

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Tyrosine-protein phosphatase non-receptor type 11 [205-593] (Homo sapiens (Human)) | BDBM25127 ((2E)-3-{3-chloro-4-[3-(3,3-dimethylbut-1-yn-1-yl)-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 450 | n/a | n/a | n/a | n/a | 6.0 | 30 |
Burnham Institute for Medical Research | Assay Description The phosphatase-catalyzed hydrolysis of 6,8-difluoro-4-methylumbelliferyl phosphate was assayed in the presence of each test compound. The fluorescen... | J Med Chem 51: 5650-62 (2008) Article DOI: 10.1021/jm800456k BindingDB Entry DOI: 10.7270/Q24T6GNQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 11 [205-593] (Homo sapiens (Human)) | BDBM24533 ((3Z)-3-[2-(2-carboxyphenyl)hydrazin-1-ylidene]-2-o...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 800 | n/a | n/a | n/a | n/a | 7.0 | 22 |
Moffitt Cancer Center | Assay Description PTP activity was measured in a black 96-well plate. Reaction was initiated by addition of the substrate DiFMUP to enzyme mixtures containing test com... | J Med Chem 51: 4948-56 (2008) Article DOI: 10.1021/jm8002526 BindingDB Entry DOI: 10.7270/Q2XD0ZZ8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 11 [205-593] (Homo sapiens (Human)) | BDBM24548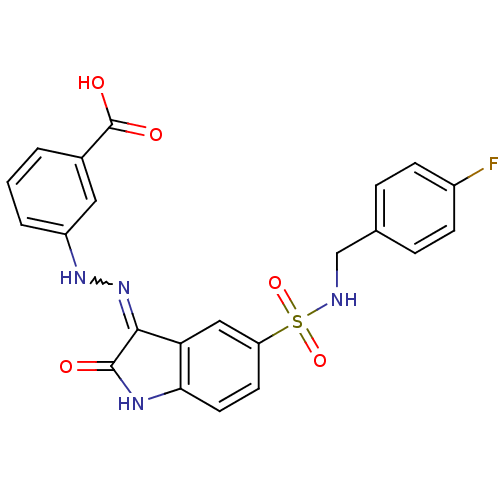 (3-{2-[(3Z)-5-{[(4-fluorophenyl)methyl]sulfamoyl}-2...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | 7.0 | 22 |
Moffitt Cancer Center | Assay Description PTP activity was measured in a black 96-well plate. Reaction was initiated by addition of the substrate DiFMUP to enzyme mixtures containing test com... | J Med Chem 51: 4948-56 (2008) Article DOI: 10.1021/jm8002526 BindingDB Entry DOI: 10.7270/Q2XD0ZZ8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 11 [205-593] (Homo sapiens (Human)) | BDBM24541 (4-{2-[(3Z)-5-(benzylsulfamoyl)-2-oxo-2,3-dihydro-1...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.30E+3 | n/a | n/a | n/a | n/a | 7.0 | 22 |
Moffitt Cancer Center | Assay Description PTP activity was measured in a black 96-well plate. Reaction was initiated by addition of the substrate DiFMUP to enzyme mixtures containing test com... | J Med Chem 51: 4948-56 (2008) Article DOI: 10.1021/jm8002526 BindingDB Entry DOI: 10.7270/Q2XD0ZZ8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 11 [205-593] (Homo sapiens (Human)) | BDBM24543 (3-{2-[(3Z)-5-{[(4-chlorophenyl)methyl]sulfamoyl}-2...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.40E+3 | n/a | n/a | n/a | n/a | 7.0 | 22 |
