 Found 35 hits Enz. Inhib. hit(s) with Target = 'Phenylalanine--tRNA ligase, mitochondrial'
Found 35 hits Enz. Inhib. hit(s) with Target = 'Phenylalanine--tRNA ligase, mitochondrial' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Phenylalanine--tRNA ligase, mitochondrial
(Homo sapiens (Human)) | BDBM50140728
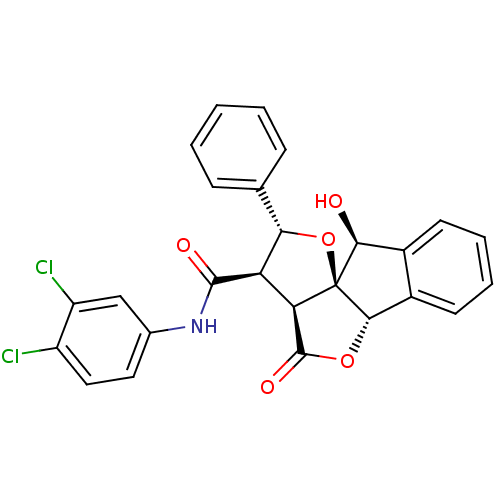
(13N-(3,4-dichlorophenyl)-2-hydroxy-11-oxo-14-pheny...)Show SMILES O[C@H]1c2ccccc2[C@@H]2OC(=O)[C@H]3[C@H]([C@@H](O[C@@]123)c1ccccc1)C(=O)Nc1ccc(Cl)c(Cl)c1 Show InChI InChI=1S/C26H19Cl2NO5/c27-17-11-10-14(12-18(17)28)29-24(31)19-20-25(32)33-23-16-9-5-4-8-15(16)22(30)26(20,23)34-21(19)13-6-2-1-3-7-13/h1-12,19-23,30H,(H,29,31)/t19-,20-,21+,22+,23+,26+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 170 | n/a | n/a | n/a | n/a | n/a | n/a |
Cubist Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Compound was evaluated for the inhibitory activity against Escherichia coli phenylalanyl-tRNA synthetase |
Bioorg Med Chem Lett 14: 1343-6 (2004)
Article DOI: 10.1016/j.bmcl.2003.11.082
BindingDB Entry DOI: 10.7270/Q2K073Q6 |
More data for this
Ligand-Target Pair | |
Phenylalanine--tRNA ligase, mitochondrial
(Homo sapiens (Human)) | BDBM50140728

(13N-(3,4-dichlorophenyl)-2-hydroxy-11-oxo-14-pheny...)Show SMILES O[C@H]1c2ccccc2[C@@H]2OC(=O)[C@H]3[C@H]([C@@H](O[C@@]123)c1ccccc1)C(=O)Nc1ccc(Cl)c(Cl)c1 Show InChI InChI=1S/C26H19Cl2NO5/c27-17-11-10-14(12-18(17)28)29-24(31)19-20-25(32)33-23-16-9-5-4-8-15(16)22(30)26(20,23)34-21(19)13-6-2-1-3-7-13/h1-12,19-23,30H,(H,29,31)/t19-,20-,21+,22+,23+,26+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 260 | n/a | n/a | n/a | n/a | n/a | n/a |
Cubist Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Compound was evaluated for the inhibitory activity against Staphylococcus aureus phenylalanyl-tRNA synthetase |
Bioorg Med Chem Lett 14: 1343-6 (2004)
Article DOI: 10.1016/j.bmcl.2003.11.082
BindingDB Entry DOI: 10.7270/Q2K073Q6 |
More data for this
Ligand-Target Pair | |
Phenylalanine--tRNA ligase, mitochondrial
(Homo sapiens (Human)) | BDBM50140729
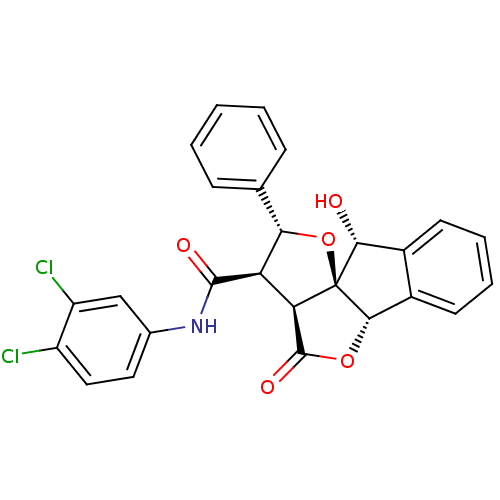
(13N-(3,4-dichlorophenyl)-2-hydroxy-11-oxo-14-pheny...)Show SMILES O[C@@H]1c2ccccc2[C@@H]2OC(=O)[C@H]3[C@H]([C@@H](O[C@@]123)c1ccccc1)C(=O)Nc1ccc(Cl)c(Cl)c1 Show InChI InChI=1S/C26H19Cl2NO5/c27-17-11-10-14(12-18(17)28)29-24(31)19-20-25(32)33-23-16-9-5-4-8-15(16)22(30)26(20,23)34-21(19)13-6-2-1-3-7-13/h1-12,19-23,30H,(H,29,31)/t19-,20-,21+,22-,23+,26+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 470 | n/a | n/a | n/a | n/a | n/a | n/a |
Cubist Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Compound was evaluated for the inhibitory activity against Staphylococcus aureus phenylalanyl-tRNA synthetase |
Bioorg Med Chem Lett 14: 1343-6 (2004)
Article DOI: 10.1016/j.bmcl.2003.11.082
BindingDB Entry DOI: 10.7270/Q2K073Q6 |
More data for this
Ligand-Target Pair | |
Phenylalanine--tRNA ligase, mitochondrial
(Homo sapiens (Human)) | BDBM50140729

