 inhibitory activity refers to the activity to inhibit one or more effects of TSP1, including angiostatic effect. The ... | Citation and Details BindingDB Entry DOI: 10.7270/Q2WH2T4B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thrombospondin-1 (Homo sapiens) | BDBM521732 (US11149066, Compound 6) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | n/a | 0.440 | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description As referred to herein, the TSP1 inhibitory activity refers to the activity to inhibit one or more effects of TSP1, including angiostatic effect. The ... | Citation and Details BindingDB Entry DOI: 10.7270/Q2WH2T4B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thrombospondin-1 (Homo sapiens) | BDBM521731 (US11149066, Compound 5) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | n/a | 0 | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description As referred to herein, the TSP1 inhibitory activity refers to the activity to inhibit one or more effects of TSP1, including angiostatic effect. The ... | Citation and Details BindingDB Entry DOI: 10.7270/Q2WH2T4B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thrombospondin-1 (Homo sapiens) | BDBM521730 (US11149066, Compound 4) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | n/a | 0.260 | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description As referred to herein, the TSP1 inhibitory activity refers to the activity to inhibit one or more effects of TSP1, including angiostatic effect. The ... | Citation and Details BindingDB Entry DOI: 10.7270/Q2WH2T4B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thrombospondin-1 (Homo sapiens) | BDBM521729 (US11149066, Compound 3) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | n/a | 0.25 | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description As referred to herein, the TSP1 inhibitory activity refers to the activity to inhibit one or more effects of TSP1, including angiostatic effect. The ... | Citation and Details BindingDB Entry DOI: 10.7270/Q2WH2T4B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thrombospondin-1 (Homo sapiens) | BDBM521728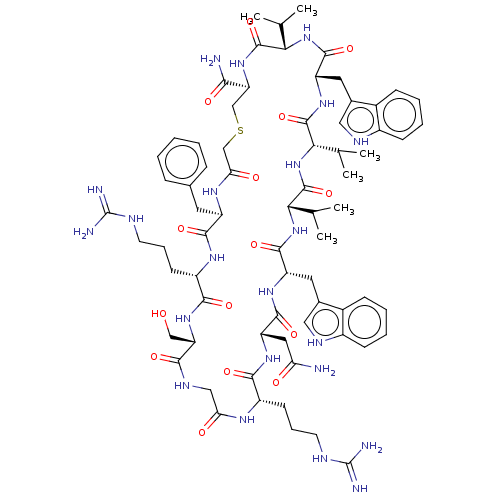 (US11149066, Compound 2) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | n/a | 0.150 | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description As referred to herein, the TSP1 inhibitory activity refers to the activity to inhibit one or more effects of TSP1, including angiostatic effect. The ... | Citation and Details BindingDB Entry DOI: 10.7270/Q2WH2T4B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thrombospondin-1 (Homo sapiens) | BDBM521727 (US11149066, Compound 1) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | n/a | 0.510 | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description As referred to herein, the TSP1 inhibitory activity refers to the activity to inhibit one or more effects of TSP1, including angiostatic effect. The ... | Citation and Details BindingDB Entry DOI: 10.7270/Q2WH2T4B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycoprotein G (Nipah virus) | BDBM50293787 ((2R,6S)-1-allyl-4-hydroxy-2,6-bis(4-nitrophenyl)-1...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a |
University of W£rzburg Curated by ChEMBL | Assay Description Inhibition of Nipah virus glycoprotein G/F-mediated cell-cell fusion expressed in african green monkey Vero cells after 24 hrs relative to untreated ... | J Med Chem 52: 4257-65 (2009) Article DOI: 10.1021/jm900411s BindingDB Entry DOI: 10.7270/Q2H1322G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycoprotein G (Nipah virus) | BDBM50293786 (CHEMBL551897 | Ethyl 4-[1-cyclopropyl-3-(2,4-dichl...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 3.00E+3 | n/a | n/a | n/a | n/a |
University of W£rzburg Curated by ChEMBL | Assay Description Inhibition of Nipah virus glycoprotein G/F-mediated cell-cell fusion expressed in african green monkey Vero cells after 24 hrs relative to untreated ... | J Med Chem 52: 4257-65 (2009) Article DOI: 10.1021/jm900411s BindingDB Entry DOI: 10.7270/Q2H1322G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycoprotein G (Nipah virus) | BDBM50293785 (CHEMBL562236 | Ethyl 4-[3-(2,4-dichlorobenzylcarba...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 4.00E+3 | n/a | n/a | n/a | n/a |
University of W£rzburg Curated by ChEMBL | Assay Description Inhibition of Nipah virus glycoprotein G/F-mediated cell-cell fusion expressed in african green monkey Vero cells after 24 hrs relative to untreated ... | J Med Chem 52: 4257-65 (2009) Article DOI: 10.1021/jm900411s BindingDB Entry DOI: 10.7270/Q2H1322G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycoprotein G (Nipah virus) | BDBM50293784 (CHEMBL559961 | Ethyl 1-[3-(2,4-dichlorobenzylcarba...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 3.00E+3 | n/a | n/a | n/a | n/a |
University of W£rzburg Curated by ChEMBL | Assay Description Inhibition of Nipah virus glycoprotein G/F-mediated cell-cell fusion expressed in african green monkey Vero cells after 24 hrs relative to untreated ... | J Med Chem 52: 4257-65 (2009) Article DOI: 10.1021/jm900411s BindingDB Entry DOI: 10.7270/Q2H1322G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycoprotein G (Nipah virus) | BDBM50293783 (7-(4-Carbamoylpiperidin-1-yl)-1-cyclopropyl-N-(2,4...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 1.50E+3 | n/a | n/a | n/a | n/a |
University of W£rzburg Curated by ChEMBL | Assay Description Inhibition of Nipah virus glycoprotein G/F-mediated cell-cell fusion expressed in african green monkey Vero cells after 24 hrs relative to untreated ... | J Med Chem 52: 4257-65 (2009) Article DOI: 10.1021/jm900411s BindingDB Entry DOI: 10.7270/Q2H1322G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thrombospondin-1 (Homo sapiens) | BDBM521741 (US11149066, Compound 75) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | n/a | 0.220 | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description As referred to herein, the TSP1 inhibitory activity refers to the activity to inhibit one or more effects of TSP1, including angiostatic effect. The ... | Citation and Details BindingDB Entry DOI: 10.7270/Q2WH2T4B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycoprotein G (Nipah virus) | BDBM50193260 (2-benzylbenzo[d]oxazol-5-amine | CHEMBL214087) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | >8.00E+3 | n/a | n/a | n/a | n/a |
University of W£rzburg Curated by ChEMBL | Assay Description Inhibition of Nipah virus glycoprotein G/F-mediated cell-cell fusion expressed in african green monkey Vero cells after 24 hrs relative to untreated ... | J Med Chem 52: 4257-65 (2009) Article DOI: 10.1021/jm900411s BindingDB Entry DOI: 10.7270/Q2H1322G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycoprotein G (Nipah virus) | BDBM50293782 (CHEMBL551697 | N-(4-Chlorobenzyl)-1-ethyl-4-oxo-7-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | >8.00E+3 | n/a | n/a | n/a | n/a |
University of W£rzburg Curated by ChEMBL | Assay Description Inhibition of Nipah virus glycoprotein G/F-mediated cell-cell fusion expressed in african green monkey Vero cells after 24 hrs relative to untreated ... | J Med Chem 52: 4257-65 (2009) Article DOI: 10.1021/jm900411s BindingDB Entry DOI: 10.7270/Q2H1322G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||