Moffitt Cancer Center | Assay Description PTP activity was measured in a black 96-well plate. Reaction was initiated by addition of the substrate DiFMUP to enzyme mixtures containing test com... | J Med Chem 51: 4948-56 (2008) Article DOI: 10.1021/jm8002526 BindingDB Entry DOI: 10.7270/Q2XD0ZZ8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 11 [205-593] (Homo sapiens (Human)) | BDBM25124 ((2E)-3-{4-[3-(adamantan-1-yl)-4-aminophenyl]-3-chl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.83E+3 | n/a | n/a | n/a | n/a | 6.0 | 30 |
Burnham Institute for Medical Research | Assay Description The phosphatase-catalyzed hydrolysis of 6,8-difluoro-4-methylumbelliferyl phosphate was assayed in the presence of each test compound. The fluorescen... | J Med Chem 51: 5650-62 (2008) Article DOI: 10.1021/jm800456k BindingDB Entry DOI: 10.7270/Q24T6GNQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 11 [205-593] (Homo sapiens (Human)) | BDBM25122 ((2E)-3-{4-[3-(adamantan-1-yl)-4-hydroxyphenyl]-3-c...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2.10E+3 | n/a | n/a | n/a | n/a | 6.0 | 30 |
Burnham Institute for Medical Research | Assay Description The phosphatase-catalyzed hydrolysis of 6,8-difluoro-4-methylumbelliferyl phosphate was assayed in the presence of each test compound. The fluorescen... | J Med Chem 51: 5650-62 (2008) Article DOI: 10.1021/jm800456k BindingDB Entry DOI: 10.7270/Q24T6GNQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 11 [205-593] (Homo sapiens (Human)) | BDBM25125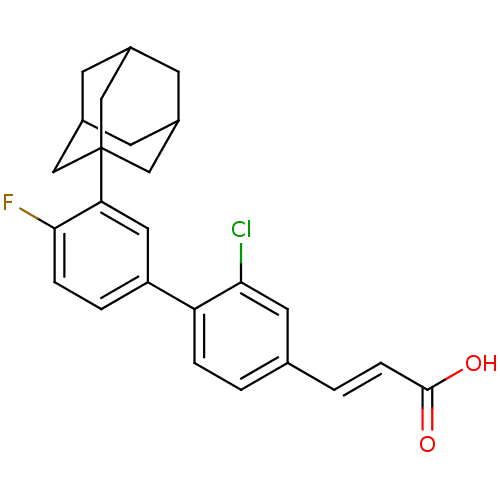 ((2E)-3-{4-[3-(adamantan-1-yl)-4-fluorophenyl]-3-ch...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.11E+3 | n/a | n/a | n/a | n/a | 6.0 | 30 |
Burnham Institute for Medical Research | Assay Description The phosphatase-catalyzed hydrolysis of 6,8-difluoro-4-methylumbelliferyl phosphate was assayed in the presence of each test compound. The fluorescen... | J Med Chem 51: 5650-62 (2008) Article DOI: 10.1021/jm800456k BindingDB Entry DOI: 10.7270/Q24T6GNQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 11 [205-593] (Homo sapiens (Human)) | BDBM25131 ((2E)-3-{3-chloro-4-[3-(3,5-dimethyladamantan-1-yl)...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2.27E+3 | n/a | n/a | n/a | n/a | 6.0 | 30 |
Burnham Institute for Medical Research | Assay Description The phosphatase-catalyzed hydrolysis of 6,8-difluoro-4-methylumbelliferyl phosphate was assayed in the presence of each test compound. The fluorescen... | J Med Chem 51: 5650-62 (2008) Article DOI: 10.1021/jm800456k BindingDB Entry DOI: 10.7270/Q24T6GNQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 11 [205-593] (Homo sapiens (Human)) | BDBM24542 (3-{2-[(3Z)-5-{[(3-chlorophenyl)methyl]sulfamoyl}-2...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.80E+3 | n/a | n/a | n/a | n/a | 7.0 | 22 |