(13N-(3,4-dichlorophenyl)-2-hydroxy-11-oxo-14-pheny...)Show SMILES O[C@@H]1c2ccccc2[C@@H]2OC(=O)[C@H]3[C@H]([C@@H](O[C@@]123)c1ccccc1)C(=O)Nc1ccc(Cl)c(Cl)c1 Show InChI InChI=1S/C26H19Cl2NO5/c27-17-11-10-14(12-18(17)28)29-24(31)19-20-25(32)33-23-16-9-5-4-8-15(16)22(30)26(20,23)34-21(19)13-6-2-1-3-7-13/h1-12,19-23,30H,(H,29,31)/t19-,20-,21+,22-,23+,26+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 510 | n/a | n/a | n/a | n/a | n/a | n/a |
Cubist Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Compound was evaluated for the inhibitory activity against Staphylococcus aureus phenylalanyl-tRNA synthetase |
Bioorg Med Chem Lett 14: 1343-6 (2004)
Article DOI: 10.1016/j.bmcl.2003.11.082
BindingDB Entry DOI: 10.7270/Q2K073Q6 |
More data for this
Ligand-Target Pair | |
Phenylalanine--tRNA ligase, mitochondrial
(Homo sapiens (Human)) | BDBM50140727
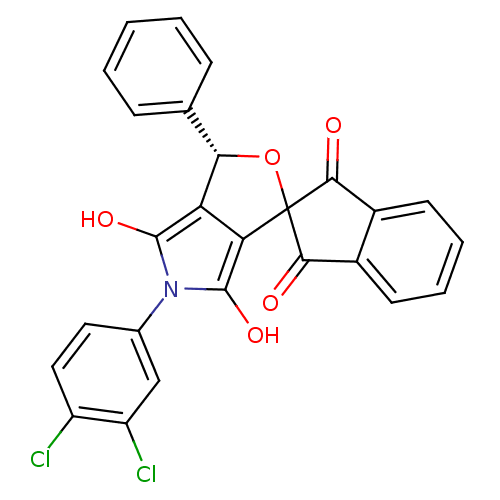
(5-(3,4-dichlorophenyl)-3-phenyl-(3R,3aR,6aS)-spiro...)Show SMILES Oc1c2[C@@H](OC3(C(=O)c4ccccc4C3=O)c2c(O)n1-c1ccc(Cl)c(Cl)c1)c1ccccc1 Show InChI InChI=1S/C26H15Cl2NO5/c27-17-11-10-14(12-18(17)28)29-24(32)19-20(25(29)33)26(34-21(19)13-6-2-1-3-7-13)22(30)15-8-4-5-9-16(15)23(26)31/h1-12,21,32-33H/t21-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Cubist Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Compound was evaluated for the inhibitory activity against Escherichia coli phenylalanyl-tRNA synthetase |
Bioorg Med Chem Lett 14: 1343-6 (2004)
Article DOI: 10.1016/j.bmcl.2003.11.082
BindingDB Entry DOI: 10.7270/Q2K073Q6 |
More data for this
Ligand-Target Pair | |
Phenylalanine--tRNA ligase, mitochondrial
(Homo sapiens (Human)) | BDBM50140725
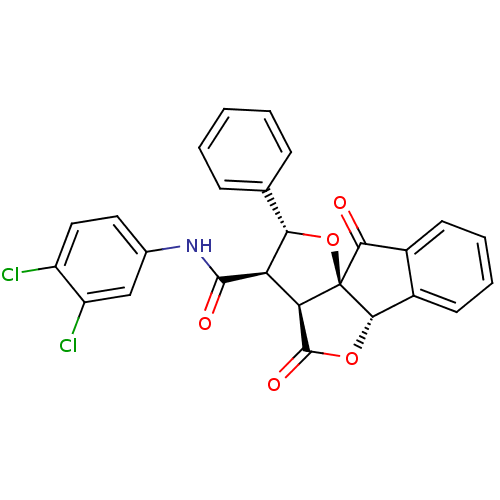
(13N-(3,4-dichlorophenyl)-2,11-dioxo-14-phenyl-(1R,...)Show SMILES Clc1ccc(NC(=O)[C@H]2[C@@H](O[C@@]34[C@@H](OC(=O)[C@@H]23)c2ccccc2C4=O)c2ccccc2)cc1Cl Show InChI InChI=1S/C26H17Cl2NO5/c27-17-11-10-14(12-18(17)28)29-24(31)19-20-25(32)33-23-16-9-5-4-8-15(16)22(30)26(20,23)34-21(19)13-6-2-1-3-7-13/h1-12,19-21,23H,(H,29,31)/t19-,20-,21+,23+,26+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Cubist Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Compound was evaluated for the inhibitory activity against Staphylococcus aureus phenylalanyl-tRNA synthetase |
Bioorg Med Chem Lett 14: 1343-6 (2004)
Article DOI: 10.1016/j.bmcl.2003.11.082
BindingDB Entry DOI: 10.7270/Q2K073Q6 |
More data for this
Ligand-Target Pair | |
Phenylalanine--tRNA ligase, mitochondrial
(Homo sapiens (Human)) | BDBM50140725

(13N-(3,4-dichlorophenyl)-2,11-dioxo-14-phenyl-(1R,...)Show SMILES Clc1ccc(NC(=O)[C@H]2[C@@H](O[C@@]34[C@@H](OC(=O)[C@@H]23)c2ccccc2C4=O)c2ccccc2)cc1Cl Show InChI InChI=1S/C26H17Cl2NO5/c27-17-11-10-14(12-18(17)28)29-24(31)19-20-25(32)33-23-16-9-5-4-8-15(16)22(30)26(20,23)34-21(19)13-6-2-1-3-7-13/h1-12,19-21,23H,(H,29,31)/t19-,20-,21+,23+,26+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Cubist Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Compound was evaluated for the inhibitory activity against Escherichia coli phenylalanyl-tRNA synthetase |
Bioorg Med Chem Lett 14: 1343-6 (2004)
Article DOI: 10.1016/j.bmcl.2003.11.082
BindingDB Entry DOI: 10.7270/Q2K073Q6 |
More data for this
Ligand-Target Pair | |
Phenylalanine--tRNA ligase, mitochondrial
(Homo sapiens (Human)) | BDBM50140726