Moffitt Cancer Center | Assay Description PTP activity was measured in a black 96-well plate. Reaction was initiated by addition of the substrate DiFMUP to enzyme mixtures containing test com... | J Med Chem 51: 4948-56 (2008) Article DOI: 10.1021/jm8002526 BindingDB Entry DOI: 10.7270/Q2XD0ZZ8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 11 [205-593] (Homo sapiens (Human)) | BDBM25129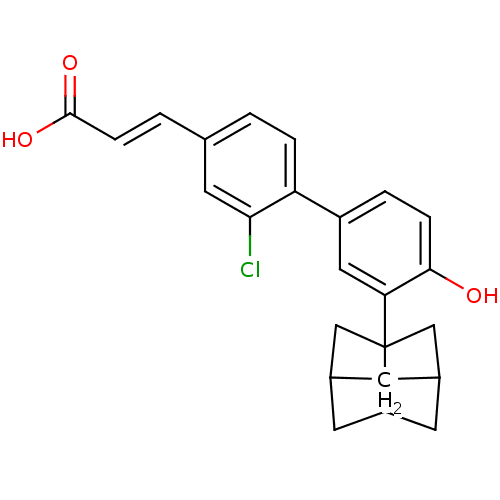 ((2E)-3-[3-chloro-4-(4-hydroxy-3-{tricyclo[3.3.1.0^...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.91E+3 | n/a | n/a | n/a | n/a | 6.0 | 30 |
Burnham Institute for Medical Research | Assay Description The phosphatase-catalyzed hydrolysis of 6,8-difluoro-4-methylumbelliferyl phosphate was assayed in the presence of each test compound. The fluorescen... | J Med Chem 51: 5650-62 (2008) Article DOI: 10.1021/jm800456k BindingDB Entry DOI: 10.7270/Q24T6GNQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 11 [205-593] (Homo sapiens (Human)) | BDBM24539 ((3Z)-N-[(4-chlorophenyl)methyl]-3-[2-(2-nitropheny...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 4.40E+3 | n/a | n/a | n/a | n/a | 7.0 | 22 |
Moffitt Cancer Center | Assay Description PTP activity was measured in a black 96-well plate. Reaction was initiated by addition of the substrate DiFMUP to enzyme mixtures containing test com... | J Med Chem 51: 4948-56 (2008) Article DOI: 10.1021/jm8002526 BindingDB Entry DOI: 10.7270/Q2XD0ZZ8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 11 [205-593] (Homo sapiens (Human)) | BDBM24537 (4-{2-[(3Z)-2-oxo-5-(propan-2-ylsulfamoyl)-2,3-dihy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.50E+3 | n/a | n/a | n/a | n/a | 7.0 | 22 |
Moffitt Cancer Center | Assay Description PTP activity was measured in a black 96-well plate. Reaction was initiated by addition of the substrate DiFMUP to enzyme mixtures containing test com... | J Med Chem 51: 4948-56 (2008) Article DOI: 10.1021/jm8002526 BindingDB Entry DOI: 10.7270/Q2XD0ZZ8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 11 [205-593] (Homo sapiens (Human)) | BDBM24536 (3-{2-[(3Z)-2-oxo-5-(propan-2-ylsulfamoyl)-2,3-dihy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 4.50E+3 | n/a | n/a | n/a | n/a | 7.0 | 22 |
Moffitt Cancer Center | Assay Description PTP activity was measured in a black 96-well plate. Reaction was initiated by addition of the substrate DiFMUP to enzyme mixtures containing test com... | J Med Chem 51: 4948-56 (2008) Article DOI: 10.1021/jm8002526 BindingDB Entry DOI: 10.7270/Q2XD0ZZ8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 11 [205-593] (Homo sapiens (Human)) | BDBM25123 ((2E)-3-{4-[3-(adamantan-1-yl)-4-acetamidophenyl]-3...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.55E+3 | n/a | n/a | n/a | n/a | 6.0 | 30 |