(13N-(3,4-dichlorophenyl)-2-hydroxy-11-oxo-14-pheny...)Show SMILES OC1c2ccccc2C2OC(=O)[C@@H]3[C@@H]([C@@H](O[C@@]123)c1ccccc1)C(=O)Nc1ccc(Cl)c(Cl)c1 Show InChI InChI=1S/C26H19Cl2NO5/c27-17-11-10-14(12-18(17)28)29-24(31)19-20-25(32)33-23-16-9-5-4-8-15(16)22(30)26(20,23)34-21(19)13-6-2-1-3-7-13/h1-12,19-23,30H,(H,29,31)/t19-,20-,21-,22?,23?,26-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Cubist Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Compound was evaluated for the inhibitory activity against Escherichia coli phenylalanyl-tRNA synthetase |
Bioorg Med Chem Lett 14: 1343-6 (2004)
Article DOI: 10.1016/j.bmcl.2003.11.082
BindingDB Entry DOI: 10.7270/Q2K073Q6 |
More data for this
Ligand-Target Pair | |
Phenylalanine--tRNA ligase, mitochondrial
(Homo sapiens (Human)) | BDBM50140726

(13N-(3,4-dichlorophenyl)-2-hydroxy-11-oxo-14-pheny...)Show SMILES OC1c2ccccc2C2OC(=O)[C@@H]3[C@@H]([C@@H](O[C@@]123)c1ccccc1)C(=O)Nc1ccc(Cl)c(Cl)c1 Show InChI InChI=1S/C26H19Cl2NO5/c27-17-11-10-14(12-18(17)28)29-24(31)19-20-25(32)33-23-16-9-5-4-8-15(16)22(30)26(20,23)34-21(19)13-6-2-1-3-7-13/h1-12,19-23,30H,(H,29,31)/t19-,20-,21-,22?,23?,26-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Cubist Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Compound was evaluated for the inhibitory activity against Escherichia coli phenylalanyl-tRNA synthetase |
Bioorg Med Chem Lett 14: 1343-6 (2004)
Article DOI: 10.1016/j.bmcl.2003.11.082
BindingDB Entry DOI: 10.7270/Q2K073Q6 |
More data for this
Ligand-Target Pair | |
Phenylalanine--tRNA ligase, mitochondrial
(Homo sapiens (Human)) | BDBM50140727

(5-(3,4-dichlorophenyl)-3-phenyl-(3R,3aR,6aS)-spiro...)Show SMILES Oc1c2[C@@H](OC3(C(=O)c4ccccc4C3=O)c2c(O)n1-c1ccc(Cl)c(Cl)c1)c1ccccc1 Show InChI InChI=1S/C26H15Cl2NO5/c27-17-11-10-14(12-18(17)28)29-24(32)19-20(25(29)33)26(34-21(19)13-6-2-1-3-7-13)22(30)15-8-4-5-9-16(15)23(26)31/h1-12,21,32-33H/t21-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Cubist Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Compound was evaluated for the inhibitory activity against Staphylococcus aureus phenylalanyl-tRNA synthetase |
Bioorg Med Chem Lett 14: 1343-6 (2004)
Article DOI: 10.1016/j.bmcl.2003.11.082
BindingDB Entry DOI: 10.7270/Q2K073Q6 |
More data for this
Ligand-Target Pair | |
Phenylalanine--tRNA ligase, mitochondrial
(Homo sapiens (Human)) | BDBM50140730
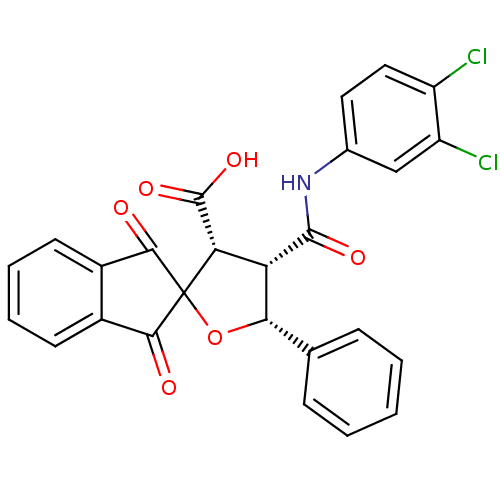
(4-(3,4-dichlorophenylcarbamoyl)-1',3'-dioxo-5-phen...)Show SMILES OC(=O)[C@@H]1[C@@H]([C@@H](OC11C(=O)c2ccccc2C1=O)c1ccccc1)C(=O)Nc1ccc(Cl)c(Cl)c1 Show InChI InChI=1S/C26H17Cl2NO6/c27-17-11-10-14(12-18(17)28)29-24(32)19-20(25(33)34)26(35-21(19)13-6-2-1-3-7-13)22(30)15-8-4-5-9-16(15)23(26)31/h1-12,19-21H,(H,29,32)(H,33,34)/t19-,20-,21-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Cubist Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Compound was evaluated for the inhibitory activity against Escherichia coli phenylalanyl-tRNA synthetase |
Bioorg Med Chem Lett 14: 1343-6 (2004)
Article DOI: 10.1016/j.bmcl.2003.11.082
BindingDB Entry DOI: 10.7270/Q2K073Q6 |
More data for this
Ligand-Target Pair | |
Phenylalanine--tRNA ligase, mitochondrial
(Homo sapiens (Human)) | BDBM50140726

(13N-(3,4-dichlorophenyl)-2-hydroxy-11-oxo-14-pheny...)Show SMILES OC1c2ccccc2C2OC(=O)[C@@H]3[C@@H]([C@@H](O[C@@]123)c1ccccc1)C(=O)Nc1ccc(Cl)c(Cl)c1 Show InChI InChI=1S/C26H19Cl2NO5/c27-17-11-10-14(12-18(17)28)29-24(31)19-20-25(32)33-23-16-9-5-4-8-15(16)22(30)26(20,23)34-21(19)13-6-2-1-3-7-13/h1-12,19-23,30H,(H,29,31)/t19-,20-,21-,22?,23?,26-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Cubist Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Compound was evaluated for the inhibitory activity against Escherichia coli phenylalanyl-tRNA synthetase |
Bioorg Med Chem Lett 14: 1343-6 (2004)
Article DOI: 10.1016/j.bmcl.2003.11.082
BindingDB Entry DOI: 10.7270/Q2K073Q6 |
More data for this
Ligand-Target Pair | |
Phenylalanine--tRNA ligase, mitochondrial
(Homo sapiens (Human)) | BDBM50140730

(4-(3,4-dichlorophenylcarbamoyl)-1',3'-dioxo-5-phen...)Show SMILES OC(=O)[C@@H]1[C@@H]([C@@H](OC11C(=O)c2ccccc2C1=O)c1ccccc1)C(=O)Nc1ccc(Cl)c(Cl)c1 Show InChI InChI=1S/C26H17Cl2NO6/c27-17-11-10-14(12-18(17)28)29-24(32)19-20(25(33)34)26(35-21(19)13-6-2-1-3-7-13)22(30)15-8-4-5-9-16(15)23(26)31/h1-12,19-21H,(H,29,32)(H,33,34)/t19-,20-,21-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Cubist Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Compound was evaluated for the inhibitory activity against Staphylococcus aureus phenylalanyl-tRNA synthetase |
Bioorg Med Chem Lett 14: 1343-6 (2004)
Article DOI: 10.1016/j.bmcl.2003.11.082
BindingDB Entry DOI: 10.7270/Q2K073Q6 |
More data for this
Ligand-Target Pair | |
Phenylalanine--tRNA ligase, mitochondrial
(Homo sapiens (Human)) | BDBM50140716
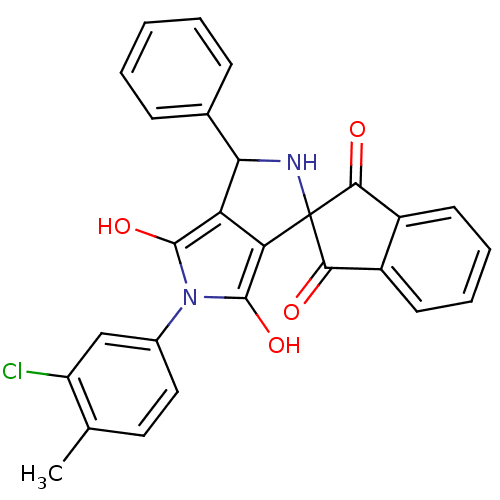
(5'-(3-chloro-4-methylphenyl)-3'-phenyl-(3'R,3a'S,6...)Show SMILES Cc1ccc(cc1Cl)-n1c(O)c2C(NC3(C(=O)c4ccccc4C3=O)c2c1O)c1ccccc1 Show InChI InChI=1S/C27H19ClN2O4/c1-14-11-12-16(13-19(14)28)30-25(33)20-21(26(30)34)27(29-22(20)15-7-3-2-4-8-15)23(31)17-9-5-6-10-18(17)24(27)32/h2-13,22,29,33-34H,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Cubist Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Inhibitory activity against phenylalanyl-tRNA synthetase from Enterococcus faecalis |
Bioorg Med Chem Lett 14: 1339-42 (2004)
Article DOI: 10.1016/j.bmcl.2003.11.081
BindingDB Entry DOI: 10.7270/Q2PR7VDS |
More data for this
Ligand-Target Pair | |
Phenylalanine--tRNA ligase, mitochondrial
(Homo sapiens (Human)) | BDBM50140713

(4-benzoyl-2-(3,4-dichlorophenyl)-6-phenyl-(3aR,6R,...)Show SMILES Oc1c2C(NC(c2c(O)n1-c1ccc(Cl)c(Cl)c1)c1ccccc1)C(=O)c1ccccc1 Show InChI InChI=1S/C25H18Cl2N2O3/c26-17-12-11-16(13-18(17)27)29-24(31)19-20(25(29)32)22(23(30)15-9-5-2-6-10-15)28-21(19)14-7-3-1-4-8-14/h1-13,21-22,28,31-32H | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Cubist Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Inhibitory activity against phenylalanyl-tRNA synthetase from Enterococcus faecalis |
Bioorg Med Chem Lett 14: 1339-42 (2004)
Article DOI: 10.1016/j.bmcl.2003.11.081
BindingDB Entry DOI: 10.7270/Q2PR7VDS |
More data for this
Ligand-Target Pair | |
Phenylalanine--tRNA ligase, mitochondrial
(Homo sapiens (Human)) | BDBM50140707
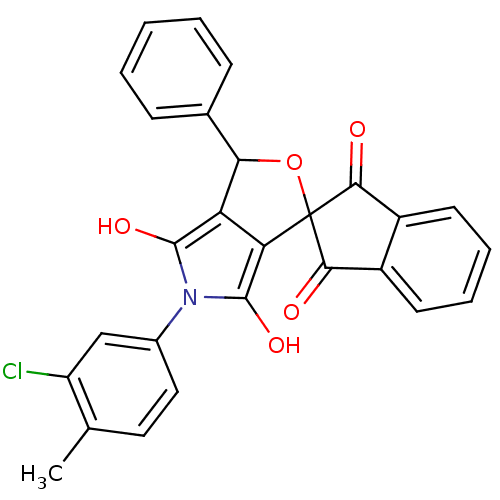
(5-(3-chloro-4-methylphenyl)-3-phenyl-(3R,3aS,6aR)-...)Show SMILES Cc1ccc(cc1Cl)-n1c(O)c2C(OC3(C(=O)c4ccccc4C3=O)c2c1O)c1ccccc1 Show InChI InChI=1S/C27H18ClNO5/c1-14-11-12-16(13-19(14)28)29-25(32)20-21(26(29)33)27(34-22(20)15-7-3-2-4-8-15)23(30)17-9-5-6-10-18(17)24(27)31/h2-13,22,32-33H,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Cubist Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Inhibitory activity against phenylalanyl-tRNA synthetase from Enterococcus faecalis |
Bioorg Med Chem Lett 14: 1339-42 (2004)
Article DOI: 10.1016/j.bmcl.2003.11.081
BindingDB Entry DOI: 10.7270/Q2PR7VDS |
More data for this
Ligand-Target Pair | |
Phenylalanine--tRNA ligase, mitochondrial
(Homo sapiens (Human)) | BDBM50140726