Burnham Institute for Medical Research | Assay Description The phosphatase-catalyzed hydrolysis of 6,8-difluoro-4-methylumbelliferyl phosphate was assayed in the presence of each test compound. The fluorescen... | J Med Chem 51: 5650-62 (2008) Article DOI: 10.1021/jm800456k BindingDB Entry DOI: 10.7270/Q24T6GNQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 11 [205-593] (Homo sapiens (Human)) | BDBM24550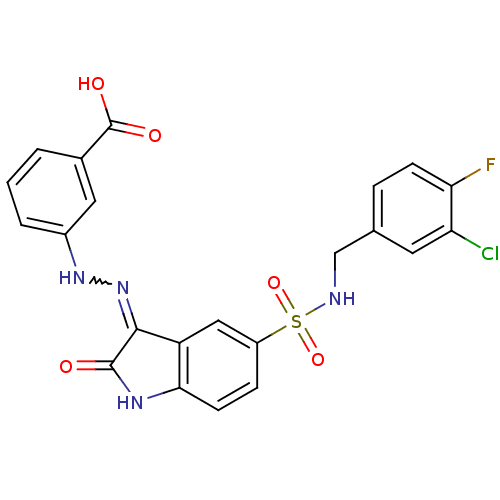 (3-{2-[(3Z)-5-{[(3-chloro-4-fluorophenyl)methyl]sul...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.80E+3 | n/a | n/a | n/a | n/a | 7.0 | 22 |
Moffitt Cancer Center | Assay Description PTP activity was measured in a black 96-well plate. Reaction was initiated by addition of the substrate DiFMUP to enzyme mixtures containing test com... | J Med Chem 51: 4948-56 (2008) Article DOI: 10.1021/jm8002526 BindingDB Entry DOI: 10.7270/Q2XD0ZZ8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 11 [205-593] (Homo sapiens (Human)) | BDBM24540 (3-{2-[(3Z)-5-{[(4-methylphenyl)methyl]sulfamoyl}-2...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.00E+3 | n/a | n/a | n/a | n/a | 7.0 | 22 |
Moffitt Cancer Center | Assay Description PTP activity was measured in a black 96-well plate. Reaction was initiated by addition of the substrate DiFMUP to enzyme mixtures containing test com... | J Med Chem 51: 4948-56 (2008) Article DOI: 10.1021/jm8002526 BindingDB Entry DOI: 10.7270/Q2XD0ZZ8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 11 [205-593] (Homo sapiens (Human)) | BDBM24551 (3-{2-[(3Z)-5-{[2-(2,4-dichlorophenyl)ethyl]sulfamo...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.20E+3 | n/a | n/a | n/a | n/a | 7.0 | 22 |
Moffitt Cancer Center | Assay Description PTP activity was measured in a black 96-well plate. Reaction was initiated by addition of the substrate DiFMUP to enzyme mixtures containing test com... | J Med Chem 51: 4948-56 (2008) Article DOI: 10.1021/jm8002526 BindingDB Entry DOI: 10.7270/Q2XD0ZZ8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 11 [205-593] (Homo sapiens (Human)) | BDBM25133 ((2E)-3-{3-chloro-4-[3-(3-ethylpentan-3-yl)-4-hydro...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 5.37E+3 | n/a | n/a | n/a | n/a | 6.0 | 30 |
Burnham Institute for Medical Research | Assay Description The phosphatase-catalyzed hydrolysis of 6,8-difluoro-4-methylumbelliferyl phosphate was assayed in the presence of each test compound. The fluorescen... | J Med Chem 51: 5650-62 (2008) Article DOI: 10.1021/jm800456k BindingDB Entry DOI: 10.7270/Q24T6GNQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 11 [205-593] (Homo sapiens (Human)) | BDBM24546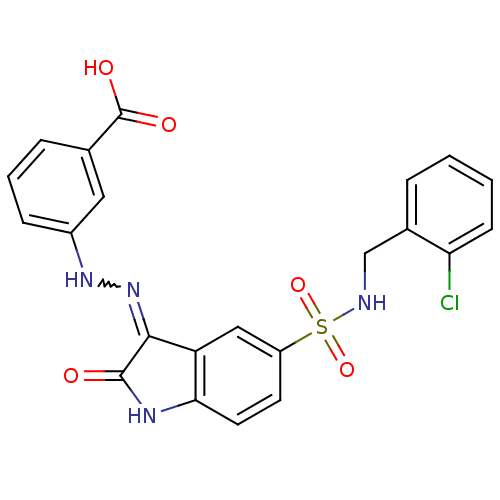 (3-{2-[(3Z)-5-{[(2-chlorophenyl)methyl]sulfamoyl}-2...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.50E+3 | n/a | n/a | n/a | n/a | 7.0 | 22 |