(13N-(3,4-dichlorophenyl)-2-hydroxy-11-oxo-14-pheny...)Show SMILES OC1c2ccccc2C2OC(=O)[C@@H]3[C@@H]([C@@H](O[C@@]123)c1ccccc1)C(=O)Nc1ccc(Cl)c(Cl)c1 Show InChI InChI=1S/C26H19Cl2NO5/c27-17-11-10-14(12-18(17)28)29-24(31)19-20-25(32)33-23-16-9-5-4-8-15(16)22(30)26(20,23)34-21(19)13-6-2-1-3-7-13/h1-12,19-23,30H,(H,29,31)/t19-,20-,21-,22?,23?,26-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Cubist Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Compound was evaluated for the inhibitory activity against Staphylococcus aureus phenylalanyl-tRNA synthetase |
Bioorg Med Chem Lett 14: 1343-6 (2004)
Article DOI: 10.1016/j.bmcl.2003.11.082
BindingDB Entry DOI: 10.7270/Q2K073Q6 |
More data for this
Ligand-Target Pair | |
Phenylalanine--tRNA ligase, mitochondrial
(Homo sapiens (Human)) | BDBM50140706

(5-(3,4-dichlorophenyl)-3-phenyl-(3R,3aR,6aS)-spiro...)Show SMILES Oc1c2C(OC3(C(=O)c4ccccc4C3=O)c2c(O)n1-c1ccc(Cl)c(Cl)c1)c1ccccc1 Show InChI InChI=1S/C26H15Cl2NO5/c27-17-11-10-14(12-18(17)28)29-24(32)19-20(25(29)33)26(34-21(19)13-6-2-1-3-7-13)22(30)15-8-4-5-9-16(15)23(26)31/h1-12,21,32-33H | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Cubist Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Inhibitory activity against phenylalanyl-tRNA synthetase from Staphylococcus aureus |
Bioorg Med Chem Lett 14: 1339-42 (2004)
Article DOI: 10.1016/j.bmcl.2003.11.081
BindingDB Entry DOI: 10.7270/Q2PR7VDS |
More data for this
Ligand-Target Pair | |
Phenylalanine--tRNA ligase, mitochondrial
(Homo sapiens (Human)) | BDBM50140704

(5-(4-chloro-3-trifluoromethylphenyl)-3-phenyl-(3R,...)Show SMILES Oc1c2C(OC3(C(=O)c4ccccc4C3=O)c2c(O)n1-c1ccc(Cl)c(c1)C(F)(F)F)c1ccccc1 Show InChI InChI=1S/C27H15ClF3NO5/c28-18-11-10-14(12-17(18)27(29,30)31)32-24(35)19-20(25(32)36)26(37-21(19)13-6-2-1-3-7-13)22(33)15-8-4-5-9-16(15)23(26)34/h1-12,21,35-36H | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Cubist Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Inhibitory activity against human phenylalanyl-tRNA synthetase was determined |
Bioorg Med Chem Lett 14: 1339-42 (2004)
Article DOI: 10.1016/j.bmcl.2003.11.081
BindingDB Entry DOI: 10.7270/Q2PR7VDS |
More data for this
Ligand-Target Pair | |
Phenylalanine--tRNA ligase, mitochondrial
(Homo sapiens (Human)) | BDBM50140706

(5-(3,4-dichlorophenyl)-3-phenyl-(3R,3aR,6aS)-spiro...)Show SMILES Oc1c2C(OC3(C(=O)c4ccccc4C3=O)c2c(O)n1-c1ccc(Cl)c(Cl)c1)c1ccccc1 Show InChI InChI=1S/C26H15Cl2NO5/c27-17-11-10-14(12-18(17)28)29-24(32)19-20(25(29)33)26(34-21(19)13-6-2-1-3-7-13)22(30)15-8-4-5-9-16(15)23(26)31/h1-12,21,32-33H | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Cubist Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Inhibitory activity against human phenylalanyl-tRNA synthetase was determined |
Bioorg Med Chem Lett 14: 1339-42 (2004)
Article DOI: 10.1016/j.bmcl.2003.11.081
BindingDB Entry DOI: 10.7270/Q2PR7VDS |
More data for this
Ligand-Target Pair | |
Phenylalanine--tRNA ligase, mitochondrial
(Homo sapiens (Human)) | BDBM50140705

(5-(2-methoxyphenyl)-3-phenylspiro[perhydrofuro[3,4...)Show SMILES COc1ccccc1-n1c(O)c2C(OC3(C(=O)c4ccccc4C3=O)c2c1O)c1ccccc1 |(13.87,-8.94,;12.54,-8.15,;11.21,-8.92,;11.21,-10.46,;9.88,-11.25,;8.54,-10.46,;8.54,-8.92,;9.88,-8.15,;9.88,-6.61,;11.08,-5.67,;12.58,-6.1,;10.55,-4.22,;10.95,-2.74,;9.67,-1.89,;8.47,-2.85,;8.31,-1.31,;9.46,-.29,;6.8,-.99,;6.03,.34,;4.49,.34,;3.72,-.99,;4.49,-2.32,;6.03,-2.32,;7.08,-3.48,;6.79,-5,;9.01,-4.29,;8.59,-5.77,;7.15,-6.31,;12.42,-2.27,;13.56,-3.31,;15.03,-2.85,;15.36,-1.36,;14.22,-.31,;12.76,-.78,)| Show InChI InChI=1S/C27H19NO6/c1-33-19-14-8-7-13-18(19)28-25(31)20-21(26(28)32)27(34-22(20)15-9-3-2-4-10-15)23(29)16-11-5-6-12-17(16)24(27)30/h2-14,22,31-32H,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Cubist Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Inhibitory activity against human phenylalanyl-tRNA synthetase was determined |
Bioorg Med Chem Lett 14: 1339-42 (2004)
Article DOI: 10.1016/j.bmcl.2003.11.081
BindingDB Entry DOI: 10.7270/Q2PR7VDS |
More data for this
Ligand-Target Pair | |
Phenylalanine--tRNA ligase, mitochondrial
(Homo sapiens (Human)) | BDBM50140711