Moffitt Cancer Center | Assay Description PTP activity was measured in a black 96-well plate. Reaction was initiated by addition of the substrate DiFMUP to enzyme mixtures containing test com... | J Med Chem 51: 4948-56 (2008) Article DOI: 10.1021/jm8002526 BindingDB Entry DOI: 10.7270/Q2XD0ZZ8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 11 [205-593] (Homo sapiens (Human)) | BDBM24544 (4-{2-[(3Z)-5-{[(4-chlorophenyl)methyl]sulfamoyl}-2...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.90E+3 | n/a | n/a | n/a | n/a | 7.0 | 22 |
Moffitt Cancer Center | Assay Description PTP activity was measured in a black 96-well plate. Reaction was initiated by addition of the substrate DiFMUP to enzyme mixtures containing test com... | J Med Chem 51: 4948-56 (2008) Article DOI: 10.1021/jm8002526 BindingDB Entry DOI: 10.7270/Q2XD0ZZ8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 11 [205-593] (Homo sapiens (Human)) | BDBM24549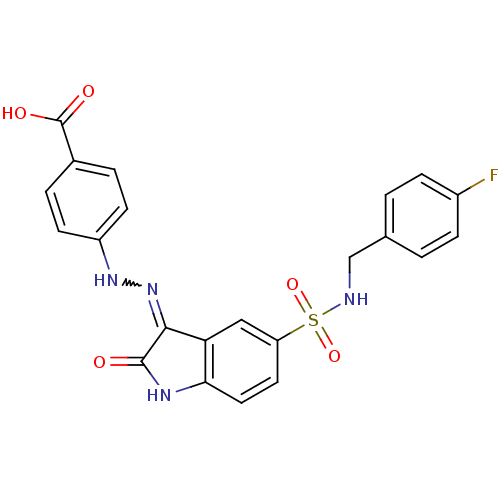 (4-{2-[(3Z)-5-{[(4-fluorophenyl)methyl]sulfamoyl}-2...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.30E+3 | n/a | n/a | n/a | n/a | 7.0 | 22 |
Moffitt Cancer Center | Assay Description PTP activity was measured in a black 96-well plate. Reaction was initiated by addition of the substrate DiFMUP to enzyme mixtures containing test com... | J Med Chem 51: 4948-56 (2008) Article DOI: 10.1021/jm8002526 BindingDB Entry DOI: 10.7270/Q2XD0ZZ8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 11 [205-593] (Homo sapiens (Human)) | BDBM25130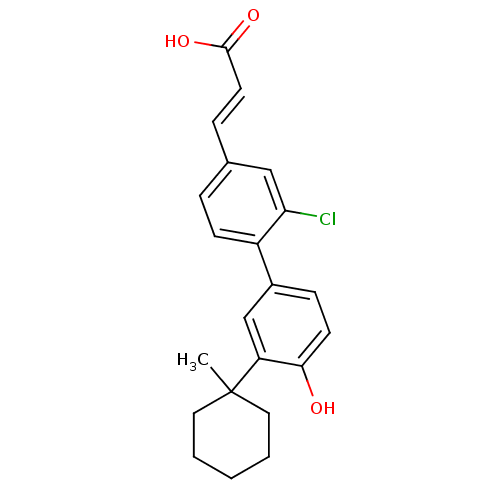 ((2E)-3-{3-chloro-4-[4-hydroxy-3-(1-methylcyclohexy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.40E+3 | n/a | n/a | n/a | n/a | 6.0 | 30 |
Burnham Institute for Medical Research | Assay Description The phosphatase-catalyzed hydrolysis of 6,8-difluoro-4-methylumbelliferyl phosphate was assayed in the presence of each test compound. The fluorescen... | J Med Chem 51: 5650-62 (2008) Article DOI: 10.1021/jm800456k BindingDB Entry DOI: 10.7270/Q24T6GNQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 11 [205-593] (Homo sapiens (Human)) | BDBM24545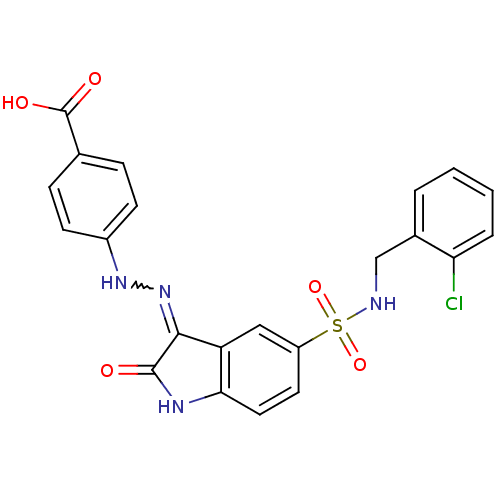 (4-{2-[(3Z)-5-{[(2-chlorophenyl)methyl]sulfamoyl}-2...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7.40E+3 | n/a | n/a | n/a | n/a | 7.0 | 22 |
Moffitt Cancer Center | Assay Description PTP activity was measured in a black 96-well plate. Reaction was initiated by addition of the substrate DiFMUP to enzyme mixtures containing test com... | J Med Chem 51: 4948-56 (2008) Article DOI: 10.1021/jm8002526 BindingDB Entry DOI: 10.7270/Q2XD0ZZ8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 11 [205-593] (Homo sapiens (Human)) | BDBM24552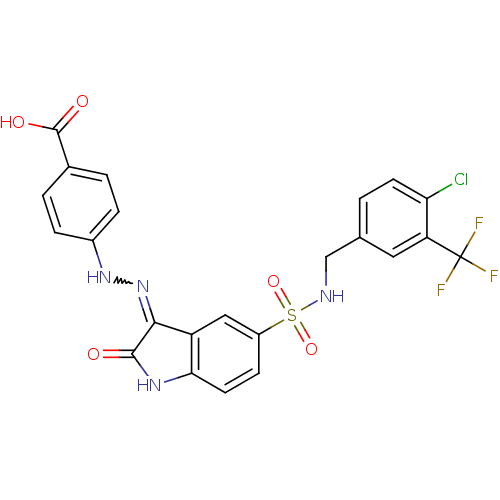 (4-{2-[(3Z)-5-({[4-chloro-3-(trifluoromethyl)phenyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8.30E+3 | n/a | n/a | n/a | n/a | 7.0 | 22 |
Moffitt Cancer Center | Assay Description PTP activity was measured in a black 96-well plate. Reaction was initiated by addition of the substrate DiFMUP to enzyme mixtures containing test com... | J Med Chem 51: 4948-56 (2008) Article DOI: 10.1021/jm8002526 BindingDB Entry DOI: 10.7270/Q2XD0ZZ8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 11 [205-593] (Homo sapiens (Human)) | BDBM25132 ((2E)-3-{3-chloro-4-[4-hydroxy-3-(2-methyl-1,3-dith...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.02E+4 | n/a | n/a | n/a | n/a | 6.0 | 30 |
Burnham Institute for Medical Research | Assay Description The phosphatase-catalyzed hydrolysis of 6,8-difluoro-4-methylumbelliferyl phosphate was assayed in the presence of each test compound. The fluorescen... | J Med Chem 51: 5650-62 (2008) Article DOI: 10.1021/jm800456k BindingDB Entry DOI: 10.7270/Q24T6GNQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 11 [205-593] (Homo sapiens (Human)) | BDBM24553 (3-{2-[(3Z)-5-({[4-chloro-3-(trifluoromethyl)phenyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.06E+4 | n/a | n/a | n/a | n/a | 7.0 | 22 |
Moffitt Cancer Center | Assay Description PTP activity was measured in a black 96-well plate. Reaction was initiated by addition of the substrate DiFMUP to enzyme mixtures containing test com... | J Med Chem 51: 4948-56 (2008) Article DOI: 10.1021/jm8002526 BindingDB Entry DOI: 10.7270/Q2XD0ZZ8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 11 [205-593] (Homo sapiens (Human)) | BDBM24538 ((3Z)-3-[2-(2-nitrophenyl)hydrazin-1-ylidene]-2-oxo...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.19E+4 | n/a | n/a | n/a | n/a | 7.0 | 22 |
Moffitt Cancer Center | Assay Description PTP activity was measured in a black 96-well plate. Reaction was initiated by addition of the substrate DiFMUP to enzyme mixtures containing test com... | J Med Chem 51: 4948-56 (2008) Article DOI: 10.1021/jm8002526 BindingDB Entry DOI: 10.7270/Q2XD0ZZ8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 11 [205-593] (Homo sapiens (Human)) | BDBM24534 ((3Z)-3-[2-(3-carboxyphenyl)hydrazin-1-ylidene]-2-o...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.58E+4 | n/a | n/a | n/a | n/a | 7.0 | 22 |
Moffitt Cancer Center | Assay Description PTP activity was measured in a black 96-well plate. Reaction was initiated by addition of the substrate DiFMUP to enzyme mixtures containing test com... | J Med Chem 51: 4948-56 (2008) Article DOI: 10.1021/jm8002526 BindingDB Entry DOI: 10.7270/Q2XD0ZZ8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 11 [205-593] (Homo sapiens (Human)) | BDBM25128 ((2E)-3-{3-chloro-4-[3-(2,6-dimethylphenyl)-4-hydro...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 1.99E+4 | n/a | n/a | n/a | n/a | 6.0 | 30 |