(5'-(3,4-dichlorophenyl)-3'-phenyl-(3'R,3a'R,6a'S)-...)Show SMILES Oc1c2C(NC3(Cc4ccccc4C3)c2c(O)n1-c1ccc(Cl)c(Cl)c1)c1ccccc1 Show InChI InChI=1S/C26H20Cl2N2O2/c27-19-11-10-18(12-20(19)28)30-24(31)21-22(25(30)32)26(13-16-8-4-5-9-17(16)14-26)29-23(21)15-6-2-1-3-7-15/h1-12,23,29,31-32H,13-14H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Cubist Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Inhibitory activity against human phenylalanyl-tRNA synthetase was determined |
Bioorg Med Chem Lett 14: 1339-42 (2004)
Article DOI: 10.1016/j.bmcl.2003.11.081
BindingDB Entry DOI: 10.7270/Q2PR7VDS |
More data for this
Ligand-Target Pair | |
Phenylalanine--tRNA ligase, mitochondrial
(Homo sapiens (Human)) | BDBM50140707

(5-(3-chloro-4-methylphenyl)-3-phenyl-(3R,3aS,6aR)-...)Show SMILES Cc1ccc(cc1Cl)-n1c(O)c2C(OC3(C(=O)c4ccccc4C3=O)c2c1O)c1ccccc1 Show InChI InChI=1S/C27H18ClNO5/c1-14-11-12-16(13-19(14)28)29-25(32)20-21(26(29)33)27(34-22(20)15-7-3-2-4-8-15)23(30)17-9-5-6-10-18(17)24(27)31/h2-13,22,32-33H,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Cubist Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Inhibitory activity against human phenylalanyl-tRNA synthetase was determined |
Bioorg Med Chem Lett 14: 1339-42 (2004)
Article DOI: 10.1016/j.bmcl.2003.11.081
BindingDB Entry DOI: 10.7270/Q2PR7VDS |
More data for this
Ligand-Target Pair | |
Phenylalanine--tRNA ligase, mitochondrial
(Homo sapiens (Human)) | BDBM50140715
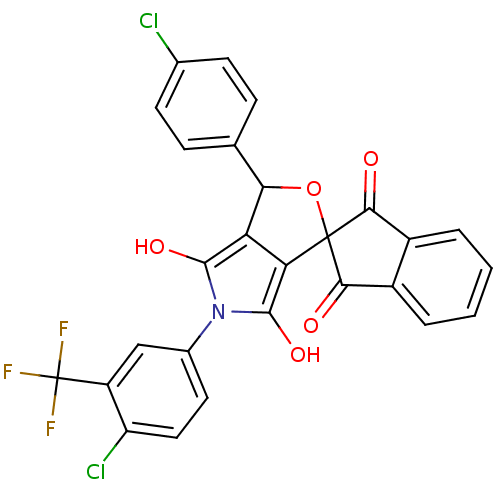
(3-(4-chlorophenyl)-5-(4-chloro-3-trifluoromethylph...)Show SMILES Oc1c2C(OC3(C(=O)c4ccccc4C3=O)c2c(O)n1-c1ccc(Cl)c(c1)C(F)(F)F)c1ccc(Cl)cc1 Show InChI InChI=1S/C27H14Cl2F3NO5/c28-13-7-5-12(6-8-13)21-19-20(26(38-21)22(34)15-3-1-2-4-16(15)23(26)35)25(37)33(24(19)36)14-9-10-18(29)17(11-14)27(30,31)32/h1-11,21,36-37H | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Cubist Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Inhibitory activity against human phenylalanyl-tRNA synthetase was determined |
Bioorg Med Chem Lett 14: 1339-42 (2004)
Article DOI: 10.1016/j.bmcl.2003.11.081
BindingDB Entry DOI: 10.7270/Q2PR7VDS |
More data for this
Ligand-Target Pair | |
Phenylalanine--tRNA ligase, mitochondrial
(Homo sapiens (Human)) | BDBM50140723

(5-(4-methoxyphenyl)-3-phenyl-(3R,3aS,6aR)-spiro[pe...)Show SMILES COc1ccc(cc1)-n1c(O)c2C(OC3(C(=O)c4ccccc4C3=O)c2c1O)c1ccccc1 Show InChI InChI=1S/C27H19NO6/c1-33-17-13-11-16(12-14-17)28-25(31)20-21(26(28)32)27(34-22(20)15-7-3-2-4-8-15)23(29)18-9-5-6-10-19(18)24(27)30/h2-14,22,31-32H,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Cubist Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Inhibitory activity against human phenylalanyl-tRNA synthetase was determined |
Bioorg Med Chem Lett 14: 1339-42 (2004)
Article DOI: 10.1016/j.bmcl.2003.11.081
BindingDB Entry DOI: 10.7270/Q2PR7VDS |
More data for this
Ligand-Target Pair | |
Phenylalanine--tRNA ligase, mitochondrial
(Homo sapiens (Human)) | BDBM50140708

(5-(2-methoxyphenyl)-3-(2-methylphenyl)-(3R,3aS,6aR...)Show SMILES COc1ccccc1-n1c(O)c2C(OC3(C(=O)c4ccccc4C3=O)c2c1O)c1ccccc1C |(13.87,-8.94,;12.54,-8.15,;11.21,-8.92,;11.21,-10.46,;9.88,-11.25,;8.54,-10.46,;8.54,-8.92,;9.88,-8.15,;9.88,-6.61,;11.08,-5.67,;12.58,-6.1,;10.55,-4.22,;10.95,-2.74,;9.67,-1.89,;8.47,-2.85,;7.08,-3.48,;6.79,-5,;6.03,-2.32,;4.49,-2.32,;3.72,-.99,;4.49,.34,;6.03,.34,;6.8,-.99,;8.31,-1.31,;9.46,-.29,;9.01,-4.29,;8.59,-5.77,;7.15,-6.31,;12.42,-2.27,;12.76,-.78,;14.22,-.31,;15.36,-1.36,;15.03,-2.85,;13.56,-3.31,;13.24,-4.81,)| Show InChI InChI=1S/C28H21NO6/c1-15-9-3-4-10-16(15)23-21-22(27(33)29(26(21)32)19-13-7-8-14-20(19)34-2)28(35-23)24(30)17-11-5-6-12-18(17)25(28)31/h3-14,23,32-33H,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Cubist Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Inhibitory activity against human phenylalanyl-tRNA synthetase was determined |
Bioorg Med Chem Lett 14: 1339-42 (2004)
Article DOI: 10.1016/j.bmcl.2003.11.081
BindingDB Entry DOI: 10.7270/Q2PR7VDS |
More data for this
Ligand-Target Pair | |
Phenylalanine--tRNA ligase, mitochondrial
(Homo sapiens (Human)) | BDBM50140717

(5-benzo[d][1,3]dioxol-5-yl-3-phenylspiro[perhydrof...)Show SMILES Oc1c2C(OC3(C(=O)c4ccccc4C3=O)c2c(O)n1-c1ccc2OCOc2c1)c1ccccc1 Show InChI InChI=1S/C27H17NO7/c29-23-16-8-4-5-9-17(16)24(30)27(23)21-20(22(35-27)14-6-2-1-3-7-14)25(31)28(26(21)32)15-10-11-18-19(12-15)34-13-33-18/h1-12,22,31-32H,13H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Cubist Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Inhibitory activity against human phenylalanyl-tRNA synthetase was determined |
Bioorg Med Chem Lett 14: 1339-42 (2004)
Article DOI: 10.1016/j.bmcl.2003.11.081
BindingDB Entry DOI: 10.7270/Q2PR7VDS |
More data for this
Ligand-Target Pair | |
Phenylalanine--tRNA ligase, mitochondrial
(Homo sapiens (Human)) | BDBM50140720

(5-(4-nitrophenyl)-3-phenyl-(3R,3aS,6aR)-spiro[perh...)Show SMILES Oc1c2C(OC3(C(=O)c4ccccc4C3=O)c2c(O)n1-c1ccc(cc1)[N+]([O-])=O)c1ccccc1 Show InChI InChI=1S/C26H16N2O7/c29-22-17-8-4-5-9-18(17)23(30)26(22)20-19(21(35-26)14-6-2-1-3-7-14)24(31)27(25(20)32)15-10-12-16(13-11-15)28(33)34/h1-13,21,31-32H | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Cubist Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Inhibitory activity against human phenylalanyl-tRNA synthetase was determined |
Bioorg Med Chem Lett 14: 1339-42 (2004)
Article DOI: 10.1016/j.bmcl.2003.11.081
BindingDB Entry DOI: 10.7270/Q2PR7VDS |
More data for this
Ligand-Target Pair | |
Phenylalanine--tRNA ligase, mitochondrial
(Homo sapiens (Human)) | BDBM50140709
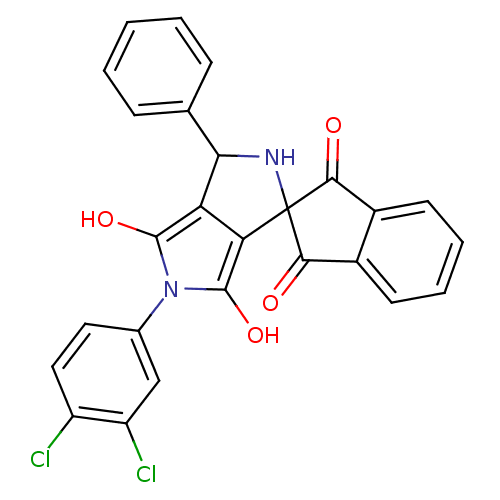
(5'-(3,4-dichlorophenyl)-3'-phenyl-(3'R,3a'S,6a'R)-...)Show SMILES Oc1c2C(NC3(C(=O)c4ccccc4C3=O)c2c(O)n1-c1ccc(Cl)c(Cl)c1)c1ccccc1 Show InChI InChI=1S/C26H16Cl2N2O4/c27-17-11-10-14(12-18(17)28)30-24(33)19-20(25(30)34)26(29-21(19)13-6-2-1-3-7-13)22(31)15-8-4-5-9-16(15)23(26)32/h1-12,21,29,33-34H | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Cubist Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Inhibitory activity against human phenylalanyl-tRNA synthetase was determined |
Bioorg Med Chem Lett 14: 1339-42 (2004)
Article DOI: 10.1016/j.bmcl.2003.11.081
BindingDB Entry DOI: 10.7270/Q2PR7VDS |
More data for this
Ligand-Target Pair | |
Phenylalanine--tRNA ligase, mitochondrial
(Homo sapiens (Human)) | BDBM50140714