Burnham Institute for Medical Research | Assay Description The phosphatase-catalyzed hydrolysis of 6,8-difluoro-4-methylumbelliferyl phosphate was assayed in the presence of each test compound. The fluorescen... | J Med Chem 51: 5650-62 (2008) Article DOI: 10.1021/jm800456k BindingDB Entry DOI: 10.7270/Q24T6GNQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 11 [205-593] (Homo sapiens (Human)) | BDBM24535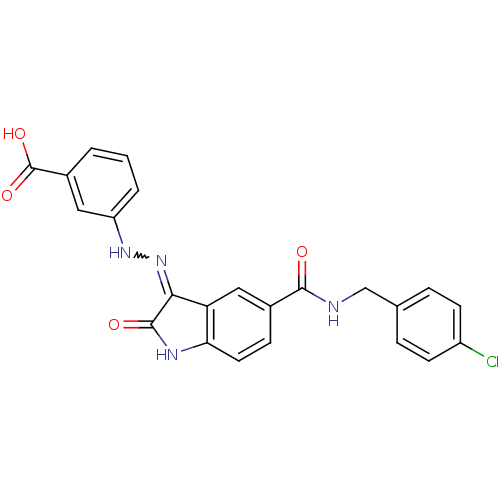 (3-{2-[(3Z)-5-{[(4-chlorophenyl)methyl]carbamoyl}-2...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2.23E+4 | n/a | n/a | n/a | n/a | 7.0 | 22 |
Moffitt Cancer Center | Assay Description PTP activity was measured in a black 96-well plate. Reaction was initiated by addition of the substrate DiFMUP to enzyme mixtures containing test com... | J Med Chem 51: 4948-56 (2008) Article DOI: 10.1021/jm8002526 BindingDB Entry DOI: 10.7270/Q2XD0ZZ8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 11 [205-593] (Homo sapiens (Human)) | BDBM24547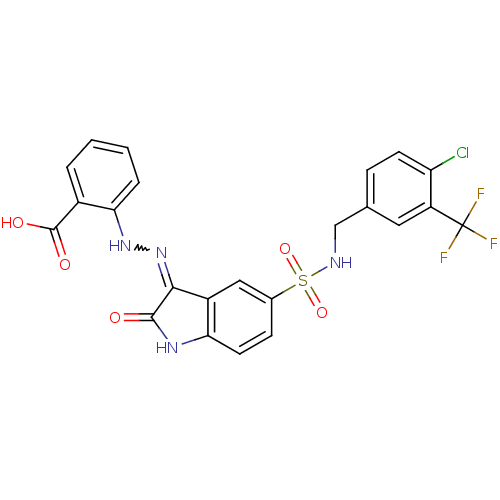 (2-{2-[(3Z)-5-({[4-chloro-3-(trifluoromethyl)phenyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.29E+4 | n/a | n/a | n/a | n/a | 7.0 | 22 |
Moffitt Cancer Center | Assay Description PTP activity was measured in a black 96-well plate. Reaction was initiated by addition of the substrate DiFMUP to enzyme mixtures containing test com... | J Med Chem 51: 4948-56 (2008) Article DOI: 10.1021/jm8002526 BindingDB Entry DOI: 10.7270/Q2XD0ZZ8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 11 [205-593] (Homo sapiens (Human)) | BDBM24531 ((3Z)-3-[2-(2-nitrophenyl)hydrazin-1-ylidene]-2-oxo...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 4.68E+4 | n/a | n/a | n/a | n/a | 7.0 | 22 |
Moffitt Cancer Center | Assay Description PTP activity was measured in a black 96-well plate. Reaction was initiated by addition of the substrate DiFMUP to enzyme mixtures containing test com... | J Med Chem 51: 4948-56 (2008) Article DOI: 10.1021/jm8002526 BindingDB Entry DOI: 10.7270/Q2XD0ZZ8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 11 [205-593] (Homo sapiens (Human)) | BDBM25126 ((2E)-3-[3-chloro-4-(4-fluorophenyl)phenyl]prop-2-e...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 5.00E+5 | n/a | n/a | n/a | n/a | 6.0 | 30 |
Burnham Institute for Medical Research | Assay Description The phosphatase-catalyzed hydrolysis of 6,8-difluoro-4-methylumbelliferyl phosphate was assayed in the presence of each test compound. The fluorescen... | J Med Chem 51: 5650-62 (2008) Article DOI: 10.1021/jm800456k BindingDB Entry DOI: 10.7270/Q24T6GNQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||