(CHEMBL27142 | methyl 5-(3,4-dichlorophenyl)-4,6-di...)Show SMILES COC(=O)C1NC(c2c(O)n(c(O)c12)-c1ccc(Cl)c(Cl)c1)c1ccccc1 Show InChI InChI=1S/C20H16Cl2N2O4/c1-28-20(27)17-15-14(16(23-17)10-5-3-2-4-6-10)18(25)24(19(15)26)11-7-8-12(21)13(22)9-11/h2-9,16-17,23,25-26H,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Cubist Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Inhibitory activity against human phenylalanyl-tRNA synthetase was determined |
Bioorg Med Chem Lett 14: 1339-42 (2004)
Article DOI: 10.1016/j.bmcl.2003.11.081
BindingDB Entry DOI: 10.7270/Q2PR7VDS |
More data for this
Ligand-Target Pair | |
Phenylalanine--tRNA ligase, mitochondrial
(Homo sapiens (Human)) | BDBM50140722
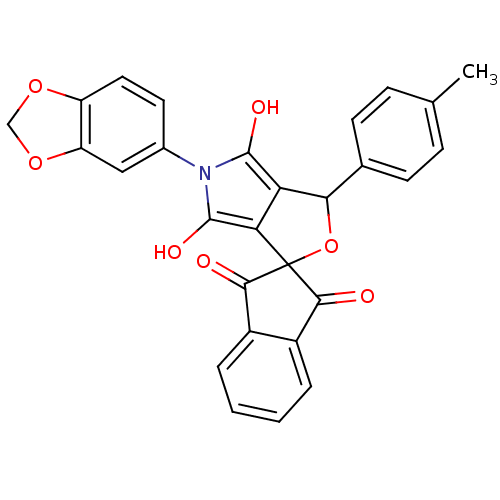
(5-benzo[d][1,3]dioxol-5-yl-3-(4-methylphenyl)-(3R,...)Show SMILES Cc1ccc(cc1)C1OC2(C(=O)c3ccccc3C2=O)c2c(O)n(c(O)c12)-c1ccc2OCOc2c1 Show InChI InChI=1S/C28H19NO7/c1-14-6-8-15(9-7-14)23-21-22(28(36-23)24(30)17-4-2-3-5-18(17)25(28)31)27(33)29(26(21)32)16-10-11-19-20(12-16)35-13-34-19/h2-12,23,32-33H,13H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Cubist Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Inhibitory activity against human phenylalanyl-tRNA synthetase was determined |
Bioorg Med Chem Lett 14: 1339-42 (2004)
Article DOI: 10.1016/j.bmcl.2003.11.081
BindingDB Entry DOI: 10.7270/Q2PR7VDS |
More data for this
Ligand-Target Pair | |
Phenylalanine--tRNA ligase, mitochondrial
(Homo sapiens (Human)) | BDBM50140716

(5'-(3-chloro-4-methylphenyl)-3'-phenyl-(3'R,3a'S,6...)Show SMILES Cc1ccc(cc1Cl)-n1c(O)c2C(NC3(C(=O)c4ccccc4C3=O)c2c1O)c1ccccc1 Show InChI InChI=1S/C27H19ClN2O4/c1-14-11-12-16(13-19(14)28)30-25(33)20-21(26(30)34)27(29-22(20)15-7-3-2-4-8-15)23(31)17-9-5-6-10-18(17)24(27)32/h2-13,22,29,33-34H,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Cubist Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Inhibitory activity against human phenylalanyl-tRNA synthetase was determined |
Bioorg Med Chem Lett 14: 1339-42 (2004)
Article DOI: 10.1016/j.bmcl.2003.11.081
BindingDB Entry DOI: 10.7270/Q2PR7VDS |
More data for this
Ligand-Target Pair | |
Phenylalanine--tRNA ligase, mitochondrial
(Homo sapiens (Human)) | BDBM50140713

(4-benzoyl-2-(3,4-dichlorophenyl)-6-phenyl-(3aR,6R,...)Show SMILES Oc1c2C(NC(c2c(O)n1-c1ccc(Cl)c(Cl)c1)c1ccccc1)C(=O)c1ccccc1 Show InChI InChI=1S/C25H18Cl2N2O3/c26-17-12-11-16(13-18(17)27)29-24(31)19-20(25(29)32)22(23(30)15-9-5-2-6-10-15)28-21(19)14-7-3-1-4-8-14/h1-13,21-22,28,31-32H | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Cubist Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Inhibitory activity against human phenylalanyl-tRNA synthetase was determined |
Bioorg Med Chem Lett 14: 1339-42 (2004)
Article DOI: 10.1016/j.bmcl.2003.11.081
BindingDB Entry DOI: 10.7270/Q2PR7VDS |
More data for this
Ligand-Target Pair | |
Phenylalanine--tRNA ligase, mitochondrial
(Homo sapiens (Human)) | BDBM50140709

(5'-(3,4-dichlorophenyl)-3'-phenyl-(3'R,3a'S,6a'R)-...)Show SMILES Oc1c2C(NC3(C(=O)c4ccccc4C3=O)c2c(O)n1-c1ccc(Cl)c(Cl)c1)c1ccccc1 Show InChI InChI=1S/C26H16Cl2N2O4/c27-17-11-10-14(12-18(17)28)30-24(33)19-20(25(30)34)26(29-21(19)13-6-2-1-3-7-13)22(31)15-8-4-5-9-16(15)23(26)32/h1-12,21,29,33-34H | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Cubist Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Inhibitory activity against phenylalanyl-tRNA synthetase from Enterococcus faecalis |
Bioorg Med Chem Lett 14: 1339-42 (2004)
Article DOI: 10.1016/j.bmcl.2003.11.081
BindingDB Entry DOI: 10.7270/Q2PR7VDS |
More data for this
Ligand-Target Pair | |
Phenylalanine--tRNA ligase, mitochondrial
(Homo sapiens (Human)) | BDBM50140711

(5'-(3,4-dichlorophenyl)-3'-phenyl-(3'R,3a'R,6a'S)-...)Show SMILES Oc1c2C(NC3(Cc4ccccc4C3)c2c(O)n1-c1ccc(Cl)c(Cl)c1)c1ccccc1 Show InChI InChI=1S/C26H20Cl2N2O2/c27-19-11-10-18(12-20(19)28)30-24(31)21-22(25(30)32)26(13-16-8-4-5-9-17(16)14-26)29-23(21)15-6-2-1-3-7-15/h1-12,23,29,31-32H,13-14H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Cubist Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Inhibitory activity against phenylalanyl-tRNA synthetase from Enterococcus faecalis |
Bioorg Med Chem Lett 14: 1339-42 (2004)
Article DOI: 10.1016/j.bmcl.2003.11.081
BindingDB Entry DOI: 10.7270/Q2PR7VDS |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data